Abstract
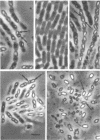
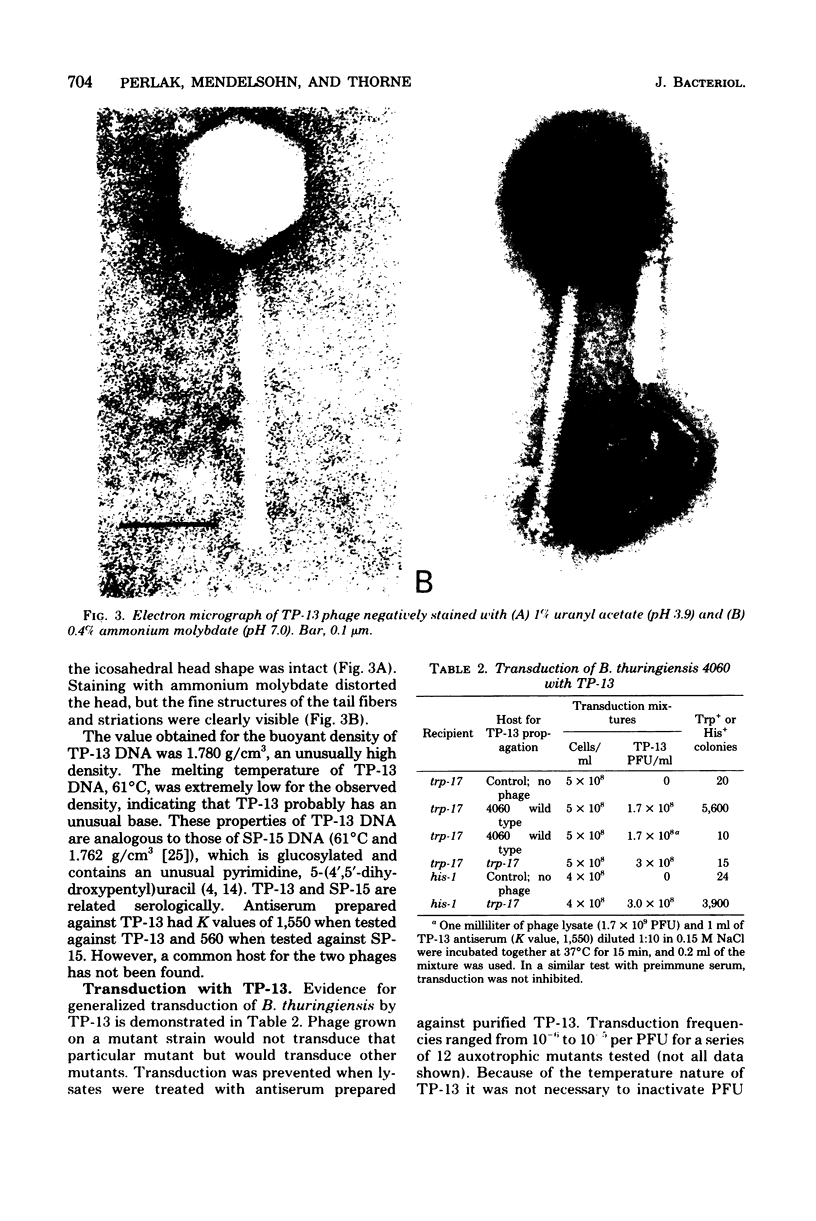
Bacteriophage TP-13, a converting phage for sporulation and crystal formation in Bacillus thuringiensis, was isolated from soil. The phage converted anoligosporogenic (sporulation frequency, 10(-8), acrystalliferous mutant to spore positive, crystal positive at a high frequency. Each plaque formed by TP-13 in a lawn of sensitive cells contained spores and crystals. These spores were heat stable, and each one was capable of producing a plaque from which TP-13 could be reisolated. Conversion of cells to sporulation and crystal formation was independent of the ho-t used for TP-13 propagation. When converted cells were cured of TP-13, they lost the ability to produce spores and crystals. Incubation of TP-13 with antiserum prepared against purified phage particles prevented conversion. TP-13 has some characteristics similar to those of SP-15 and PBS-1, including large size, morphology, and adsorption specificity of motile cells. TP-13 mediated generalized transduction in several strains of B. thuringiensis at frequencies of 10(-6) to 10(-5). Comparison of cotransduction values indicated that TP-13 transduced considerably larger segments of deoxyribonucleic acid than CP-51 or TP-10, two other transducing phages for B. thuringiensis.
