Abstract
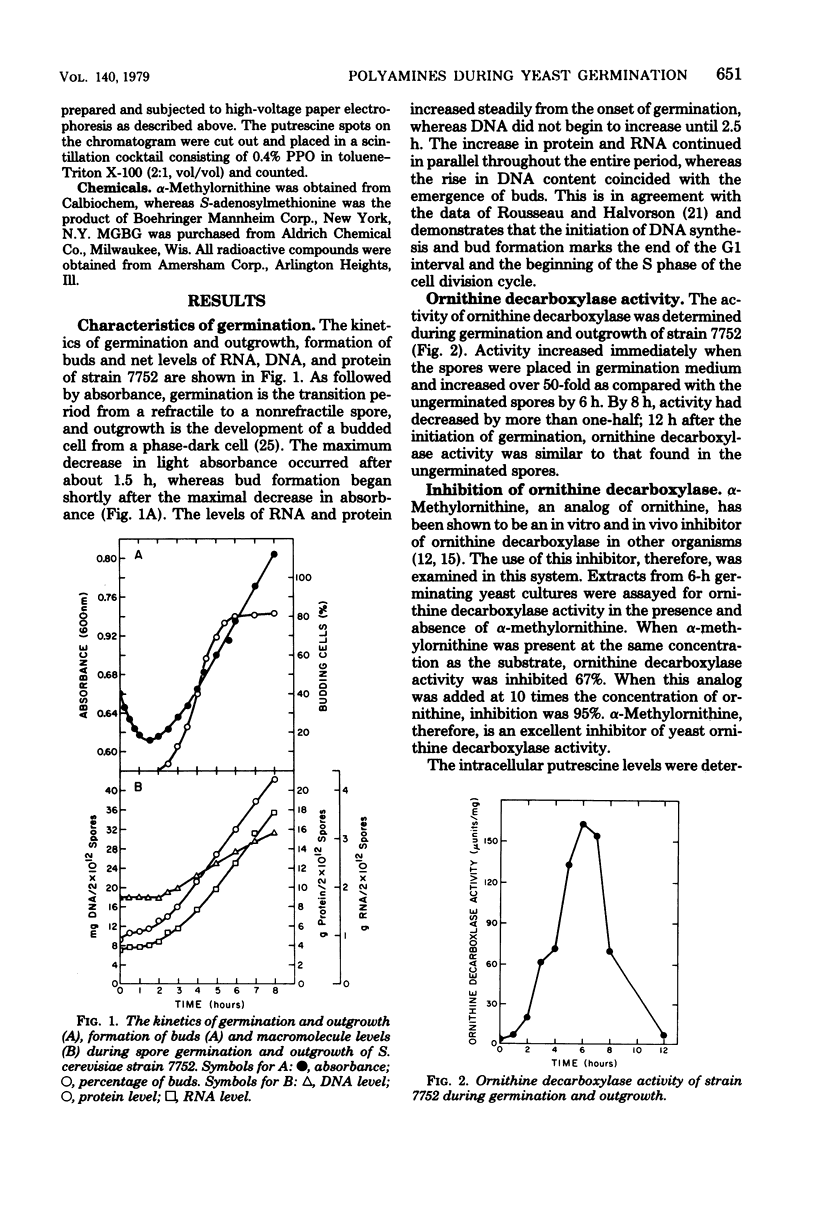
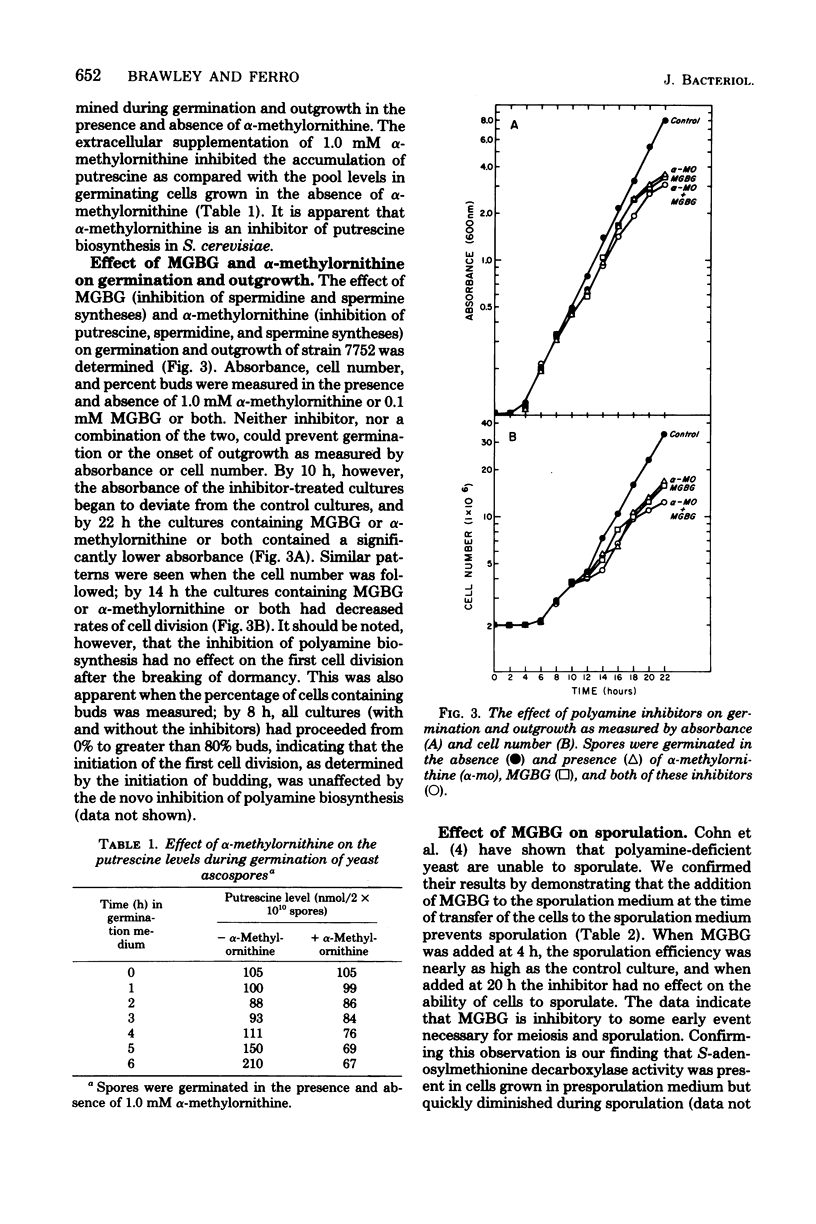
The role of the diamine putrescine during germination and outgrowth of ascospores of Saccharomyces cerevisiae was examined. Ornithine decarboxylase activity increased and declined rapidly during germination and outgrowth; peak activity was attained after the cells had proceeded through the G1 interval of the cell cycle, whereas minimal activity was present at the completion of the first cell division. alpha-Methylornithine inhibited both ornithine decarboxylase activity and the in vivo accumulation of putrescine. In the presence of alpha-methylornithireak dormancy and proceed through one cell division. Subsequent cellular growth, however, was retarded but not completely inhibited. The supplementation of Methylglyoxal bis(guanylhydrazone) to sporulation medium greatly inhibited this sexual process. These data suggest that the synthesis of putrescine is not required for the breaking of spore dormancy, but that polyamine biosynthesis may be essential for meiosis and sporulation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choih S. J., Ferro A. J., Shapiro S. K. Function of S-adenosylmethionine in germinating yeast ascospores. J Bacteriol. 1977 Jul;131(1):63–68. doi: 10.1128/jb.131.1.63-68.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choih S. J., Ferro A. J., Shapiro S. K. Relationship between polyamines and macromolecules in germinating yeast ascospores. J Bacteriol. 1978 Jan;133(1):424–426. doi: 10.1128/jb.133.1.424-426.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn M. S., Tabor C. W., Tabor H. Isolation and characterization of Saccharomyces cerevisiae mutants deficient in S-adenosylmethionine decarboxylase, spermidine, and spermine. J Bacteriol. 1978 Apr;134(1):208–213. doi: 10.1128/jb.134.1.208-213.