Abstract
There has been a good deal of controversy over whether attention influences area V1—the first cortical area onto which information from the retina is projected. Attention to motion has been found to modulate monkey area MT and the human homolog of MT/MST. Here we show that activation of V1 by attention to motion is task dependent. Our stimulus consisted of a group of translating random dots superimposed over another group of random dots executing expansion motion. Subjects were instructed to pay attention selectively to the translation, expansion, or neither in particular (passive condition). The activity in the human MT/MST homolog measured by functional magnetic resonance imaging (fMRI) was significantly higher in both the translation and the expansion conditions than in the passive condition, while the activity in area V1 was significantly higher only in the translation condition. These results show that attention to motion modulates area V1, and more interestingly that high-level cognitive processing such as attention may directly or indirectly determine the retroactive extent of feedback within the motion pathway in a manner dependent on the type of motion attended.
A considerable amount of research has suggested that attention influences relatively high-level processing, but not the feature analysis level. Treisman and her colleagues built a feature integration theory in which one role of attention is to integrate visual features that are independently processed at lower level stages (1). Neurobiologically, it has been found that the responses of cells in area V4 and the inferior temporal area (IT) of macaque monkeys to an unattended stimulus are dramatically reduced (2). However, such a response reduction in cells was not found in V1, where local measurements in various features occur. On the other hand, the activity of orientation-tuned cells in V1 was found to be enhanced when subjects (macaque monkeys) attended to a specific orientation (3).
With regard to the effect of attention on motion processing, at least one type of motion has been found to be driven by attention (4, 5). This finding suggests that attention is quite influential in motion processing. It has been found that attention modulates MT and MST in monkeys by means of electrophysiology (6) and the human MT/MST homolog by functional magnetic resonance imaging (fMRI) (7).
Recently, by means of both psychophysics and fMRI, it was found that when attention was directed to one of the edges of a moving wedge, the wedge appeared to move in the direction perpendicular to the attended edge—the same direction as the local component motion direction that has been suggested to be measured along that edge in V1 (8). In that case, activity in both V1 and the MT/MST human homolog increased above non-attention levels.
Then a question arises: Does attention to all types of motion activate V1? If not, what conditions activate V1? The present study has sought to answer these questions. We found that the modulation of V1 depends the type of motion to which attention is directed.
METHODS
Subjects.
Two adult females and four adult males served as subjects. Five subjects were naive about the purpose of the experiment. None of the observers had any known history of neurological disease. Informed consent was obtained from all the subjects after the nature and possible consequences of the studies were explained.
Stimulus.
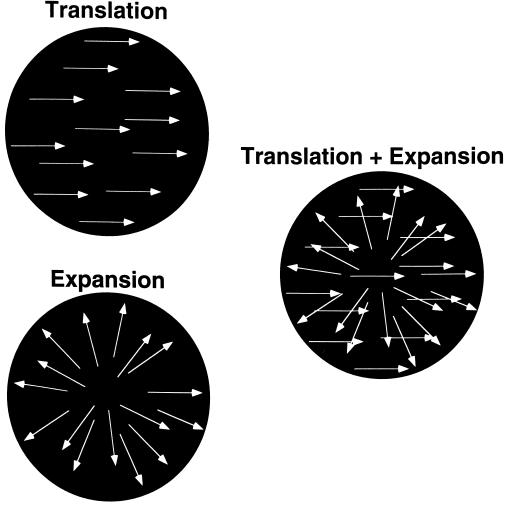
The stimulus (Fig. 1) consisted of a group of unidirectionally translating random dots superimposed over another group of random dots executing expansion motion. The white random dots (48.0 cd/m2) moved against a black circular background (0.5 cd/m2) whose diameter was 8.0 deg in angular subtence. The speed of the dots translating unidirectionally and those producing expansion were both 15 deg/sec. The experiment used 120 translating dots and 120 expanding dots. Individual dots appeared for ≈150 msec and then disappeared, to be replaced by others at random locations. The stimuli were projected by means of a Sony CRT projector situated in a control room, through a window, and onto a white screen inside the shielded room housing the fMRI machine.
Figure 1.
