Abstract
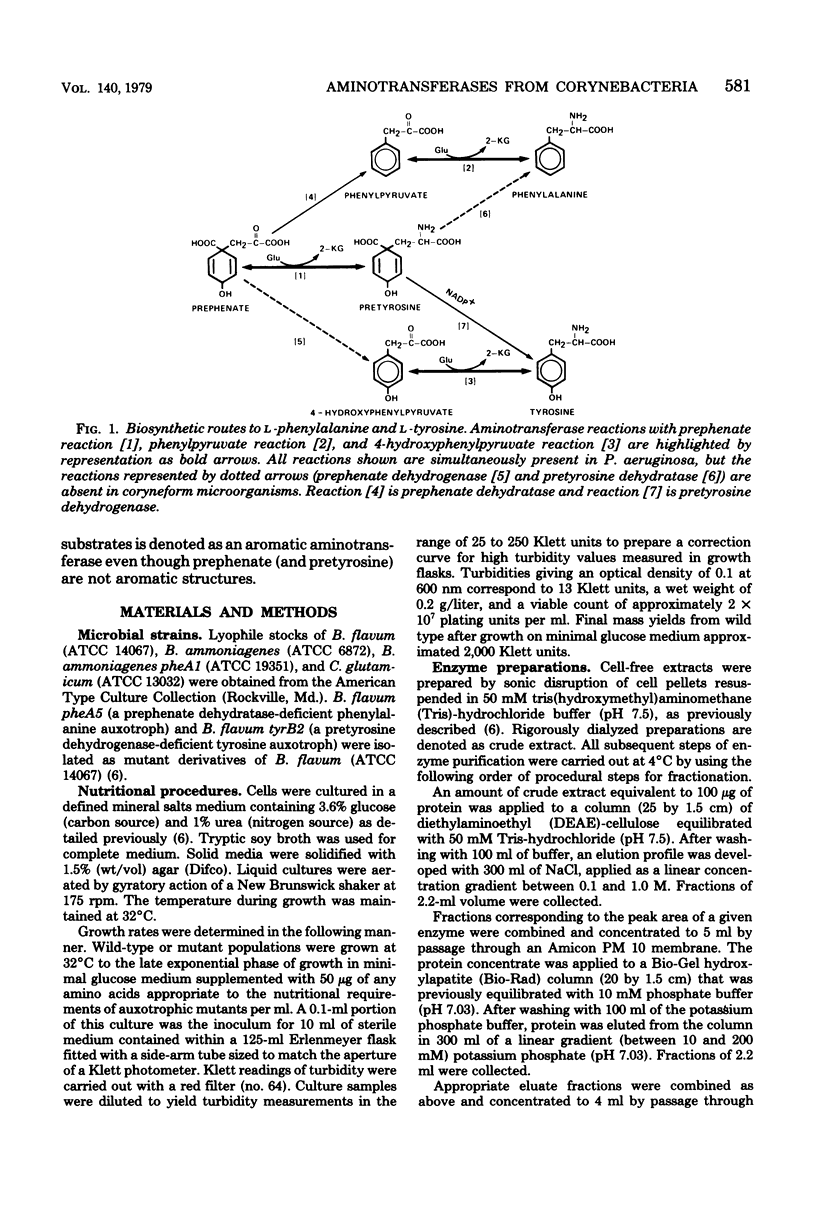
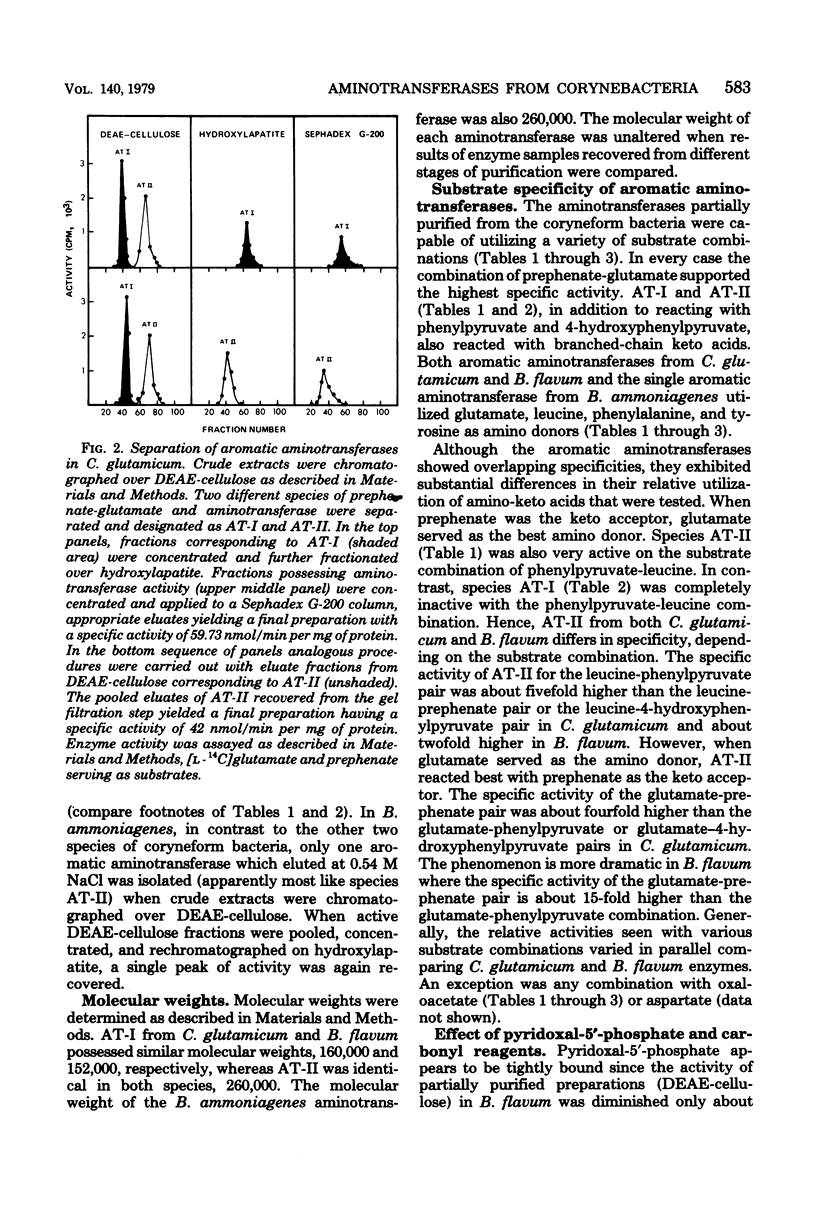
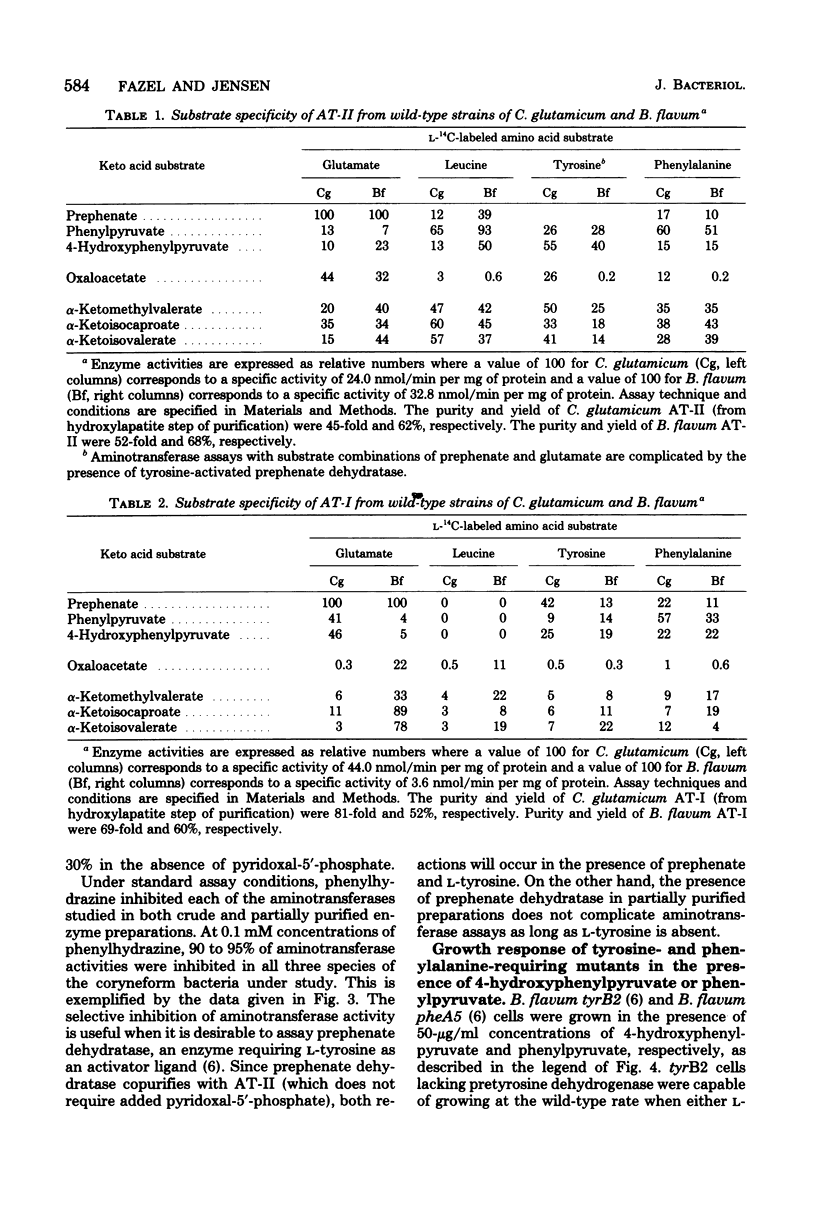
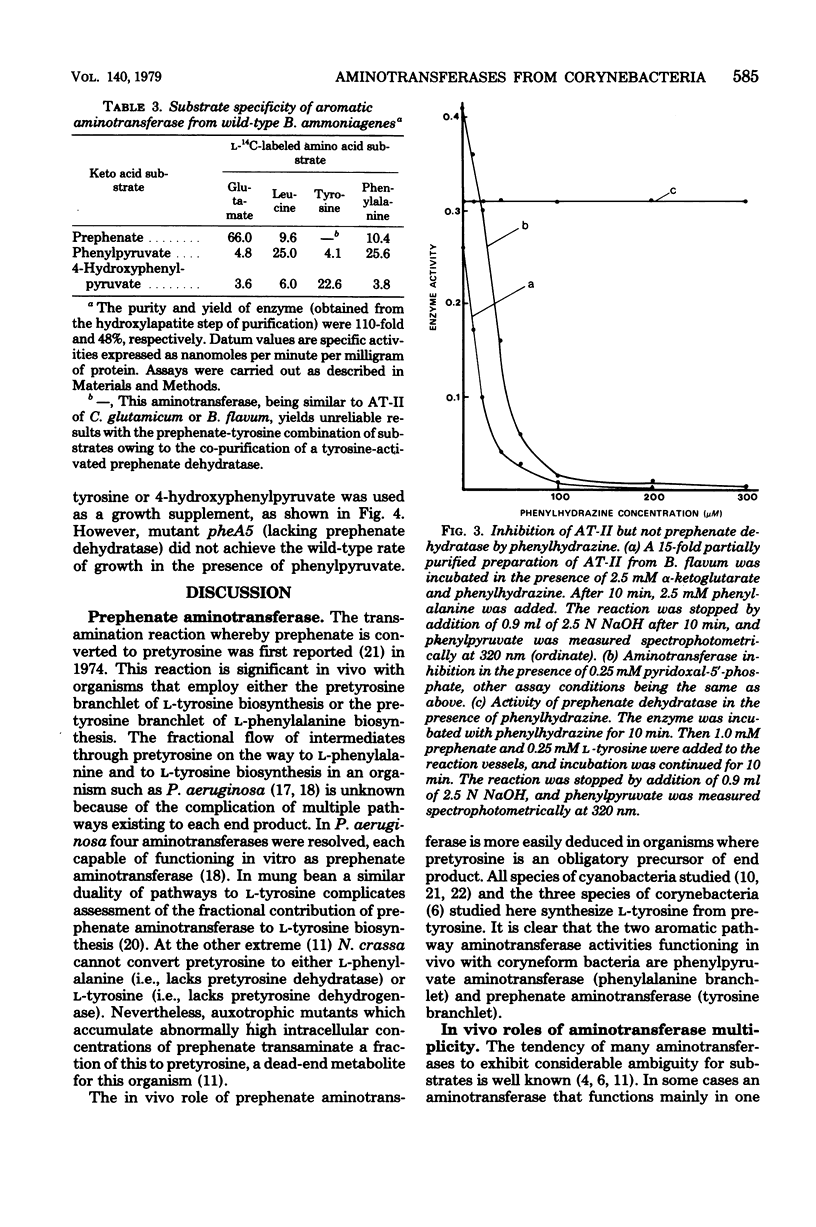
Species of coryneform bacteria (Corynebacterium glutamicum, Brevibacterium flavum, and B. ammoniagenes) are capable of transaminating all three of the aromatic pathway intermediates; prephenate, phenylpyruvate, and 4-hydroxy-phenylpyruvate. Two molecular species of aromatic aminotransferase (denoted aminotransferase I and aminotransferase II) were partially purified from C. glutamicum and B. flavum, whereas a single aromatic aminotransferase was isolated from B. ammoniagenes. In both C. glutamicum and B. flavum, aromatic aminotransferase I and aromatic aminotransferase II have molecular weights of about 155,000 and 260,000 respectively. The two aromatic aminotransferases from C. glutamicum and B. flavum, although exhibiting a similar spectrum of overlapping specificities, differ substantially in substrate preference. Pyridoxal-5'-phosphate is tightly associated with these aminotransferases, since little loss of activity was detected when partially purified enzyme preparations were assayed in the absence of exogenous pyridoxal-5'-phosphate. The aminotransferases are quite sensitive to inhibition by phenylhydrazine. This has practical application when assay of prephenate dehydratase is desired in the