Abstract
Foraging rats learned to avoid footshock that was present in a part of a circular arena that was either stable or rotating slowly in a lighted room. The rotation dissociated spatial information in the separate reference frames of the room and arena. After learning to avoid the shocked region in either condition, in the absence of shock, memory for this place was expressed by simultaneous avoidance of an area defined in the reference frame of the room as well as of an area defined in the reference frame of the rotating arena. Spatial memories in these distinct reference frames were acquired, retrieved, and extinguished autonomously.
There is no single sensory organ for spatial information and thus no receptor surface onto which external space can be mapped. Yet, mammals have spatial knowledge; they organize arbitrarily complex spatial information into a coherent reference frame. Such internal spatial reference frames or “cognitive maps” (1, 2) are used to guide behavior efficiently.
The spatial information in a cognitive map must derive from many sources. Places and directions only can be specified within an arbitrary reference frame defined by arbitrarily perceived information. Rodent navigation in open fields has been studied extensively in the effort to understand spatial cognition. Consider a standard laboratory situation: an arena within a room. A location on the arena can be specified in the reference frame of the enclosing room according to the geometric relations amongst the many inaccessible but perceptible room features: the north-east quadrant of the room, for example. Equally well, the place may be defined in reference to the geometric as well as the substratal (e.g., chemoreceptive or tactile) features of the arena itself. In contrast to these exteroceptive reference frames, the place also may be defined within a distinct interoceptive reference frame, the idiothetic frame defined by self-motion: for example, three steps forward from the start position. Although categorizing spatial reference frames according to the distinct types of information on which they depend allows us to study the organization of spatial cognition systematically, the reader should keep in mind that these different sources of information and thus the different reference frames naturally overlap and coexist.
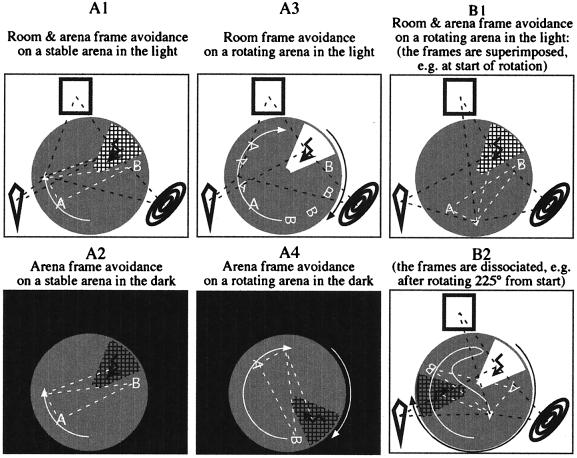
Places defined in the different reference frames are physically equivalent when the environment is stable. Allothetic orientation requires exteroception, the detection of cues in the external world. In contrast, idiothetic orientation explicitly does not depend on sensing spatial features of the world; it derives from proprioceptive and vestibular signals and motor efference copies generated by self-induced movements from a start position. We consider optic and haptic flow to contribute to idiothesis because they only inform about movement. Although the distinction is conceptually straightforward, it is difficult to achieve experimentally. We have managed a partial separation by using light or darkness of the experimental room and stability or continuous slow rotation of the arena on which a place avoidance task was performed (ref. 3; Fig. 1).
Figure 1.