Schematic illustration of the test stimulus used in this experiment.
fMRI Parameters.
Local cerebral oxygenation changes were measured with echo-planar imaging using a 1.5 T whole body scanner (Siemens Vision) with a CP head coil. Acquisition parameters were as follows: TE (echo time), 66 ms; TR (repetition time), 2.1 sec; in-plane resolution (pixel size), 1.80 × 1.80 mm2; flip angle, 90°; matrix, 128 × 128; slice thickness, 5 mm; field of view, 230 mm; number of slices, 7.
Procedure.
The whole procedure consisted of three scans of the main experiment and two tests to functionally locate area V1 and the human homolog of area MT/MST. The two tests preceded the main experiment. The first test was performed to map V1 with the method of stimulus phase encoding and Fourier analysis (9–10). The second test was conducted to localize the MT/MST human homolog by activating regions most responsive to low-contrast moving stimuli (11). In the main experiment, the color of the fixation-cross signaled the type of motion to which attention should be directed. The color changed every 29.4 sec (14 images × 2.1 sec TR). The initial red cross instructed the subject to direct attention only to unidirectional translation (translation condition). The red cross was replaced by a green cross, which instructed the subject to direct attention only to expansion (expansion condition). The green cross was replaced by a yellow cross, which instructed the subject to direct attention to no particular motion (passive condition). A brief beep was sounded at the moment of cross-color change to alert the subject to the change of condition. This sequence of three conditions was repeated three times in each scan. Throughout the experiment, the test stimulus was presented with a fixation cross displayed in the center.
Eye-Movement Measurement.
To minimize eye movements within the scanner, practice trials were run in all three conditions outside the scanner before the actual sessions. Whenever significant eye movements were detected, subjects were reminded not to move their eyes. This training was repeated 100–800 times for each subject until no significant eye movement was elicited. The Ober2 eye movement parallel system was used for measuring eye movements throughout the duration of the experiment, including imaging intervals, because it can function within a high magnetic field. To reduce artifacts due to head motion, subjects’ heads were stabilized with bite bars, and the data was motion-corrected (12, 13).
RESULTS
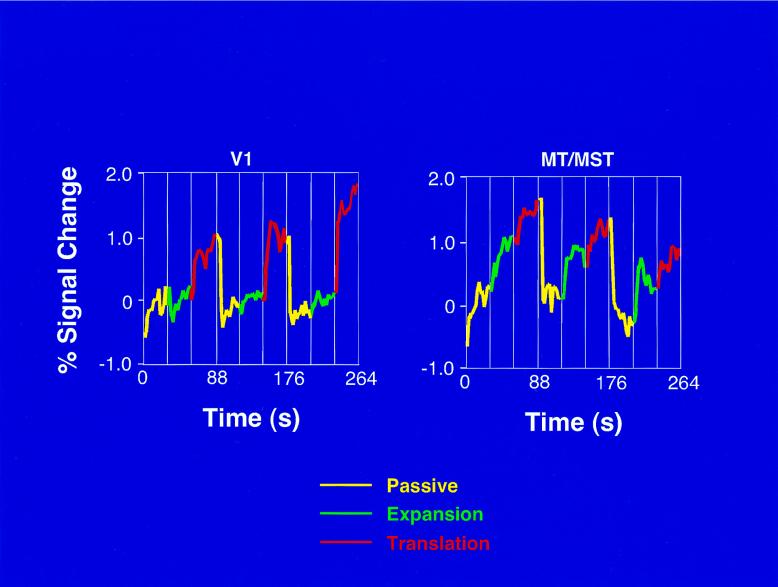
Fig. 2 shows the time courses of the MR signals for area V1 and the MT/MST homolog averaged across five subjects and three scans. V1 was more active in the translation condition than in the passive condition, while there was no significant difference in the amount of activation between the expansion and passive conditions. On the other hand, area MT/MST was more active in both the translation and expansion conditions than in the passive condition.
Figure 2.
Time courses of the MR signals averaged across five subjects and three scans, showing both area V1 and the MT/MST homolog during each of three conditions. The graph shows that there is a 3- to 5-sec time lag between the onset of a condition and the corresponding MR signal changes.