Full text
PDF

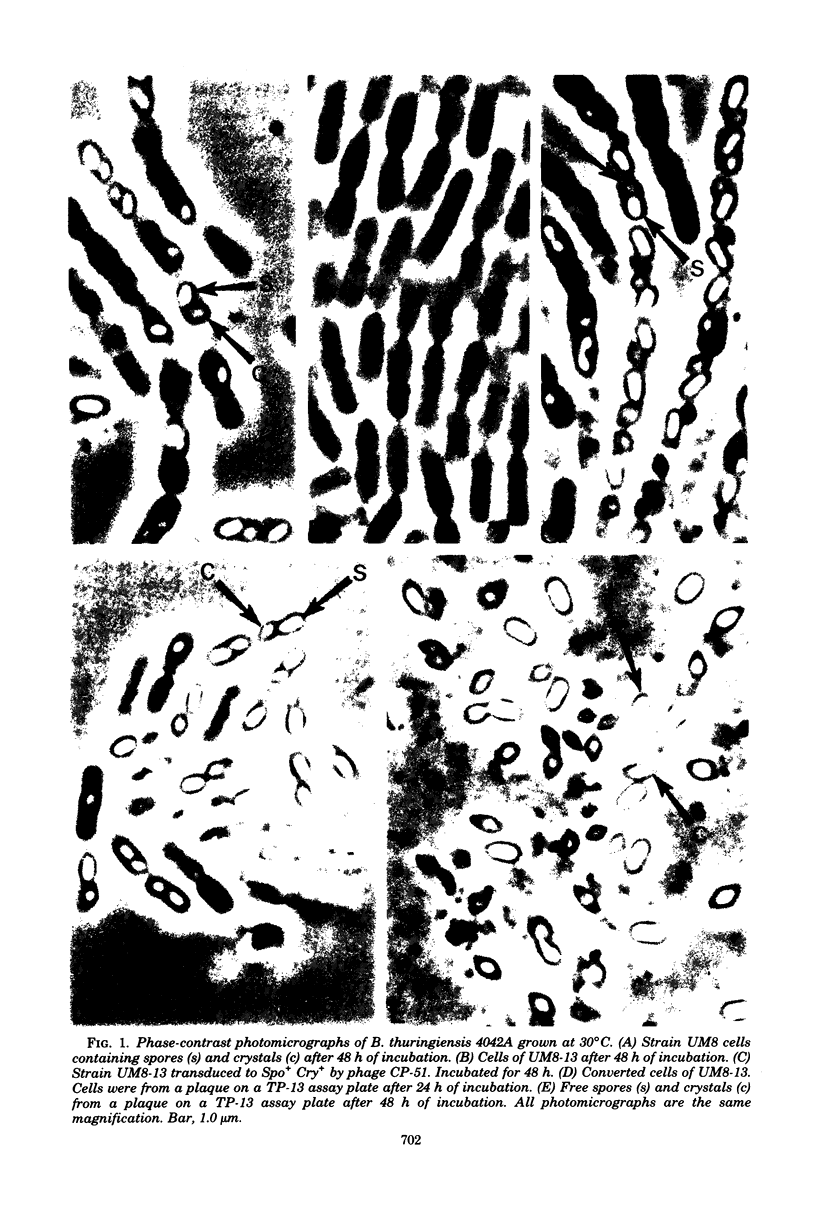



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bramucci M. G., Keggins K. M., Lovett P. S. Bacteriophage PMB12 conversion of the sporulation defect in RNA polymerase mutants of Bacillus subtilis. J Virol. 1977 Oct;24(1):194–200. doi: 10.1128/jvi.24.1.194-200.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramucci M. G., Keggins K. M., Lovett P. S. Bacteriophage conversion of spore-negative mutants to spore-positive in Bacillus pumilus. J Virol. 1977 Apr;22(1):194–202. doi: 10.1128/jvi.22.1.194-202.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandon C., Gallop P. M., Marmur J., Hayashi H., Nakanishi K. Structure of a new pyrimidine from Bacillus subtilis phage SP-15 nucleic acid. Nat New Biol. 1972 Sep 20;239(90):70–71. doi: 10.1038/newbio239070a0. [DOI] [PubMed] [Google Scholar]
- Currier T. C., Nester E. W. Isolation of covalently closed circular DNA of high molecular weight from bacteria. Anal Biochem. 1976 Dec;76(2):431–441. doi: 10.1016/0003-2697(76)90338-9. [DOI] [PubMed] [Google Scholar]
- Delafield F. P., Somerville H. J., Rittenberg S. C. Immunological homology between crystal and spore protein of Bacillus thuringiensis. J Bacteriol. 1968 Sep;96(3):713–720. doi: 10.1128/jb.96.3.713-720.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserling F. A. The structure of Bacillus subtilis bacteriophage PBS 1. J Ultrastruct Res. 1967 Feb;17(3):342–347. doi: 10.1016/s0022-5320(67)80053-4. [DOI] [PubMed] [Google Scholar]
- Iyer V. CONCENTRATION AND ISOLATION OF AUXOTROPHIC MUTANTS OF SPOREFORMING BACTERIA. J Bacteriol. 1960 Feb;79(2):309–310. doi: 10.1128/jb.79.2.309-310.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keggins K. M., Nauman R. K., Lovett P. S. Sporulation-converting bacteriophages for Bacillus pumilus. J Virol. 1978 Sep;27(3):819–822. doi: 10.1128/jvi.27.3.819-822.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovett P. S., Young F. E. Identification of Bacillus subtilis NRRL B-3275 as a strain of Bacillus pumilus. J Bacteriol. 1969 Nov;100(2):658–661. doi: 10.1128/jb.100.2.658-661.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol. 1962 Jul;5:109–118. doi: 10.1016/s0022-2836(62)80066-7. [DOI] [PubMed] [Google Scholar]
- Marmur J., Brandon C., Neubort S., Ehrlich M., Mandel M., Konvicka J. Unique properties of nucleic acid from Bacillus subtilis phage SP-15. Nat New Biol. 1972 Sep 20;239(90):68–70. doi: 10.1038/newbio239068a0. [DOI] [PubMed] [Google Scholar]