Combinations of light or darkness and stability or rotation during the place avoidance task dissociate spatial information within the room and arena reference frames. The figures represent overhead views of a circular arena (gray) in a room with extra-arena visual landmarks. Stable unidentified features of the arena surface are indicated by “A” and “B”. The rat is required to avoid shock (zigzagged arrow) in an unmarked region, which in the room frame is indicated by white and in the arena frame by cross hatching. The white arrow on the arena represents the rat’s movement as seen from overhead in the room frame. The arrow head and tail correspond to the current and start positions, respectively. (A1) When the arena is stable in a lit room, the two reference frames overlap. The rat can learn the room frame distances between the room landmarks and the punished region and thus can determine its position with respect to the shock area and these landmarks. Broken black lines denote that these relations can be known and utilized. In addition, the rat can determine its position with respect to the arena features and the shock area by determining these distances from idiothetic (self-motion) cues. Broken white lines denote that these relations can be known and utilized. (A2) Information in the room frame is removed by darkness, leaving only useful arena frame information. (A3) Rotating the arena and reinforcing a room frame avoidance preserves the utility of information in the room frame while it renders information in the arena frame unstable for predicting the shock location. Note that, although the rat’s movement is the same as in A1 and A2, the path is drawn longer because, in the room frame perspective, it includes the arena rotation (≈90°, represented by the black arrow). (A4) Rotating the arena in the dark and reinforcing avoidance of a specific area on the arena leaves only arena frame information to direct the avoidance. As in A3, the rat’s path is shown during 90° of rotation (white arrow outside the arena). (B1) The current experiments study avoidance during rotation in the light. The room and arena reference frames overlap only before the rotation begins (illustrated) or at the moment that each revolution is completed. The rat can know it’s position with respect to the room cues and the room frame shock area. At the same time, it can know a different set of relations, it’s position with respect to cues on the arena surface, and the shock area in the arena frame. (B2) When the room and arena reference frames are dissociated, the set of room-based relations is maintained only within the reference frame of the room. Similarly, the set of relations based on self-motion across the arena is maintained only within the reference frame of the arena. Notice that the shock area in the arena frame is now in a place in the room that the rat previously visited. Even if the rat stops locomoting, it will be brought by the arena rotation into the shock area defined by the room. To avoid shock, the rat must move against the rotation, but this will bring it closer to the arena shock location. As the rotation approaches a complete revolution, the shock area on the arena approaches the shock area in the room; thus, the rat must pass between these converging areas through the unpunished center of the arena to avoid shock.
Rats retrieved pellets scattered on a featureless arena while avoiding a place that was defined in both the allothetic frame of the room and the idiothetic frame of the arena. Room frame avoidance required visual cues in the room whereas arena frame avoidance required self-motion information because there were no stable arena frame features to specify the place to be avoided. Uncontrolled arena cues like feces and urine accumulated during a session, but self-motion information must have provided the metric (2, 4) to judge the spatial relations amongst these cues if they were useful in guiding the avoidance.
This raises an issue of terminology. The words “idiothesis” and “path integration”, although common in the literature on the neural mechanisms of rodent navigation, have been used rather loosely. Idiothesis was specified (5) to refer to navigation based on idiothetic or self-motion information (6, 7) that derives from internally generated signals of both substratal movement (e.g., from proprioceptors, motor efference copies, and optic flow) as well as inertial motion (i.e., linear and angular acceleration detected by the otoliths and semicircular canals, respectively). Path integration or “dead-reckoning” (4), on the other hand, is navigation based on, but not limited to, idiothesis. A path-integrating subject from time to time must correct its idiothetic sense of position by referencing its distance to known stable landmarks. These landmarks may be either substrate-based local cues or more distant orientation beacons.
A previous study (3) formed the basis of the present work. Rats learned to avoid both a room and an arena frame location when trained on a stable arena in the light (Fig. 1A1). During subsequent darkness (Fig. 1A2), the avoidance extinguished after 45 min and did not reappear when the lights were switched on. During rotation in darkness (Fig. 1A4), the avoidance learned on the stable arena in the light, was expressed in the arena frame but not in the room frame, and took >30 min to extinguish. After extinction of this arena frame avoidance, when the lights were turned on, only the room frame avoidance reappeared and persisted for another 30 min.