DISCUSSION
There was a possibility that attention to unidirectional translation imposed a heavier task load than attention to expansion, and as a result, V1 was more activated when attention was directed to unidirectional translation than in the passive condition, while no significant difference was found in amounts of activations between the attention to expansion condition and the passive condition. To check this, we made the number of translating dots be 180 and the number of the expanding dots be 60 so that the unidirectional translation was much easier to attend to. The other aspects of the procedure were identical to those in the previous experiment. The same subjects participated in this experiment, and all of them reported that attention to unidirectional translation was much easier than attention to expansion. In spite of this change, basically the same results were obtained as in the previous experiment, suggesting that the activation of V1 was a result of attention to unidirectional translation and not due to a heavier task load.
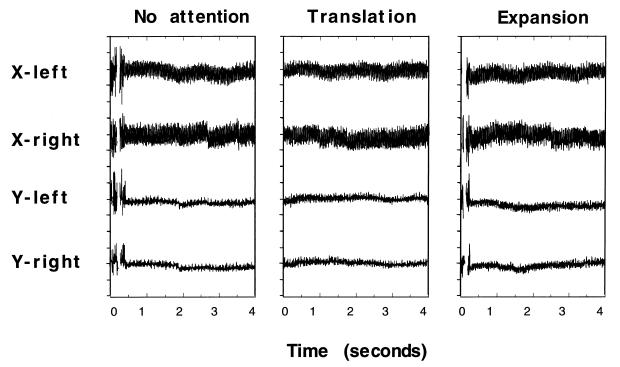
Careful monitoring of eye movements showed that in all three conditions, there were no significant differences in either patterns or amounts of eye movements between the three conditions with five subjects. [One subject showed significantly larger amplitudes of eye movements in the attention-to-translation condition than in the other two conditions. To avoid confounding the activation in V1 due to attention to translation with that due to the larger amplitudes of eye movements, the data produced by this subject was excluded from the further analysis, although the data was consistent with those by others.] This result indicates that the higher activation in V1 during the translation condition as compared with the passive condition cannot be a mere result of eye movements in the translation condition.
These results lead to an important implication: the modulated cortical areas are determined by the type of motion to which attention is directed.
Why does attention to unidirectional translation modulate an area as early as V1 while attention to expansion does not? This could be answered by hypothesizing that attention directly determines the retroactive extent of feedback within the confined pathway of motion processing (8).
Another possibility is that attention indirectly determines the extent of feedback by presenting a bias to a specific type of global motion, which differentially modulates V1 activity (14). Feedforward signals for at least opponent motion directions have been found to suppress each other in MT (15). Feedback signals for different motion directions could undergo similar suppression. When attention is directed to expansion, enhancement for the many motion signals comprising expansion may feed back to V1. However, these signals may suppress each other on their way down to V1. As a result, little or no enhancement due to attention would be obtained at V1. On the other hand, when attention is directed to one-way translation, the feedback signals, in this case composed of a single motion direction, would not suppress each other. As a result, V1 activity would be greater on attention to translation.
An earlier study showed the task-dependent effects of attention on a more global scale and in different pathways (16). Attention to form activated areas in the ventral pathway, known for its role in form perception and pattern recognition. Attention to speed activated areas in the dorsal pathway, known for its role in spatial localization (17). Our study suggests that the nature of the attentional task somehow determines the retroactive extent of feedback within the confined pathway of motion processing, rather than affecting different pathways.
Massive feedback projections are known to exist from higher cortical areas to lower visual areas, including areas V1, MT, and MST (18). The present findings may demonstrate the specificity of feedback through those projections in activating the stages necessary to accomplish a task required by high-level cognitive processing. Alternately, feedback signals of different motion directions that are suppressed on their way down to V1 may cause the differential activation of V1 according to the type of motion attended.
Figure 3.
Eye movements of a representative subject recorded during fMRI scanning in the no-attention, attention-to-translation, and attention-to-expansion conditions. Each tick on the y axis represents a 30-min arc. The deflections in the records were caused by eye blinks. The data contain random noise introduced by the simultaneous acquisition of MR images, although eye movement patterns can be recognized. The horizontal eye movements were larger than the vertical. This may be because eye movements were influenced by the wedge that moved only horizontally. However, there was no significant difference in the overall patterns among the three conditions. All the other subjects showed similar traces. The horizontal and vertical positions of both left and right eyes were sampled at 200Hz.
