Abstract
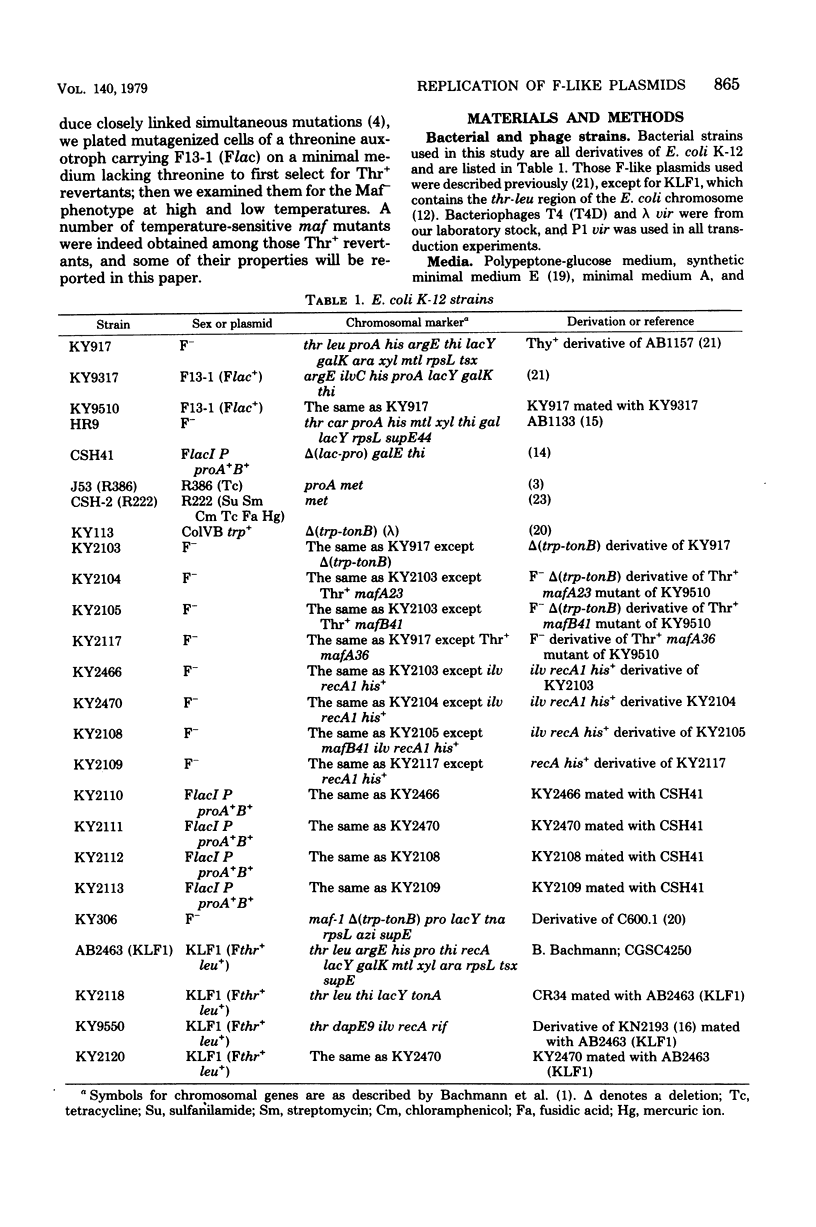
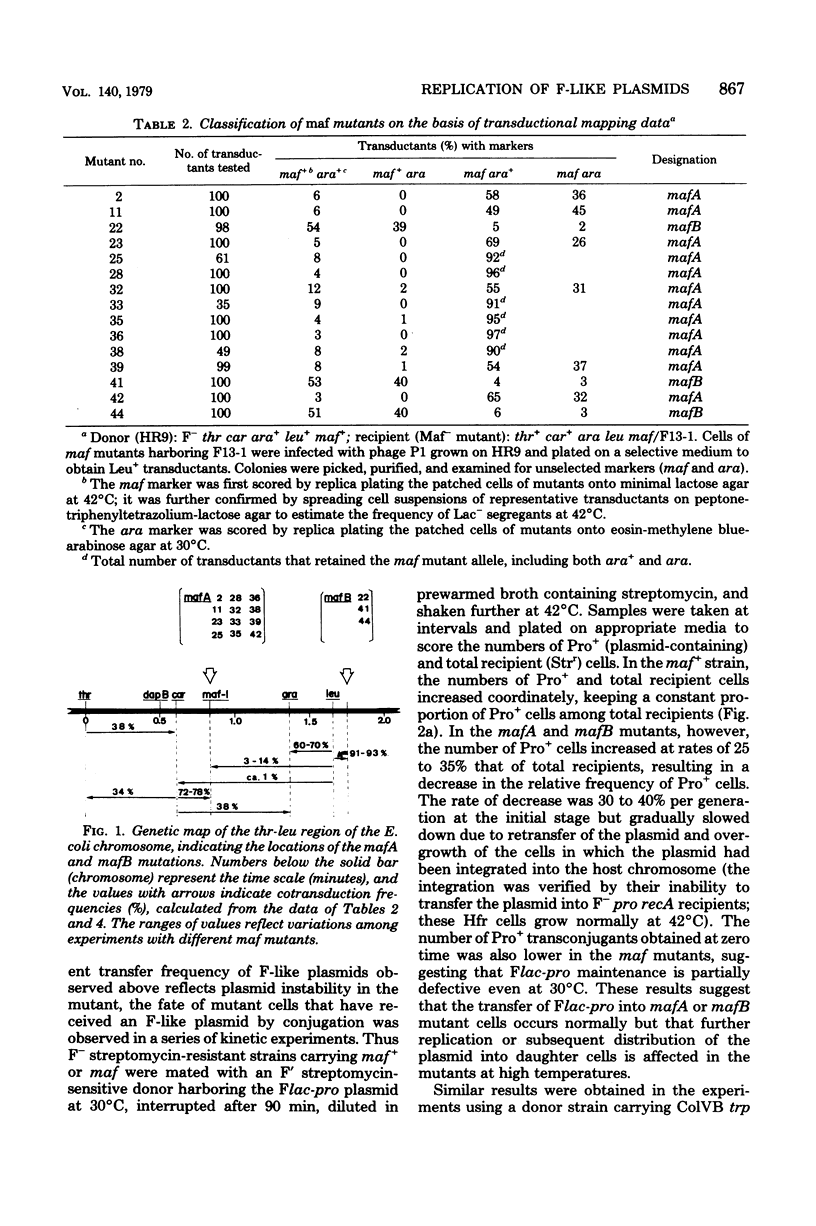
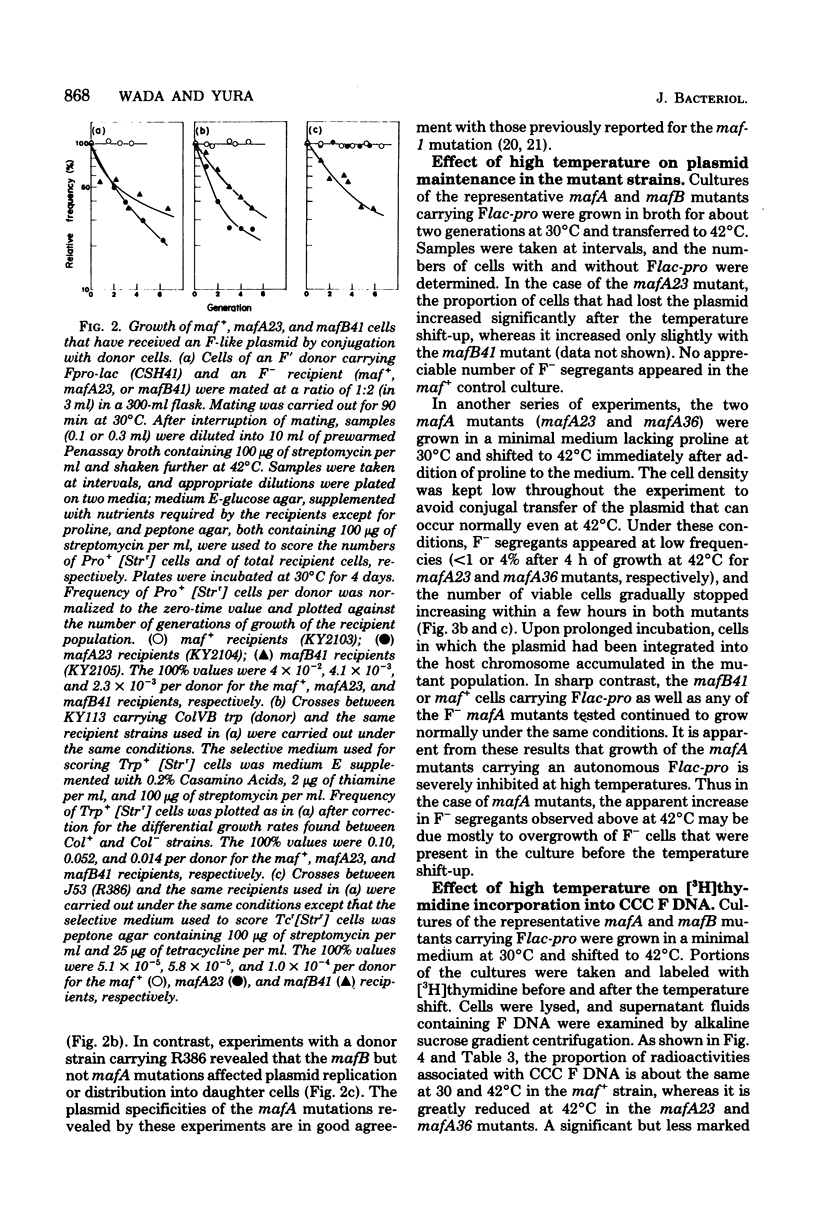
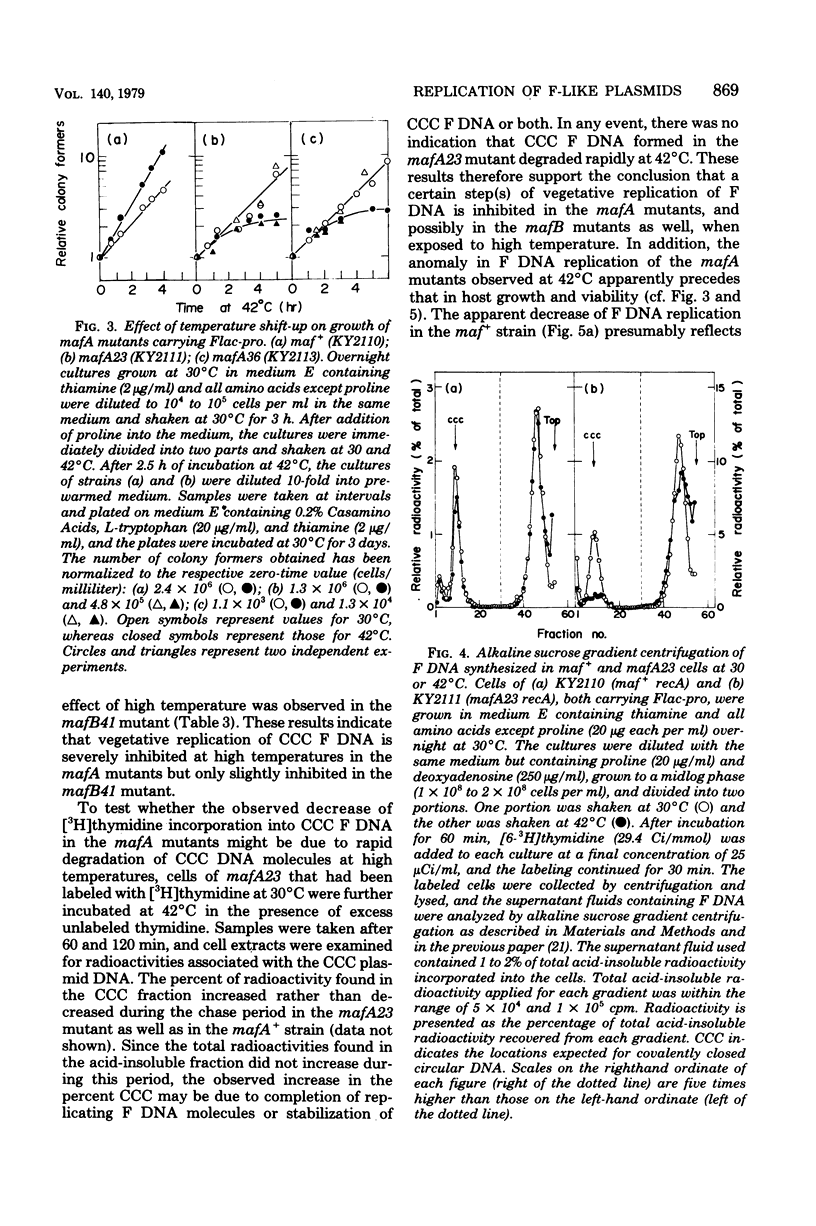
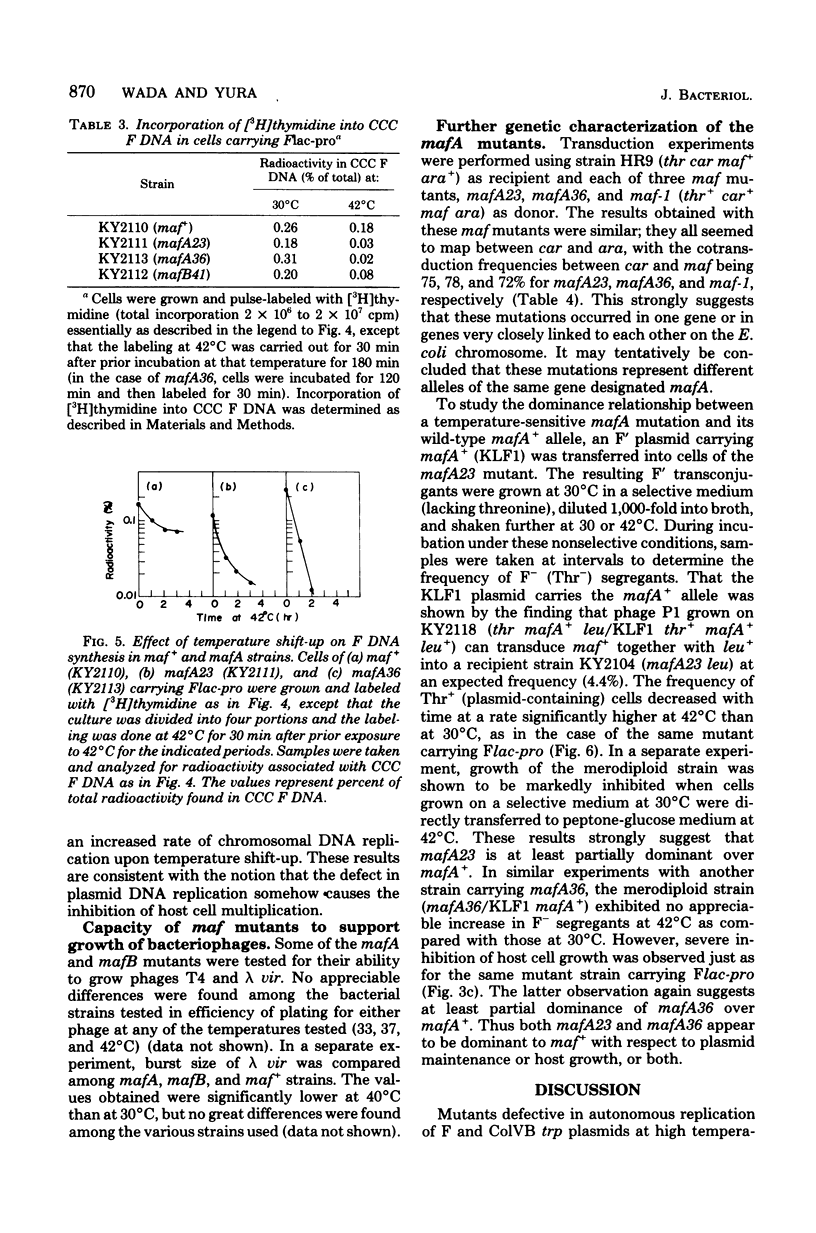
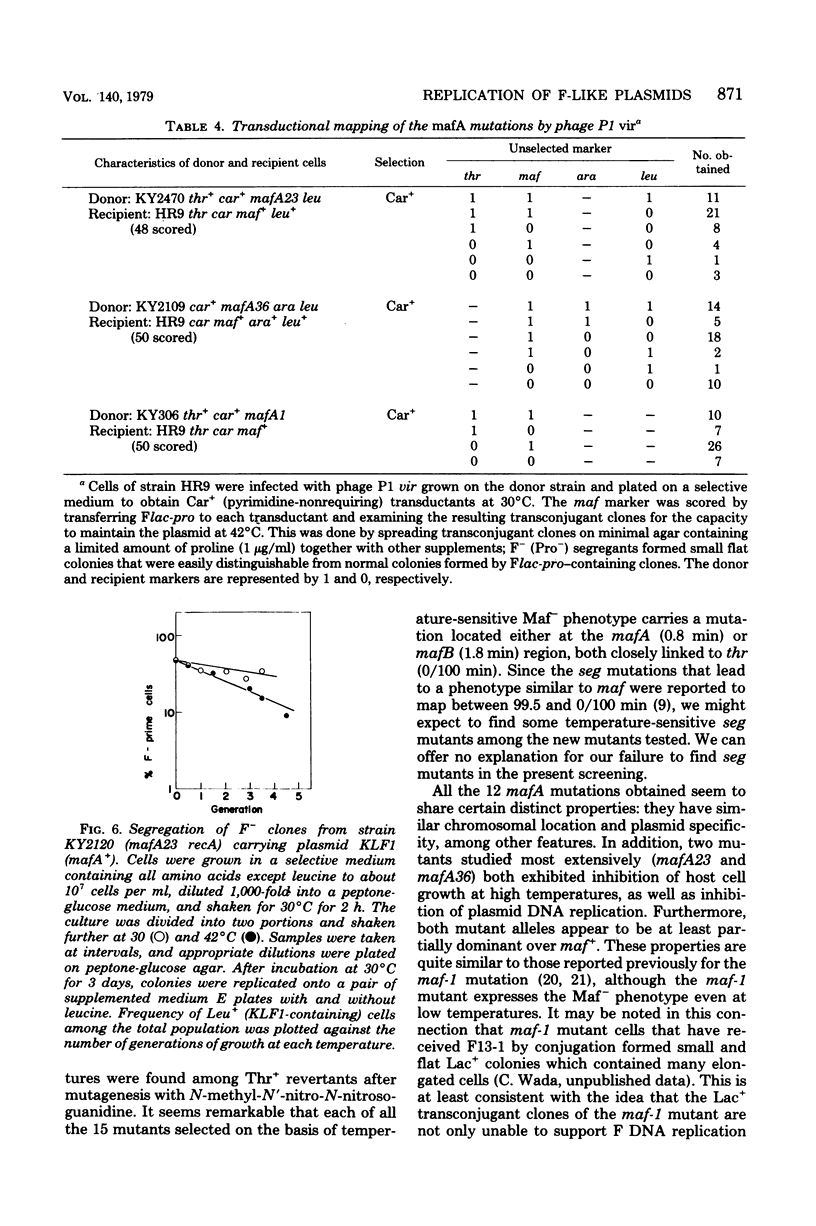
Mutants of Escherichia coli K-12 defective in replication of F-like plasmids at a high temperature (42 degrees C) were found among threonine-independent (Thr+) revertants of a threonine-requiring F' stain after localized mutagenesis with N-methyl-N'-nitro-N-nitrosoguanidine. Transduction experiments with phage P1 permitted us to divide these mutations into two classes with respect to man location; some mutations were located between thr and ara at about 0.8 min, very close to maf-1 reported previously (Wada et al., J. Mol. Biol. 108:25-41, 1976 and the others probably were located between leu and azi at about 1.8 min. The former class of mutants designated mafA exhibited the same plasmid specificity as maf-1; replication of plasmids F and ColVB trp, but not R386 or R222, were affected at a high temperature. By contrast, the