Unlike learning in a stable environment where avoidance in both the room and arena frames was acquired, learning on a rotating arena in the light (Fig. 1A3) did not permit the formation of arena frame avoidance memories because no arena frame position consistently was associated with punishment. After learning a room frame avoidance on the rotating arena in the light, there was avoidance neither in the room nor in the arena frames when the lights (and shock) were turned off. A robust room frame avoidance reappeared when just the lights were turned on again.
Because, depending on the conditions, rats acquired, retrieved, and extinguished spatial memories from one or the other or both reference frames of the place avoidance task, these data suggested that spatial memories defined in different reference frames were functionally autonomous. An interesting set of questions then arises: How do these memories interact when their individual expression leads to different physical places (Fig. 1B)? Is spatial behavior directed by a unitary representation of space, or is there a functional hierarchy in the expression of coexistent spatial memories from different reference frames?
Using a within-subject, within-session design, the present experiments demonstrate that, indeed, spatial avoidance memories are acquired in both the allothetic reference frame of the room and the idiothetic reference frame of the arena in the normal condition when a single physical location consistently is defined in the different reference frames. During subsequent slow rotation of the arena, when these distinct memories correspond to different physical locations, the rats avoided two different regions that corresponded to the two reference frames. Though both allothetic and idiothetic spatial memories were expressed, the allothetic memories were dominant. Some of these data have been presented in preliminary form (8, 9).
METHODS
Subjects and Surgery.
Rats were treated in accordance with National Institutes of Health and Czech guidelines. Eight male rats of the Long Evans strain were obtained from the breeding colony of the Institute of Physiology, Academy of Sciences, Prague. Once they weighed 300 g, they were trained to forage for food pellets that were scattered randomly on an elevated circular metal arena 80 cm in diameter that could be rotated about its center by using an electric motor (3). After a few days, under thiopental (50 mg/kg) anesthesia, a 4-cm long, uninsulated silver wire 200 μm in diameter was implanted s.c. at the back of the neck and was attached to a connector cemented to the rat’s skull.
Behavioral Training.
Avoidance training began after a week’s recovery. A counter-balanced cable was attached to the connector to power the shock and an infrared light-emitting diode that was attached between the rat’s shoulders by a latex harness. A custom personal computer-based system tracked the light-emitting diode position in the reference frame of the room every 100 ms by using an overhead television camera. The room frame position of a second LED on the outside of the arena also was tracked and was used to calculate the rat’s position in the reference frame of the arena (10).
A “prohibited sector” was defined in both reference frames as a 45° partial sector centered in one of the four quadrants. The sectors only extended inward to within 24 cm of the arena center (30% of the arena radius), thus preserving the possibility of escape when the arena frame sector approached the stable room frame sector. A rat in between these converging areas could escape through the unpunished central area (see Fig. 1B).
Whenever the rat entered the prohibited sector for >0.5 s, 50 Hz current (<0.6 mA) was delivered for 0.5 s between the implanted wire and the high impedance contact between the rat’s feet and the grounded arena floor. The shock was repeated after 3 s if the animal did not leave the prohibited area. The shock condition only was intended to be unpleasant, and, once trained, the rats continued to forage over the unpunished surface of the arena without signs of fear.
Analysis.
Daily acquisition sessions with the shock activated were given over 4 days; each lasted 12 min. Immediately afterward, the shock was disconnected, and retrieval of the avoidance was tested until the avoidance was extinguished. The avoidance was assessed every 2 min by calculating the percentage of the path length (and time) spent in the to-be-avoided area divided by the total path length (and time) spent in the four corresponding areas of each quadrant. The extinction criterion was met when this was >13% for 3 consecutive 2-min epochs. The quality of the avoidance was quantified by calculating the percentage of the path length in each 10° bin sector of the arena surface exclusive of the central unpunished annulus. The bin with the minimum distance (MIN) was taken as the center of the avoided region. The accuracy of the avoidance was measured by the absolute value of the angular deviation (DEV) of the most avoided sector from the center of the punished sector. The width of the avoided region (WIDTH) was defined as 1 SD on either side of the minimum bin. In one sense, this quantifies the specificity of the avoidance. Taken another way, WIDTH is a measure of the cautiousness of the avoiding rat. Each measure was determined separately for the room and arena reference frames when they were dissociated by slow constant (clockwise) rotation of the arena at 1 revolution per minute in the light. Throughout the text, the values corresponding to the room and arena reference frames are subscripted accordingly. Measures from the two reference frames were compared by two-tailed paired t tests.