presence of aromatic aminotransferase activity since potentially trivial interference can be negated by selective phenylhydrazine inhibition of aromatic aminotransferase activity. At 0.1 mM concentrations of phenylhydrazine, 90% inhibitions of aminotransferase activities were achieved in partially purified preparations of B. flavum and C. glutamicum.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode R., Birnbaum D. Die Enzyme der Biosynthese aromatischer Aminosäuren bei Hansenula henricii: Nachweis und Charakterisierung der Enzyme des Pretyrosin-Weges. Z Allg Mikrobiol. 1979;19(2):83–88. doi: 10.1002/jobm.3630190203. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- COTTON R. G., GIBSON F. THE BIOSYNTHESIS OF PHENYLALANINE AND TYROSINE; ENZYMES CONVERTING CHORISMIC ACID INTO PREPHENIC ACID AND THEIR RELATIONSHIPS TO PREPHENATE DEHYDRATASE AND PREPHENATE DEHYDROGENASE. Biochim Biophys Acta. 1965 Apr 12;100:76–88. doi: 10.1016/0304-4165(65)90429-0. [DOI] [PubMed] [Google Scholar]
- Collier R. H., Kohlhaw G. Nonidentity of the aspartate and the aromatic aminotransferase components of transaminase A in Escherichia coli. J Bacteriol. 1972 Oct;112(1):365–371. doi: 10.1128/jb.112.1.365-371.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazel A. M., Jensen R. A. Obligatory biosynthesis of L-tyrosine via the pretyrosine branchlet in coryneform bacteria. J Bacteriol. 