Abstract
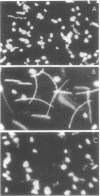
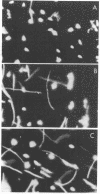
Salmonella typhimurium and Escherichia coli from anaerobic cultures displayed tactic responses to gradients of nitrate, fumarate, and oxygen when the appropriate electron transport pathway was present. Such responses were named "electron acceptor taxis" because they are elicited by terminal electron acceptors. Mutant strains of S. typhimurium and E. coli were used to establish that functioning electron transport pathways to nitrate and fumarate are required for taxis to these compounds. Aerotaxis in S. typhimurium was blocked by 1.0 mM KCN, which inhibited oxygen uptake. Similarly, a functioning electron transport pathway was shown to be essential for the tumbling response of S. typhimurium and E. coli to intense light (290 to 530 nm). Some inhibitors and uncouplers of respiration were repellents of S. typhimurium. We propose that behavioral responses to light or electron acceptors involve electron transport-mediated perturbations of the proton motive force.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler J. Chemotaxis in bacteria. Science. 1966 Aug 12;153(3737):708–716. doi: 10.1126/science.153.3737.708. [DOI] [PubMed] [Google Scholar]
- Adler J. Chemotaxis in bacteria. Annu Rev Biochem. 1975;44:341–356. doi: 10.1146/annurev.bi.44.070175.002013. [DOI] [PubMed] [Google Scholar]
- Adler J., Epstein W. Phosphotransferase-system enzymes as chemoreceptors for certain sugars in Escherichia coli chemotaxis. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2895–2899. doi: 10.1073/pnas.71.7.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aksamit R. R., Koshland D. E., Jr Identification of the ribose binding protein as the receptor for ribose chemotaxis in Salmonella typhimurium. Biochemistry. 1974 Oct 22;13(22):4473–4478. doi: 10.1021/bi00719a001. [DOI] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Isolation, characterization and complementation of Salmonella typhimurium chemotaxis mutants. J Mol Biol. 1975 Sep 15;97(2):225–235. doi: 10.1016/s0022-2836(75)80036-2. [DOI] [PubMed] [Google Scholar]
- Aswad D., Koshland D. E., Jr Role of methionine in bacterial chemotaxis. J Bacteriol. 1974 May;118(2):640–645. doi: 10.1128/jb.118.2.640-645.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARACCHINI O., SHERRIS J. C. The chemotactic effect of oxygen on bacteria. J Pathol Bacteriol. 1959 Apr;77(2):565–574. doi: 10.1002/path.1700770228. [DOI] [PubMed] [Google Scholar]
- Berg H. C., Brown D. A. Chemotaxis in Escherichia coli analysed by three-dimensional tracking. Nature. 1972 Oct 27;239(5374):500–504. doi: 10.1038/239500a0. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Chemotaxis in bacteria. Annu Rev Biophys Bioeng. 1975;4(00):119–136. doi: 10.1146/annurev.bb.04.060175.001003. [DOI] [PubMed] [Google Scholar]
- Cox G. B., Gibson F. Studies on electron transport and energy-linked reactions using mutants of Escherichia coli. Biochim Biophys Acta. 1974 Apr 30;346(1):1–25. doi: 10.1016/0304-4173(74)90010-x. [DOI] [PubMed] [Google Scholar]
- Grinius L., Brazenaite J. Membrane potential generation in mutants of Escherichia coli with alterations in the membrane ATPase complex. studies on intact cells. FEBS Lett. 1976 Feb 15;62(2):186–189. doi: 10.1016/0014-5793(76)80049-x. [DOI] [PubMed] [Google Scholar]
- Haddock B. A., Jones C. W. Bacterial respiration. Bacteriol Rev. 1977 Mar;41(1):47–99. doi: 10.1128/br.41.1.47-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harayama S., Iino T. Phototaxis and membrane potential in the photosynthetic bacterium Rhodospirillum rubrum. J Bacteriol. 1977 Jul;131(1):34–41. doi: 10.1128/jb.131.1.34-41.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Koiwai O., Kozuka M. Studies on bacterial chemotaxis. II. Effect of cheB and cheZ mutations on the methylation of methyl-accepting chemotaxis protein of Escherichia coli. J Biochem. 1979 May;85(5):1213–1223. [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Kellermann O., Szmelcman S. Active transport of maltose in Escherichia coli K12. Involvement of a "periplasmic" maltose binding protein. Eur J Biochem. 1974 Aug 15;47(1):139–149. doi: 10.1111/j.1432-1033.1974.tb03677.x. [DOI] [PubMed] [Google Scholar]
- Kim K. E., Chang G. W. Trimethylamine oxide reduction by Salmonella. Can J Microbiol. 1974 Dec;20(12):1745–1748. doi: 10.1139/m74-269. [DOI] [PubMed] [Google Scholar]