RESULTS
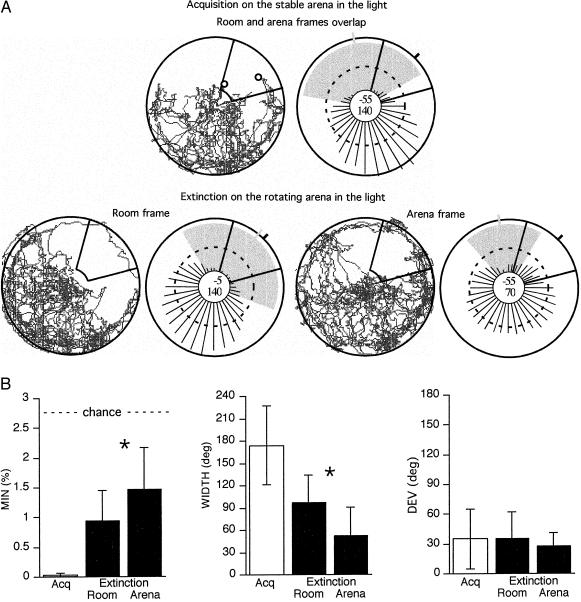
The rats were familiarized with the task and then were trained to avoid a new location. The conditioned spatial avoidance was learned on the stable arena in the light when the room and arena frames were not dissociated physically. The task was well learned before testing retrieval; only 0.88 ± 0.83 shocks (per 12-min session) were received during the acquisition phases immediately preceding the retrieval test. The room and arena reference frames then were dissociated by rotation in the light, and the shock was turned off to test whether the avoidance was retrieved in the separate room and arena reference frames. The session ended when the extinction criterion was met in both reference frames. Fig. 2A shows the track from a rat that simultaneously avoided separate room and arena frame locations.
Figure 2.
(A) An example of one rat’s avoidance behavior is shown by pairs of circles, the left representing the track and the right representing the corresponding radial histogram of the angular spatial distribution of the movements. (Top row) An avoidance was conditioned on the stable arena by shocking the rat for entering the outlined area in the north-east. Two shocks were given after 4.5 and 8.5 min (marked by circles on the track). The length of the radial lines in the histograms indicate the percentage of the total distance (21 m) that was moved in each 10° partial sector. The continuous circle in the center marks 0%. The broken circle corresponds to chance (2.8%), and the heavy circle at the arena outline corresponds to 6.7%. The heavy mark on the arena outline indicates the center of the punished region. The gray shaded area shows the avoided region, the most avoided sector of which (MIN) is marked by the short gray line. The deviation of this from the center of the punished area (DEV) is given by the top number in the middle of the histogram. The other number is WIDTH. Like the other rats, this one foraged away from the punished area. (Bottom row) The shock was turned off to test retrieval of the avoidance, and the arena was rotated continuously to dissociate the room and arena reference frames. The behavior in the first 20 min is shown. In the room frame, a 140° area centered on the to-be-avoided area (DEV = −5°) was avoided. This area was first entered after 14 min. The total distance moved in the room frame was 34 m. In the arena frame, a 70° area was avoided. It was somewhat displaced (DEV = −55°) from the to-be-avoided area, which was first entered after 6 min. The total distance moved in the arena frame (less the passive movement in the room frame) was 32 m. (B) Summary of avoidance during acquisition (Acq; open bars) on the stable arena and the first 20 min of extinction (solid bars) in the room and arena reference frames dissociated by rotation. During extinction, avoidance in the room frame was less specific (more cautious) than in the arena frame (WIDTH). However, the strength of the avoidance (MIN) was only slightly better, and the accuracy (DEV) in either frame was not different from the accuracy when the shock was on. Error bars, SD; ∗, significant differences based on paired t tests (MIN, P = 0.07; WIDTH, P = 0.03).