1979 Jun;138(3):805–815. doi: 10.1128/jb.138.3.805-815.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelfand D. H., Steinberg R. A. Escherichia coli mutants deficient in the aspartate and aromatic amino acid aminotransferases. J Bacteriol. 1977 Apr;130(1):429–440. doi: 10.1128/jb.130.1.429-440.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Pierson D. L. Evolutionary implications of different types of microbial enzymology for L-tyrosine biosynthesis. Nature. 1975 Apr 24;254(5502):667–671. doi: 10.1038/254667a0. [DOI] [PubMed] [Google Scholar]
- Jensen R. A., Zamir L., Saint Pierre M., Patel N., Pierson D. L. Isolation and preparation of pretyrosine, accumulated as a dead-end metabolite by Neurospora crassa. J Bacteriol. 1977 Dec;132(3):896–903. doi: 10.1128/jb.132.3.896-903.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mavrides C., Orr W. Multispecific aspartate and aromatic amino acid aminotransferases in Escherichia coli. J Biol Chem. 1975 Jun 10;250(11):4128–4133. [PubMed] [Google Scholar]
- McGilvray D., Umbarger H. E. Regulation of transaminase C synthesis in Escherichia coli: conditional leucine auxotrophy. J Bacteriol. 1974 Nov;120(2):715–723. doi: 10.1128/jb.120.2.715-723.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. V., Jr, Thompson E. B. Radioactive assay for tyrosine aminotransferase. Anal Biochem. 1972 Jun;47(2):487–494. doi: 10.1016/0003-2697(72)90142-x. [DOI] [PubMed] [Google Scholar]