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber J. E., Halvorson H. O. Regulation of sporulation in yeast. Curr Top Dev Biol. 1972;7:61–83. doi: 10.1016/s0070-2153(08)60069-1. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Saccharomyces cerevisiae cell cycle. Bacteriol Rev. 1974 Jun;38(2):164–198. doi: 10.1128/br.38.2.164-198.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heby O., Gray J. W., Lindl P. A., Marton L. J., Wilson C. B. Changes in L-ornithine decarboxylase activity during the cell cycle. Biochem Biophys Res Commun. 1976 Jul 12;71(1):99–105. doi: 10.1016/0006-291x(76)90254-0. [DOI] [PubMed] [Google Scholar]
- Heller J. S., Fong W. F., Canellakis E. S. Induction of a protein inhibitor to ornithine decarboxylase by the end products of its reaction. Proc Natl Acad Sci U S A. 1976 Jun;73(6):1858–1862. doi: 10.1073/pnas.73.6.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Mizutani A. A new method for isolation of polyamines from animal tissues. Anal Biochem. 1973 Dec;56(2):408–416. doi: 10.1016/0003-2697(73)90206-6. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mamont P. S., Böhlen P., McCann P. P., Bey P., Schuber F., Tardif C. Alpha-methyl ornithine, a potent competitive inhibitor of ornithine decarboxylase, blocks proliferation of rat hepatoma cells in culture. Proc Natl Acad Sci U S A. 1976 May;73(5):1626–1630. doi: 10.1073/pnas.73.5.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills D. Effect of pH on adenine and amino acid uptake during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Oct;112(1):519–526. doi: 10.1128/jb.112.1.519-526.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton N. E., Abdel-Monem M. M. Inhibitors of polyamine biosynthesis. 4. Effects of alpha-methyl-(+/-)-ornithine and methylglyoxal bis(guanylhydrazone) on growth and polyamine content of L1210 leukemic cells of mice. J Med Chem. 1977 Feb;20(2):249–253. doi: 10.1021/jm00212a012. [DOI] [PubMed] [Google Scholar]