On average, it took 22 ± 17 (±SD) and 26 ± 20 min to meet the extinction criteria in the room and arena frames, respectively. These to-criterion-times were not different (t7 = 0.36, P = 0.73). To facilitate comparisons between avoidance in the room and arena frames, we evaluated the first 20 min of extinction, during which time, the rats still were avoiding the prohibited sector in both reference frames (compare the MIN values to 2.78, which is expected for homogeneous foraging). Fig. 2B summarizes the results of these first 20 min of extinction. Regions in both the room and arena frames were avoided (MINroom = 0.94 ± 0.52; WIDTHroom = 98 ± 37°; MINarena = 1.5 ± 0.69; WIDTHarena = 53 ± 38°), but the room frame avoidance was slightly more expressed (MIN: t7 = 2.1, P = 0.07); WIDTH: t7 = 2.7, P = 0.03). However, the accuracy in the two frames was similar to each other (DEVroom = 35 ± 27°; DEVarena = 28 ± 14°; t7 = 0.65, P = 0.54), and to the accuracy during acquisition (DEV = 35 ± 30°). Thus, the rats learned to avoid a place that could be defined in either or both the room and arena frames.
An additional question must be answered to fully interpret these results. Did the more substantial room frame avoidance reflect a true dominance of the room frame memories, or was it simply caused by poorer acquisition of the arena frame avoidance? A second experiment was performed to answer this question. The same rats were retrained on the rotating arena in the light to avoid both a new allothetic and a new idiothetic frame location; that is, shock was delivered at both a place in the room and a place on the arena. After 12 min, the shock was disconnected, and retrieval in both reference frames was evaluated for 20 min in the absence of shock.
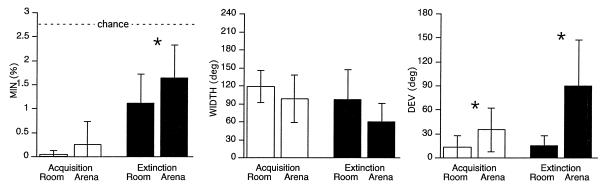
The avoidance during acquisition was similar but not identical in the two reference frames (Fig. 3). The numbers of shocks that were received were similar in the two reference frames (room: 3.9 ± 2.3; arena: 4.4 ± 4.8; t7 = 0.45, P = 0.66) but were more than what was received in the first experiment on the stable arena (room: t7 = 5.3, P = 0.001; arena: t7 = 2.4, P = 0.05). The width of the avoided regions were similar (WIDTHroom = 119 ± 27°; WIDTHarena = 99 ± 40°; t7 =1.67, p = 0.14) to each other, but both were smaller than the avoided region when the arena was stable (room: t7 = 2.8, P = 0.03; arena: t7 = 3.7, P = 0.008). Improved accuracy in the room frame was the only difference between the avoidance acquired in the two frames. The absolute deviations from the punished sector were smaller in the room frame compared with the arena frame (DEVroom = 14 ± 14°; DEVarena = 35 ± 27°; t7 = 2.5, P = 0.04) and the acquisition on the stable arena (t7 = 2.2, p = 0.06). The arena frame accuracy (DEVarena) was not different from that on the stable arena (t7 = 0.0, P = 1.0). Thus, explicitly reinforcing the avoidance of both a room and arena frame location did not disturb avoidance behavior; on the contrary, in response to the increased demand to explicitly chart locations in both reference frames, performance was improved. The general increase of the specificity of avoidance can be attributed to a general enhancement of motivation, attention, or caution. However, distinct processes must have contributed to the avoidance in the room and arena frames because accuracy in the room frame was specifically enhanced.