- Kondoh H., Ball C. B., Adler J. Identification of a methyl-accepting chemotaxis protein for the ribose and galactose chemoreceptors of Escherichia coli. Proc Natl Acad Sci U S A. 1979 Jan;76(1):260–264. doi: 10.1073/pnas.76.1.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshland D. E., Jr A response regulator model in a simple sensory system. Science. 1977 Jun 3;196(4294):1055–1063. doi: 10.1126/science.870969. [DOI] [PubMed] [Google Scholar]
- Larsen S. H., Adler J., Gargus J. J., Hogg R. W. Chemomechanical coupling without ATP: the source of energy for motility and chemotaxis in bacteria. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1239–1243. doi: 10.1073/pnas.71.4.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R. M. Bacterial motility and chemotaxis: the molecular biology of a behavioral system. CRC Crit Rev Biochem. 1978;5(4):291–341. doi: 10.3109/10409237809177145. [DOI] [PubMed] [Google Scholar]
- Macnab R. M., Koshland D. E., Jr The gradient-sensing mechanism in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2509–2512. doi: 10.1073/pnas.69.9.2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macnab R., Koshland D. E., Jr Bacterial motility and chemotaxis: light-induced tumbling response and visualization of individual flagella. J Mol Biol. 1974 Apr 15;84(3):399–406. doi: 10.1016/0022-2836(74)90448-3. [DOI] [PubMed] [Google Scholar]
- Mesibov R., Ordal G. W., Adler J. The range of attractant concentrations for bacterial chemotaxis and the threshold and size of response over this range. Weber law and related phenomena. J Gen Physiol. 1973 Aug;62(2):203–223. doi: 10.1085/jgp.62.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki K., Wilson T. H. Proton translocation associated with anaerobic transhydrogenation from glycerol 3-phosphate to fumarate in Escherichia coli. Biochem Biophys Res Commun. 1978 Aug 29;83(4):1570–1575. doi: 10.1016/0006-291x(78)91400-6. [DOI] [PubMed] [Google Scholar]
- Miller J. B., Koshland D. E., Jr Sensory electrophysiology of bacteria: relationship of the membrane potential to motility and chemotaxis in Bacillus subtilis. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4752–4756. doi: 10.1073/pnas.74.11.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S., Diehn B. Cytochrome c oxidase as the receptor molecule for chemoaccumulation (chemotaxis) of Euglena toward oxygen. Science. 1978 May 5;200(4341):548–549. doi: 10.1126/science.205948. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Properties of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):517–526. doi: 10.1128/jb.117.2.517-526.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ordal G. W., Goldman D. J. Chemotaxis away from uncouplers of oxidative phosphorylation in Bacillus subtilis. Science. 1975 Sep 5;189(4205):802–805. doi: 10.1126/science.808854. [DOI] [PubMed] [Google Scholar]
- SHERRIS J. C., PRESTON N. W., SHOESMITH J. G. The influence of oxygen and arginine on the motility of a strain of Pseudomonas sp. J Gen Microbiol. 1957 Feb;16(1):86–96. doi: 10.1099/00221287-16-1-86. [DOI] [PubMed] [Google Scholar]
- Silverman M., Simon M. Chemotaxis in Escherichia coli: methylation of che gene products. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3317–3321. doi: 10.1073/pnas.74.8.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer M. E., Guest J. R. Isolation and properties of fumarate reductase mutants of Escherichia coli. J Bacteriol. 1973 May;114(2):563–570. doi: 10.1128/jb.114.2.563-570.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer M. S., Goy M. F., Adler J. Sensory transduction in Escherichia coli: a requirement for methionine in sensory adaptation. Proc Natl Acad Sci U S A. 1977 Jan;74(1):183–187. doi: 10.1073/pnas.74.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spudich J. L., Koshland D. E., Jr Quantitation of the sensory response in bacterial chemotaxis. Proc Natl Acad Sci U S A. 1975 Feb;72(2):710–713. doi: 10.1073/pnas.72.2.710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strange P. G., Koshland D. E., Jr Receptor interactions in a signalling system: competition between ribose receptor and galactose receptor in the chemotaxis response. Proc Natl Acad Sci U S A. 1976 Mar;73(3):762–766. doi: 10.1073/pnas.73.3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor B. L., Koshland D. E., Jr Intrinsic and extrinsic light responses of Salmonella typhimurium and Escherichia coli. J Bacteriol. 1975 Aug;123(2):557–569. doi: 10.1128/jb.123.2.557-569.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]