latter mutants designated mafB were defective in replication of nay of these plasmids at a high temperature. When a culture of mafA mutants carrying an F' plasmid was transferred from 30 to 42 degrees C, the plasmid replication as determined by incorporation of [3H]thymidine into covalently closed circular F DNA was markedly inhibited. Under certain conditions, the temperature shift-up caused severe growth inhibition of the mutant cells. Examination of merodiploids (mafA/FmafA+) for plasmid maintenance suggested that the two mafA mutations tested (mafA23 and mafA36) were both dominant, at least partially, over the wild-type mafA+ allele. These properties of the mafA mutants, manifested at the restrictive temperature, are similar to those previously reported for the maf-1 mutant. Taken together with other evidence it is likely that these mutations affect either the same gene (mafA) or a set of closely linked genes, playing a specific role in autonomous plasmid replication in E. coli.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B., Taylor A. L. Recalibrated linkage map of Escherichia coli K-12. Bacteriol Rev. 1976 Mar;40(1):116–167. doi: 10.1128/br.40.1.116-167.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuzin F., Jacob F. Mutations de l'épisome F d'Escherichia coli K 12. II. Mutants à réplication thermosensible. Ann Inst Pasteur (Paris) 1967 Apr;112(4):397–418. [PubMed] [Google Scholar]
- Dennison S. Naturally occurring R factor, derepressed for pilus synthesis, belonging to the same compatibility group as the sex factor F of Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):416–422. doi: 10.1128/jb.109.1.416-422.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hathaway B. G., Bergquist P. L. Temperature-sensitive mutations affecting the replication of F-prime factors in Escherichia coli K 12. Mol Gen Genet. 1973 Dec 31;127(4):297–306. doi: 10.1007/BF00267100. [DOI] [PubMed] [Google Scholar]
- Hiraga S. Novel F prime factors able to replicate in Escherichia coli Hfr strains. Proc Natl Acad Sci U S A. 1976 Jan;73(1):198–202. doi: 10.1073/pnas.73.1.