Acknowledgments
This study was partially supported by the National Science Foundation to T.W. (SBR-9631573). A.M.H. was supported by the Naval Research Young Investigator Award given to Paolo Gaudiano (N00014-96-1-0772).
References
- 1.Treisman A, Gelade G. Cogn Psychol. 1981;12:97–136. doi: 10.1016/0010-0285(80)90005-5. [DOI] [PubMed] [Google Scholar]
- 2.Moran J, Desimone R. Science. 1985;229:782–784. doi: 10.1126/science.4023713. [DOI] [PubMed] [Google Scholar]
- 3.Motter B C. J Neurophysiol. 1993;70:909–919. doi: 10.1152/jn.1993.70.3.909. [DOI] [PubMed] [Google Scholar]
- 4.Cavanagh P. Science. 1992;257:1563–1565. doi: 10.1126/science.1523411. [DOI] [PubMed] [Google Scholar]
- 5.Lu Z-L, Sperling G. Nature (London) 1995;377:237–239. doi: 10.1038/377237a0. [DOI] [PubMed] [Google Scholar]
- 6.Treue S, Maunsell J H R. Nature (London) 1996;382:539–541. doi: 10.1038/382539a0. [DOI] [PubMed] [Google Scholar]
- 7.O’Craven K M, Rosen B R, Kwong K K, Treisman A, Savoy R L. Neuron. 1997;18:591–598. doi: 10.1016/s0896-6273(00)80300-1. [DOI] [PubMed] [Google Scholar]
- 8.Watanabe T, Sasaki Y, Miyauchi S, Putz B, Fujimaki N, Nielsen M, Takino R, Miyakawa S. J Neurophysiol. 1997;79:2218–2221. doi: 10.1152/jn.1998.79.4.2218. [DOI] [PubMed] [Google Scholar]
- 9.Engel S A, Rumelhart D E, Wandell B A, Lee A T, Glover G H, Chichilnisky E J, Shadlen M N. Nature (London) 1994;369:525. doi: 10.1038/369525a0. [DOI] [PubMed] [Google Scholar]
- 10.Sereno M I, Dale A M, Reppas J B, Kwong K K, Belliveau J W, Brady T J, Rosen B R, Tootell R B H. Science. 1994;268:889–893. doi: 10.1126/science.7754376. [DOI] [PubMed] [Google Scholar]
- 11.Tootell R, Reppas J B, Dale A M, Look R B, Sereno M I, Malach R, Brady T J, Rosen B R. J Neurosci. 1995;375:139–141. doi: 10.1038/375139a0. [DOI] [PubMed] [Google Scholar]
- 12.Jiang A, Kennedy D N, Baker J R, Weisskoff R M, Tootell R B H, Woods R P, Benson R R, Kwong K K, Brady T J, Rosen B R, et al. Hum Brain Mapp. 1995;3:1–12. [Google Scholar]
- 13.Woods R P, Cherry S R, Mazziotta J C. J Comput Assist Tomogr. 1992;16:620–33. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]
- 14.Harner, A. & Watanabe, T. (1998) Invest. Ophthalmol. Visual Sci. (Suppl.).
- 15.Snowden G R, Treue S, Erickson R E, Andersen R A. J Neurosci. 1991;11:2768–2785. doi: 10.1523/JNEUROSCI.11-09-02768.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corbetta M, Miezin F M, Shulman G L, Peterson S E. J Neurosci. 1991;11:2383–2402. doi: 10.1523/JNEUROSCI.11-08-02383.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ungerleider L G, Mishkin M. In: The Analysis of Visual Behavior. Angle D A, Goodale M A, Mansfield R J W, editors. Cambridge, MA: MIT Press; 1982. [Google Scholar]
- 18.van Essen D C, Anderson C H, Felleman D J. Science. 1992;255:419–422. doi: 10.1126/science.1734518. [DOI] [PubMed] [Google Scholar]