Figure 3.
Summary of acquisition (open bars) and extinction (solid bars) of avoidance in the room and arena reference frames dissociated by rotation. (Acquisition) The only difference between avoidance in the two reference frames was better accuracy (DEV) in the room frame. (Extinction) The strength (MIN) and accuracy (DEV) of avoidance in the room frame was better than in the arena frame whereas the specificity (WIDTH) was similar. Error bars, SD; ∗, significant differences based on paired t tests (Extinction MIN, P = 0.01; Acquisition DEV, P = 0.04; Extinction DEV, P = 0.006).
Further evidence of this distinction comes from comparing performance in the room and arena frames during the 20-min extinction test (Fig. 3). The strength (MINroom = 1.1 ± 0.56; MINarena = 1.6 ± 0.67; t7 = 3.3, P = 0.01) and accuracy (DEVroom = 18 ± 12°; DEVarena = 90 ± 57°; t7 = 3.9, P = 0.006) of the avoidance was greater in the room frame, but the specificity did not differ (WIDTHroom = 93 ± 53°; WIDTHarena = 60 ± 31°; t7 = 1.5, P = 0.18).
The 20-min extinction test was divided into 10-min halves to determine whether the avoidance in the room frame was better because avoidance in the arena frame extinguished faster. According to t tests, all measures of avoidance were similar both within (room versus arena) and between the first and second 10 min. The only remaining explanation for the slightly poorer avoidance in the arena frame is that the avoidance based on room cues was learned more accurately than the avoidance based on the arena cues.
DISCUSSION
To summarize the results, in the normal circumstance of stable, equivalent spatial reference frames, spatial memories defined in an explicitly allothetic framework are distinct and equivalent in strength to those memories in the arena frame that necessarily were based on substratal idiothesis. When memories from these two frames indicate different physical locations, both memories are expressed, but the substratal memories are less accurate.
These experiments demonstrate that the different memories for a spatial location are autonomous. It is currently unknown whether the different memories involve distinct brain loci. Extensive experimentation indicates that the hippocampus is critical for the sort of allothetic memories that are necessary to support the room frame avoidance. Whether the hippocampal navigational system is also critical for the arena frame avoidance is an open question. It originally was assumed that spatial behavior based solely on idiothetic information did not require the hippocampus (2), but a true test of pure idiothetic navigation has not been reported yet. Recently (11, 12, 13), it was argued that the hippocampus creates a spatial representation that is built up from path integration; however, preliminary evidence that hippocampal lesions do not disrupt path integration in a burrowing task (14) suggests that the path integration system itself is extra-hippocampal. Candidate structures include the striatum (15), the retrosplenial cortex (16), and the lateral mammillary nuclei (17), of which the latter two are closely related to the hippocampus proper.
The dissociation and simultaneous expression of room and arena frame avoidance is strong evidence of separate underlying neural systems. We imagine that the arena frame avoidance was based on a path integration process by which idiothetic information provided the necessary metric—say, idiothetic distance—between perceptible cues on the arena surface. Path integration is subject to cumulative errors that can be corrected by periodically referencing some stable landmark, a process called “taking a fix” (4, 18). Without taking a fix to correct pure idiothetic path integration, it is difficult to explain how the arena frame avoidance in our previous study (3) was able to persist up to 1 hour in some cases. Notably, however, the duration of accurate path integration without taking a fix has not been characterized. Ongoing experiments in our lab (9, 19) in which the arena floor is shuffled to remove the information provided by arena surface markings are allowing us to examine idiothetic navigation in the absence of useful exteroceptive information. Preliminary estimates set the extent of accurate idiothesis to ≈7 m or <2 min of pellet chasing.
