Abstract
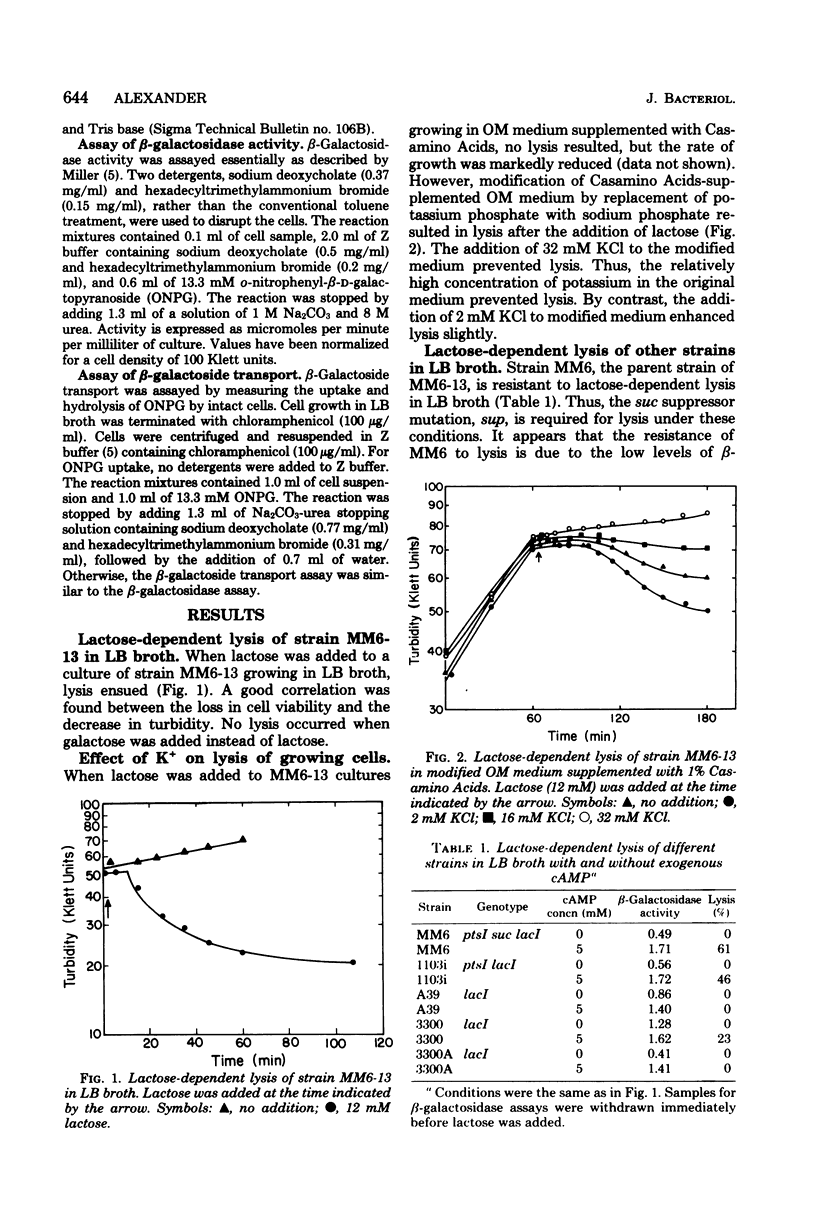
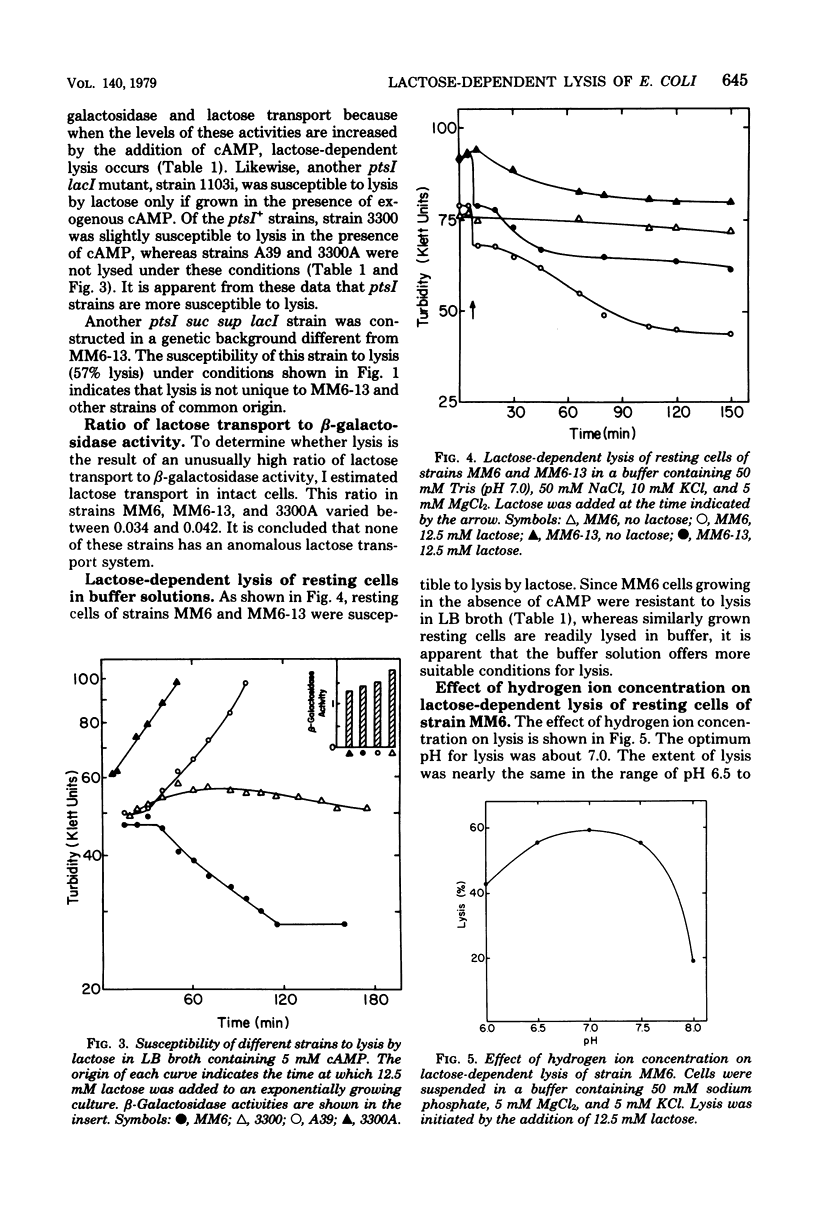
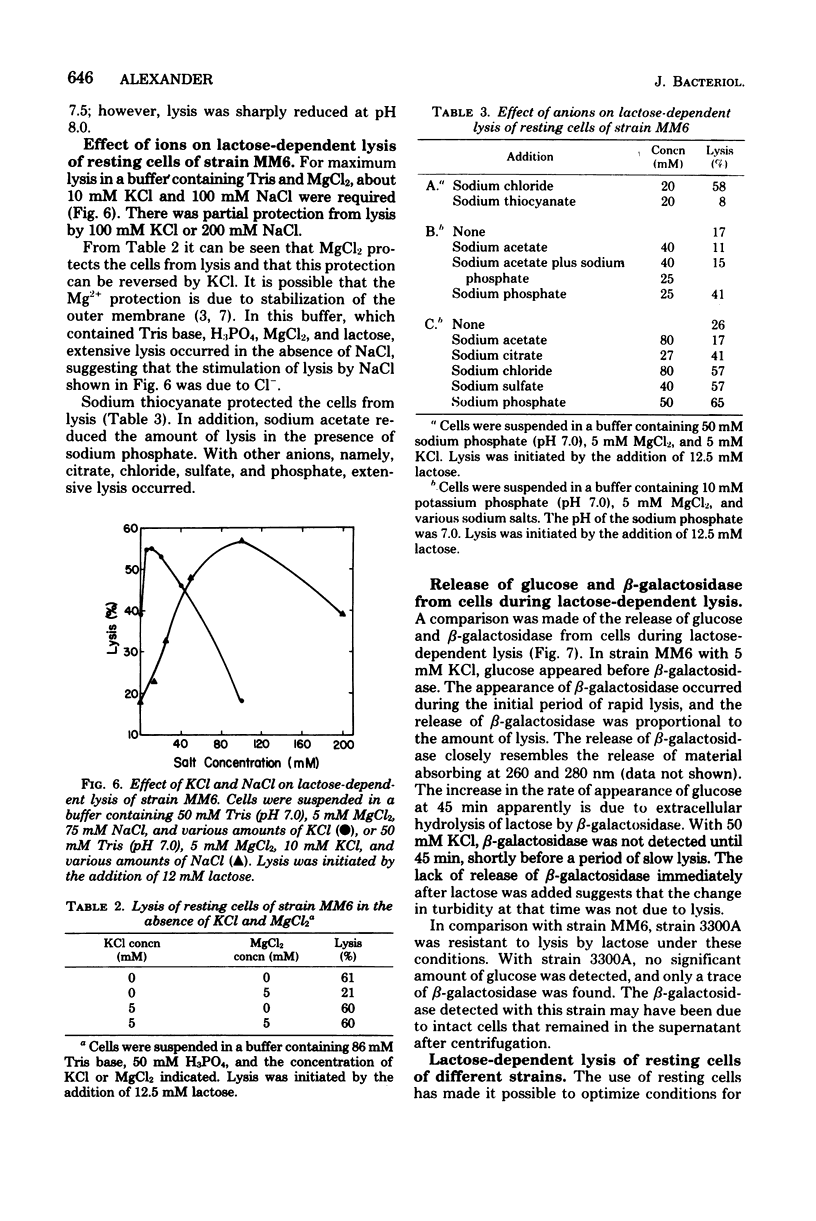
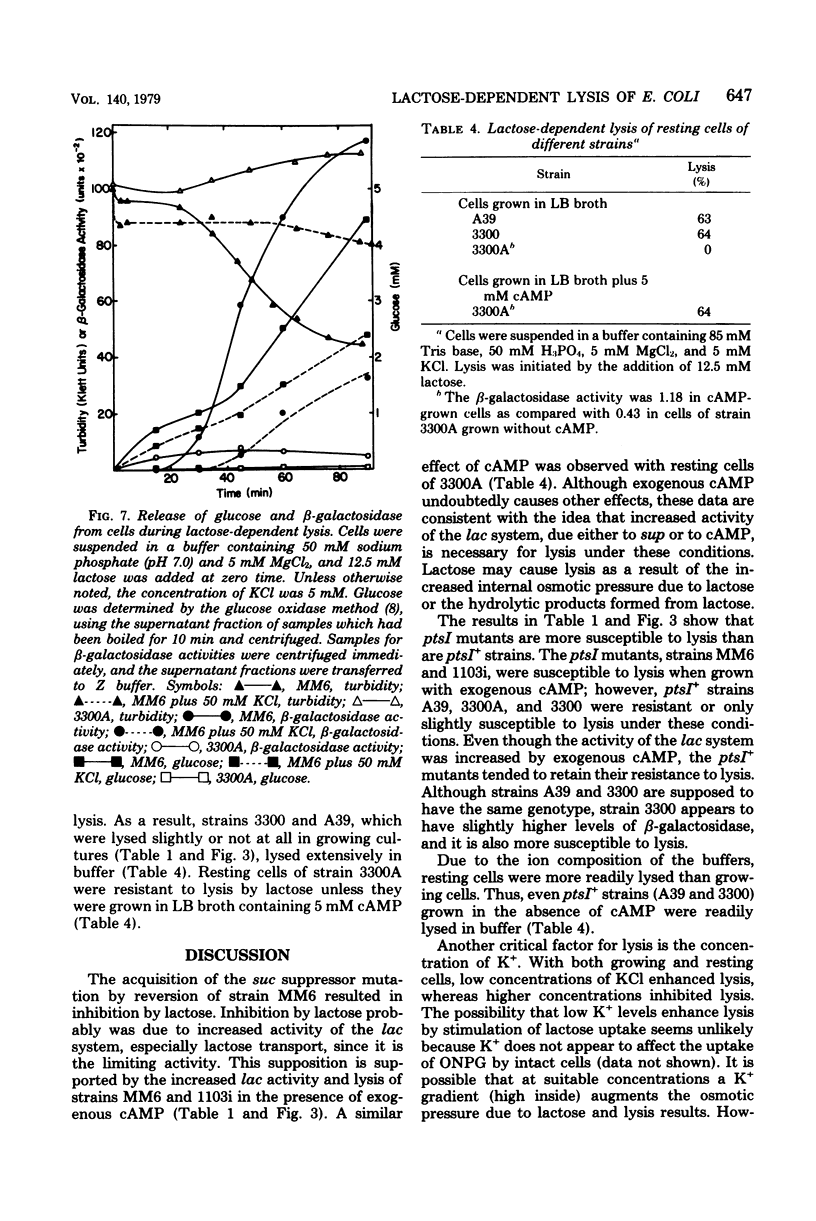
Growth of Escherichia coli strain MM6-13 (ptsI suc lacI sup), which as a suppressor of the succinate-negative phenotype, was inhibited by lactose. Cells growing in yeast extract-tryptone-sodium chloride medium (LB broth) were lysed upon the addition of lactose. In Casamino Acids-salts medium, lactose inhibited growth, but due to the high K+ content no lysis occurred. Lysis required high levels of beta-galctosidase and lactose transport activity. MM6, the parental strain of MM6-13, has lower levels of both of these activities