- McCuen R. W., Thorne C. B. Genetic mapping of genes concerned with glutamyl polypeptide production by Bacillus licheniformis and a study of their relationship to the development of competence for transformation. J Bacteriol. 1971 Sep;107(3):636–645. doi: 10.1128/jb.107.3.636-645.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meenakshi K., Jayaraman K. On the formation of crystal proteins during sporulation in Bacillus thuringiensis var. thuringiensis. Arch Microbiol. 1979 Jan 16;120(1):9–14. doi: 10.1007/BF00413265. [DOI] [PubMed] [Google Scholar]
- Schesser J. H., Bulla L. A., Jr Toxicity of Bacillus thuringiensis spores to the tobacco hornworm, Manduca sexta. Appl Environ Microbiol. 1978 Jan;35(1):121–123. doi: 10.1128/aem.35.1.121-123.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville H. J., Delafield F. P., Rittenberg S. C. Biochemical homology between crystal and spore protein of Bacillus thuringiensis. J Bacteriol. 1968 Sep;96(3):721–726. doi: 10.1128/jb.96.3.721-726.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville H. J. Formation of the parasporal inclusion of Bacillus thuringiensis. Eur J Biochem. 1971 Jan;18(2):226–237. doi: 10.1111/j.1432-1033.1971.tb01235.x. [DOI] [PubMed] [Google Scholar]
- Stahly D. P., Dingman D. W., Bulla L. A., Jr, Aronson A. I. Possible origin and function of the parasporal crystal in Bacillus thuringiensis. Biochem Biophys Res Commun. 1978 Oct 16;84(3):581–588. doi: 10.1016/0006-291x(78)90745-3. [DOI] [PubMed] [Google Scholar]
- TAYLOR M. J., THORNE C. B. TRANSDUCTION OF BACILLUS LICHENIFORMIS AND BACILLUS SUBTILIS BY EACH OF TWO PHAGES. J Bacteriol. 1963 Sep;86:452–461. doi: 10.1128/jb.86.3.452-461.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne C. B. Transduction in Bacillus thuringiensis. Appl Environ Microbiol. 1978 Jun;35(6):1109–1115. doi: 10.1128/aem.35.6.1109-1115.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyeryar F. J., Jr, Taylor M. J., Lawton W. D., Goldberg I. D. Cotransduction and cotransformation of genetic markers in Bacillus subtilis and Bacillus licheniformis. J Bacteriol. 1969 Nov;100(2):1027–1036. doi: 10.1128/jb.100.2.1027-1036.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton D. B., Thorne C. B. Comparison of Bacillus cereus bacteriophages CP-51 and CP-53. J Virol. 1971 Aug;8(2):242–253. doi: 10.1128/jvi.8.2.242-253.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yousten A. A. A method for the isolation of asporogenic mutants of Bacillus thuringiensis. Can J Microbiol. 1978 Apr;24(4):492–494. doi: 10.1139/m78-081. [DOI] [PubMed] [Google Scholar]