Abstract
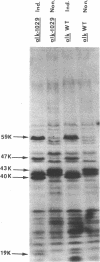
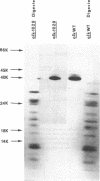
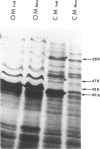
Pseudomonas putida strains carrying the plasmid alk genes will grow on n-alkanes. Induced alk+ strains contain membrane activities for alkane hydroxylation and dehydrogenation of aliphatic primary alcohols. P. putida cytoplasmic and outer membranes can be separated by sucrose gradient centrifugation after disruption of cells by either mild detergent lysis or passage through a French press. Both the membrane component of alkane hydroxylase and membrane alcohol dehydrogenase fractionated with the cytoplasmic membrane. Induction of the alk regulon resulted in the appearance of at least three new plasmid-determined cytoplasmic membrane peptides of about 59,000 (59K), 47,000 (47K), and 40,000 (40K) daltons as well as the disappearance of a pair of chromosomally encoded outer membrane peptides of about 43,000 daltons. The 40K peptide is the membrane component of alkane hydroxylase and the product of the plasmid alkB gene because the alkB1029 mutation altered the properties of alkane hydroxylase in whole cells, reduced its thermal stability in cell extracts, and led to increased electrophoretic mobility of the inducible 40K peptide. These results are consistent with a model for vectorial oxidation of n-alkanes in the cytoplasmic membrane of P. putida.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedik M., Fennewald M., Shapiro J. Transposition of a beta-lactamase locus from RP1 into Pseudomonas putida degradative plasmids. J Bacteriol. 1977 Feb;129(2):809–814. doi: 10.1128/jb.129.2.809-814.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Fennewald M., Shapiro J., Huettner C. Fractionation of inducible alkane hydroxylase activity in Pseudomonas putida and characterization of hydroxylase-negative plasmid mutations. J Bacteriol. 1977 Nov;132(2):614–621. doi: 10.1128/jb.132.2.614-621.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Shapiro J. Induction of alkane hydroxylase proteins by unoxidized alkane in Pseudomonas putida. J Bacteriol. 1975 Aug;123(2):759–760. doi: 10.1128/jb.123.2.759-760.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S., Shapiro J. Plasmid-determined alcohol dehydrogenase activity in alkane-utilizing strains of Pseudomonas putida. J Bacteriol. 1976 May;126(2):794–798. doi: 10.1128/jb.126.2.794-798.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collins M. L., Niederman R. A. Membranes of Rhodospirillum rubrum: isolation and physicochemical properties of membranes from aerobically grown cells. J Bacteriol. 1976 Jun;126(3):1316–1325. doi: 10.1128/jb.126.3.1316-1325.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennewald M., Benson S., Oppici M., Shapiro J. Insertion element analysis and mapping of the Pseudomonas plasmid alk regulon. J Bacteriol. 1979 Sep;139(3):940–952. doi: 10.1128/jb.139.3.940-952.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennewald M., Shapiro J. Regulatory mutations of the Pseudomonas plasmid alk regulon. J Bacteriol. 1977 Nov;132(2):622–627. doi: 10.1128/jb.132.2.622-627.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Lyle R. D. Chemical alterations in cell envelopes of polymyxin-resistant Pseudomonas aeruginosa isolates. J Bacteriol. 1979 Jun;138(3):839–845. doi: 10.1128/jb.138.3.839-845.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grund A., Shapiro J., Fennewald M., Bacha P., Leahy J., Markbreiter K., Nieder M., Toepfer M. Regulation of alkane oxidation in Pseudomonas putida. J Bacteriol. 1975 Aug;123(2):546–556. doi: 10.1128/jb.123.2.546-556.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock R. E., Nikaido H. Outer membranes of gram-negative bacteria. XIX. Isolation from Pseudomonas aeruginosa PAO1 and use in reconstitution and definition of the permeability barrier. J Bacteriol. 1978 Oct;136(1):381–390. doi: 10.1128/jb.136.1.381-390.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KING T. E. RECONSTITUTION OF RESPIRATORY CHAIN ENZYME SYSTEMS. XI. USE OF ARTIFICIAL ELECTRON ACCEPTORS IN THE ASSAY OF SUCCINATE-DEHYDROGENATING ENZYMES. J Biol Chem. 1963 Dec;238:4032–4036. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Miura Y., Okazaki M., Hamada S. I., Murakawa S. I., Yugen R. Assimilation of liquid hydrocarbon by microorganisms. I. Mechanism of hydrocarbon uptake. Biotechnol Bioeng. 1977 May;19(5):701–714. doi: 10.1002/bit.260190507. [DOI] [PubMed] [Google Scholar]
- Nieder M., Shapiro J. Physiological function of the Pseudomonas putida PpG6 (Pseudomonas oleovorans) alkane hydroxylase: monoterminal oxidation of alkanes and fatty acids. J Bacteriol. 1975 Apr;122(1):93–98. doi: 10.1128/jb.122.1.93-98.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Peterson J. A., Basu D., Coon M. J. Enzymatic omega-oxidation. I. Electon carriers in fatty acid and hydrocarbon hydroxylation. J Biol Chem. 1966 Nov 10;241(21):5162–5164. [PubMed] [Google Scholar]
- Ruettinger R. T., Griffith G. R., Coon M. J. Characterization of the omega-hydroxylase of Pseudomonas oleovorans as a nonheme iron protein. Arch Biochem Biophys. 1977 Oct;183(2):528–537. doi: 10.1016/0003-9861(77)90388-5. [DOI] [PubMed] [Google Scholar]
- Trust T. J., Millis N. F. Activation of the C2to C19 monocarboxylic acids by Pseudomonas. J Bacteriol. 1971 Mar;105(3):1216–1218. doi: 10.1128/jb.105.3.1216-1218.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eyk J., Bartels T. J. Paraffin Oxidation in Pseudomonas aeruginosa II. Gross Fractionation of the Enzyme System into Soluble and Particulate Components. J Bacteriol. 1970 Dec;104(3):1065–1073. doi: 10.1128/jb.104.3.1065-1073.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WADDELL W. J. A simple ultraviolet spectrophotometric method for the determination of protein. J Lab Clin Med. 1956 Aug;48(2):311–314. [PubMed] [Google Scholar]
- WEISSBACH A., HURWITZ J. The formation of 2-keto-3-deoxyheptonic acid in extracts of Escherichia coli B. I. Identification. J Biol Chem. 1959 Apr;234(4):705–709. [PubMed] [Google Scholar]