- OGUR M., ROSEN G. The nucleic acids of plant tissues; the extraction and estimation of desoxypentose nucleic acid and pentose nucleic acid. Arch Biochem. 1950 Feb;25(2):262–276. [PubMed] [Google Scholar]
- Ono M., Inoue H., Suzuki F., Takeda Y. Studies on ornithine decarboxylase from the liver of thioacetamide-treated rats. Purification and some properties. Biochim Biophys Acta. 1972 Sep 19;284(1):285–297. doi: 10.1016/0005-2744(72)90067-8. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Pösö H., Sinervirta R., Jänne J. S-adenosylmethionine decarboxylase from baker's yeast. Biochem J. 1975 Oct;151(1):67–73. doi: 10.1042/bj1510067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau P., Halvorson H. O., Bulla L. A., Jr, St Julian G. Germination and outgrowth of single spores of Saccharomyces cerevisiae viewed by scanning electron and phase-contrast microscopy. J Bacteriol. 1972 Mar;109(3):1232–1238. doi: 10.1128/jb.109.3.1232-1238.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau P., Halvorson H. O. Macromolecular synthesis during the germanation of Saccharomyces cerevisiae spores. J Bacteriol. 1973 Mar;113(3):1289–1295. doi: 10.1128/jb.113.3.1289-1295.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens L., McKinnon I. M., Winther M. Polyamine and ornithine metabolism during the germination of conidia of Aspergillus nidulans. Biochem J. 1976 Aug 15;158(2):235–241. doi: 10.1042/bj1580235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tingle M. A., Küenzi M. T., Halvorson H. O. Germination of yeast spores lacking mitochondrial deoxyribonucleic acid. J Bacteriol. 1974 Jan;117(1):89–93. doi: 10.1128/jb.117.1.89-93.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. A., Morris D. R. Polyamine auxotrophs of Saccharomyces cerevisiae. J Bacteriol. 1978 Apr;134(1):214–220. doi: 10.1128/jb.134.1.214-220.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]