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Jamieson A. F., Bergquist P. L. Genetic mapping of chromosomal mutations affecting the replication of the F-factor of Escherichia coli. Mol Gen Genet. 1976 Oct 18;148(2):221–223. doi: 10.1007/BF00268388. [DOI] [PubMed] [Google Scholar]
- Kingsbury D. T., Helinski D. R. Temperature-sensitive mutants for the replication of plasmids in Escherichia coli. I. Isolation and specificity of host and plasmid mutations. Genetics. 1973 May;74(1):17–31. doi: 10.1093/genetics/74.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama A. H., Yura T. Plasmid mutations affecting self-maintenance and host growth in Escherichia coli. J Bacteriol. 1975 Apr;122(1):80–88. doi: 10.1128/jb.122.1.80-88.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Low K. B. Escherichia coli K-12 F-prime factors, old and new. Bacteriol Rev. 1972 Dec;36(4):587–607. doi: 10.1128/br.36.4.587-607.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Uchida H. Initiation of the DNA replication of bacteriophage lambda in Escherichia coli K12. J Mol Biol. 1977 Jun 15;113(1):1–25. doi: 10.1016/0022-2836(77)90038-9. [DOI] [PubMed] [Google Scholar]
- Sato T., Horiuchi T., Nagata T. Genetic analyses of an amber mutation in Escherichia coli K-12, affecting deoxyribonucleic acid ligase and viability. J Bacteriol. 1975 Dec;124(3):1089–1096. doi: 10.1128/jb.124.3.1089-1096.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler J., Adelberg E. A. Temperature dependence of sex-factor maintenance in Escherichia coli K-12. J Bacteriol. 1972 Jan;109(1):447–449. doi: 10.1128/jb.109.1.447-449.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terawaki Y., Kakizawa Y., Takayasu H., Yoshikawa M. Temperature sensitivity of cell growth in Escherichia coli associated with the temperature sensitive R(KM) factor. Nature. 1968 Jul 20;219(5151):284–285. doi: 10.1038/219284a0. [DOI] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- WATANABE T., FUKASAWA T. Episome-mediated transfer of drug resistance in Enterobacteriaceae. I. Transfer of resistance factors by conjugation. J Bacteriol. 1961 May;81:669–678. doi: 10.1128/jb.81.5.669-678.