- Nester E. W. Cross pathway regulation: effect of histidine on the synthesis and activity of enzymes of aromatic acid biosynthesis in Bacillus subtilis. J Bacteriol. 1968 Nov;96(5):1649–1657. doi: 10.1128/jb.96.5.1649-1657.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel N., Pierson D. L., Jensen R. A. Dual enzymatic routes to L-tyrosine and L-phenylalanine via pretyrosine in Pseudomonas aeruginosa. J Biol Chem. 1977 Aug 25;252(16):5839–5846. [PubMed] [Google Scholar]
- Patel N., Stenmark-Cox S. L., Jensen R. A. Enzymological basis of reluctant auxotrophy for phenylalanine and tyrosine in Pseudomonas aeruginosa. J Biol Chem. 1978 May 10;253(9):2972–2978. [PubMed] [Google Scholar]
- Powell J. T., Morrison J. F. Role of the Escherichia coli aromatic amino acid aminotransferase in leucine biosynthesis. J Bacteriol. 1978 Oct;136(1):1–4. doi: 10.1128/jb.136.1.1-4.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenmark S. L., Pierson D. L., Jensen R. A., Glover G. I. Blue-green bacteria synthesise L-tyrosine by the pretyrosine pathway. Nature. 1974 Feb 1;247(5439):290–292. doi: 10.1038/247290a0. [DOI] [PubMed] [Google Scholar]
- Umbarger H. E. Amino acid biosynthesis and its regulation. Annu Rev Biochem. 1978;47:532–606. doi: 10.1146/annurev.bi.47.070178.002533. [DOI] [PubMed] [Google Scholar]