The possibility that the same locations in space are represented by separable memories raises an interesting issue that now can be studied experimentally. The data showed that rats possess different useful, autonomous representations of their environment. Here, we were able to show that these representations were defined in different reference frames, and, thus, when we speak of how neural circuits encode space, the issue of multiple reference frames arises. This already was suggested in recordings of postsubicular and anterior thalamic “head direction” cells (20), which maintained their directional firing while the rat walked along a corridor between two chambers. The firing was fixed in reference to a cue card in the first chamber. However, once in the second chamber, the discharge switched to the direction indicated by a similar card placed in a different reference direction. It also was shown that hippocampal “place cells” can both fire in cell-specific reference frame-specific locations (10, 11) and can switch between reference frames when the task changes (21) within a complex task (22) and when conflicts between idiothetic and allothetic information appear (23). Despite such changes in stimulus control, these data support the idea that the hippocampus behaves as a coherently organized spatial representation because all of the cells tend to behave in the same way within a recording session.
Data from a recent series of place cell experiments by Eichenbaum and colleagues (24, 25) present a different view that is mutually complementary with the present behavioral data. The set of visual, tactile, and olfactory cues on the floor of a plus maze were dissociated from the set of visual cues surrounding the maze by turning the maze and enclosure cues by 90° in opposite directions. Subsets of place cells responded to the arena cues, others responded to the room cues, and still some responded to the conjunction of the two. Further, they showed that only a large minority (37%) of the cells that were recorded simultaneously responded in the same manner, which suggests that, at the level of the hippocampus, spatial information from separate multiple, coexistent reference frames is represented. It is, however, not clear whether these unit responses indicate that the hippocampus organizes distinct spatial reference frames or, rather, that it encodes sets of fundamental relations that only later will be organized into a framework that can be used to organize behavior.
This important issue is unresolved in part because it is not certain whether the discharge characteristics of place cells reflect the rat’s spatial cognitive state, and to settle this issue, it will take studying the cellular responses during tasks in which momentary spatial cognition can be assessed (10). It already has been shown that place cell discharge is disorganized by arena rotation in the light but not in the dark (8), but this was demonstrated during random searching when the rat was not required to use any particular spatial information. Preliminary results in our lab (26) show that, when the rat is reinforced for attending to room frame information, rotation of the arena is much less disturbing to firing fields, which may be defined in either or both the room and arena frames. By using the behavioral methods of the present study, distant allothetic, substratal allothetic, and pure idiothetic spatial information can be made useful or irrelevant to the rat. How these conditions interact with the rat’s attentional system to define the reference frame(s) in which the place cell population discharges is now being examined along with its behavioral consequences. We expect that such studies will go a long way toward understanding the mechanisms by which a reference frame is constructed from different kinds of spatial information, on the one hand, and, on the other, once this information is incorporated into a spatial representation, what kinds of behavior the representations can support.
Acknowledgments
We thank H. Mittelstaedt for valuable comments on an earlier draft of this paper and A. Zahalka for technical assistance. This work was supported by Granting Agency of the Czech Republic Grant 309/97/0555.