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada C., Hiraga S., Yura T. A mutant of Escherichia coli incapable of supporting vegetative replication of F-like plasmids. J Mol Biol. 1976 Nov;108(1):25–41. doi: 10.1016/s0022-2836(76)80092-7. [DOI] [PubMed] [Google Scholar]
- Wada C., Yura T., Hiraga S. Replication of Fpoh+ plasmid in mafA mutants of Escherichia coli defective in plasmid maintenance. Mol Gen Genet. 1977 Apr 29;152(3):211–217. doi: 10.1007/BF00268820. [DOI] [PubMed] [Google Scholar]
- Wada C., Yura T. Phenethyl alcohol resistance in Escherichia coli. II. Replication of F factor in the resistant strain C600. Genetics. 1971 Nov;69(3):257–287. [PMC free article] [PubMed] [Google Scholar]
- Yamagata H., Uchida H. Spectinomycin resistance mutations affecting the stability of sex-factors in Escherichia coli. J Mol Biol. 1972 Jun 28;67(3):533–535. doi: 10.1016/0022-2836(72)90472-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Kaji A. Replication of thermosensitive Rts1 plasmid deoxyribonucleic acid at the nonpermissive temperature. J Bacteriol. 1977 Oct;132(1):90–99. doi: 10.1128/jb.132.1.90-99.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