and was resistant to lysis under these conditions. When MM6 was grown in LB broth with exogenous cyclic adenosine monophosphate, however, beta-galactosidase and lactose transport activities were greatly increased, and lysis occurred upon the addition of lactose. Resting cells of both MM6 and MM6-13 were lysed by lactose in buffers containing suitable ions. In the presence of MG2+, lysis was enhanced by 5 mM KCl and 100 mM NaCl. Higher slat concentrations (50 mM KCl or 200 mM NaCl) provided partial protection from lysis. In the absence of Mg2+, lysis occurred without KCl. Lactose-dependent lysis occurred in buffers containing anions such as sulafte, chloride, phosphate, or citrate; however, thiocyanate or acetate protected the cells from lysis. These data indicate that both cations and anions, as well as the levels of lactose transport and beta-galactosidase activity, are important in lysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. K., Tyler B. Genetic analysis of succinate utilization in enzyme I mutants of the phosphoenolpyruvate: sugar phosphotransferase system in Escherichia coli. J Bacteriol. 1975 Oct;124(1):252–261. doi: 10.1128/jb.124.1.252-261.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennedy C. K. Induction of colicin production by high temperature or inhibition of protein synthesis. J Bacteriol. 1971 Oct;108(1):10–19. doi: 10.1128/jb.108.1.10-19.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- Lo T. C., Rayman M. K., Sanwal B. D. Transport of succinate in Escherichia coli. I. Biochemical and genetic studies of transport in whole cells. J Biol Chem. 1972 Oct 10;247(19):6323–6331. [PubMed] [Google Scholar]
- SOLS A., DE LA FUENTE G. Hexokinase and other enzymes of sugar metabolism in the intestine. Methods Med Res. 1961;9:302–309. [PubMed] [Google Scholar]
- Wang R. J., Morse H. G., Morse M. L. Carbohydrate Accumulation and Metabolism in Escherichia coli: Characteristics of the Reversions of ctr Mutations. J Bacteriol. 1970 Dec;104(3):1318–1324. doi: 10.1128/jb.104.3.1318-1324.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]