References
- 1.Tolman E C. Psychol Rev. 1948;55:189–208. doi: 10.1037/h0061626. [DOI] [PubMed] [Google Scholar]
- 2.O’Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford: Clarendon; 1978. [Google Scholar]
- 3.Bures J, Fenton A A, Kaminsky Yu, Rossier J, Sacchetti B, Zinyuk L. Philos Trans R Soc London B. 1997;352:1515–1524. doi: 10.1098/rstb.1997.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallistel C R. The Organization of Learning. Cambridge, MA: MIT Press; 1990. [Google Scholar]
- 5.Mittelstaedt M-L, Glasauer S. Zool J Physiol. 1991;95:427–435. [Google Scholar]
- 6.Mittelstaedt M-L. In: Information Processing in the Visual Systems of Arthropods. Wehner R, editor. Berlin: Springer; 1972. pp. 275–279. [Google Scholar]
- 7.Mittelstaedt H, Mittelstaedt M-L. Fortschr Zool. 1973;21:46–58. [Google Scholar]
- 8.Sacchetti B, Wesierska M, Fenton A A, Bures J. Soc Neurosci Abstr. 1997;23:1592. [Google Scholar]
- 9.Bures J, Fenton A A, Kaminsky Yu, Wesierska M, Zahalka A. Neuropharmacology. 1998;37:689–699. doi: 10.1016/s0028-3908(98)00031-8. [DOI] [PubMed] [Google Scholar]
- 10.Bures J, Fenton A A, Kaminsky Yu, Zinyuk L. Proc Natl Acad Sci USA. 1997;94:243–250. doi: 10.1073/pnas.94.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gothard K M, Skaggs W E, Moore K M, McNaughton B L. J Neurosci. 1996;16:823–835. doi: 10.1523/JNEUROSCI.16-02-00823.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McNaughton B L, Barnes C A, Gerrard J L, Gothard K M, Jung M W, Knierim J J, Kudrimoti H, Qin Y, Skaggs W E, Suster M, Weaver K L. J Exp Biol. 1996;199:173–185. doi: 10.1242/jeb.199.1.173. [DOI] [PubMed] [Google Scholar]
- 13.Whishaw I Q, McKenna J E, Maaswinkel H. Curr Opin Neurobiol. 1997;7:228–234. doi: 10.1016/s0959-4388(97)80011-6. [DOI] [PubMed] [Google Scholar]
- 14.Alyan S H, Paul B M, Ellsworth E, White R D, McNaughton B L. Soc Neurosci Abstr. 1997;23:504. [Google Scholar]
- 15.Wiener S I. J Neurosci. 1993;13:3802–3817. doi: 10.1523/JNEUROSCI.13-09-03802.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen L L, Lin L-H, Barnes C A, McNaughton B L. Exp Brain Res. 1994;101:24–34. doi: 10.1007/BF00243213. [DOI] [PubMed] [Google Scholar]
- 17.Leonhard C L, Stackman R W, Taube J S. Soc Neurosci Abstr. 1996;22:1873. [Google Scholar]
- 18.Knierim J J, Kudrimoti H S, McNaughton B L. J Neurosci. 1995;15:1648–1659. doi: 10.1523/JNEUROSCI.15-03-01648.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuchlik, A., Kaminsky, Yu, Zahalka, A. & Bures, J. (1998) Eur. J. Neurosci. 10, Suppl., 259 (abstr.).
- 20.Taube J S, Burton H L. J Neurophysiol. 1995;74:1953–1971. doi: 10.1152/jn.1995.74.5.1953. [DOI] [PubMed] [Google Scholar]
- 21.Markus E J, Qin Y, Leonard B, Skaggs W E, McNaughton B L, Barnes C A. J Neurosci. 1995;15:7079–7094. doi: 10.1523/JNEUROSCI.15-11-07079.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gothard K M, Skaggs W E, McNaughton B L. J Neurosci. 1996;16:8027–8040. doi: 10.1523/JNEUROSCI.16-24-08027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jeffery K J. Neuropharmacology. 1998;37:677–687. doi: 10.1016/s0028-3908(98)00053-7. [DOI] [PubMed] [Google Scholar]
- 24.Tanila H, Shapiro M L, Eichenbaum H. Hippocampus. 1997;7:613–623. doi: 10.1002/(SICI)1098-1063(1997)7:6<613::AID-HIPO4>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 25.Shapiro M L, Tanila H, Eichenbaum H. Hippocampus. 1997;7:624–642. doi: 10.1002/(SICI)1098-1063(1997)7:6<624::AID-HIPO5>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Zinyuk, L. E., Kubik, S., Kaminsky, Yu. & Bures, J. (1998) Eur. J. Neurosci. 10 Suppl., 440 (abstr.).