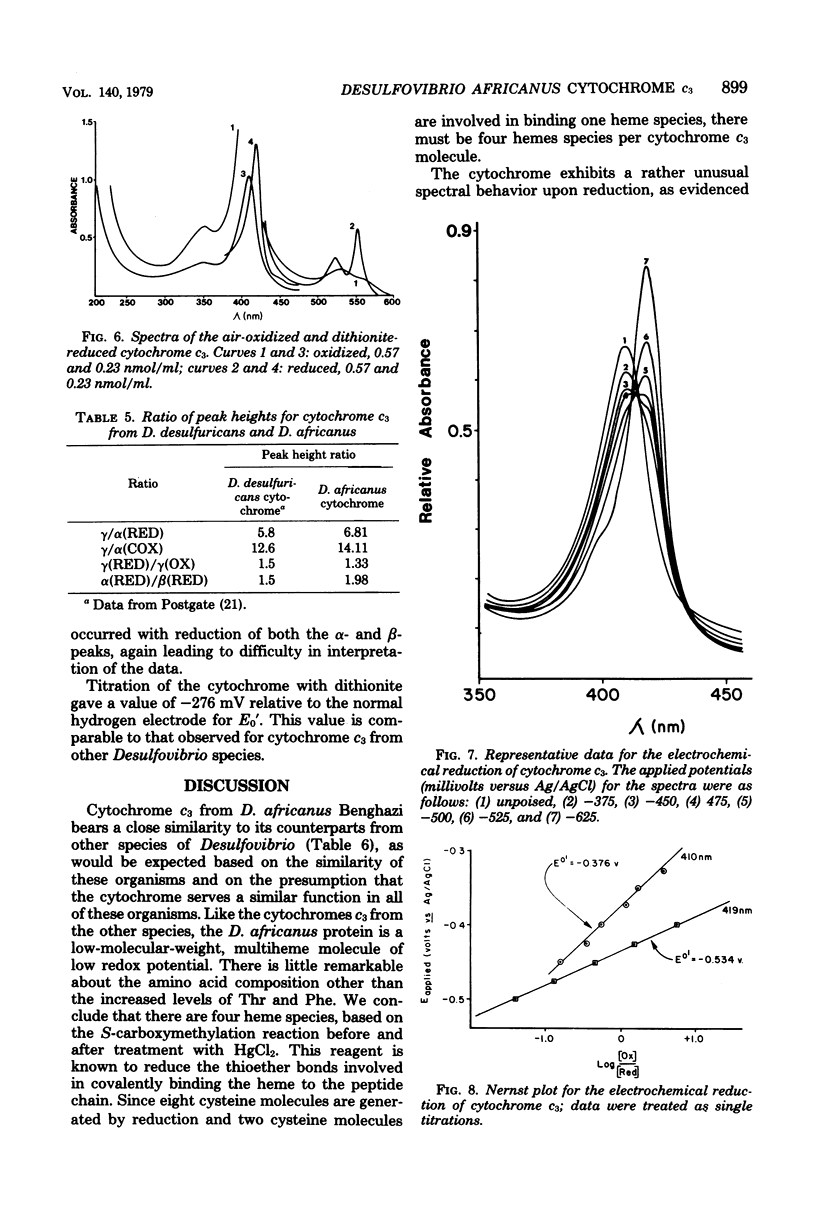
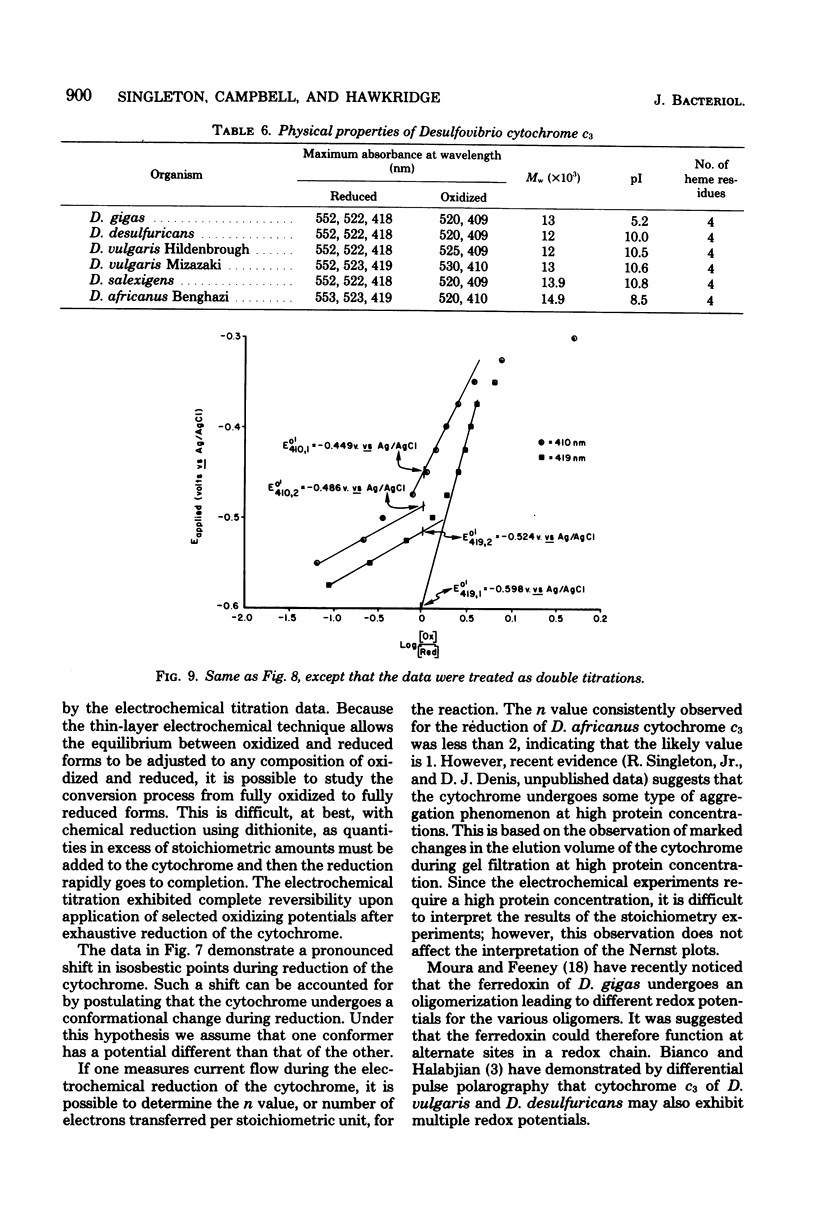
Abstract
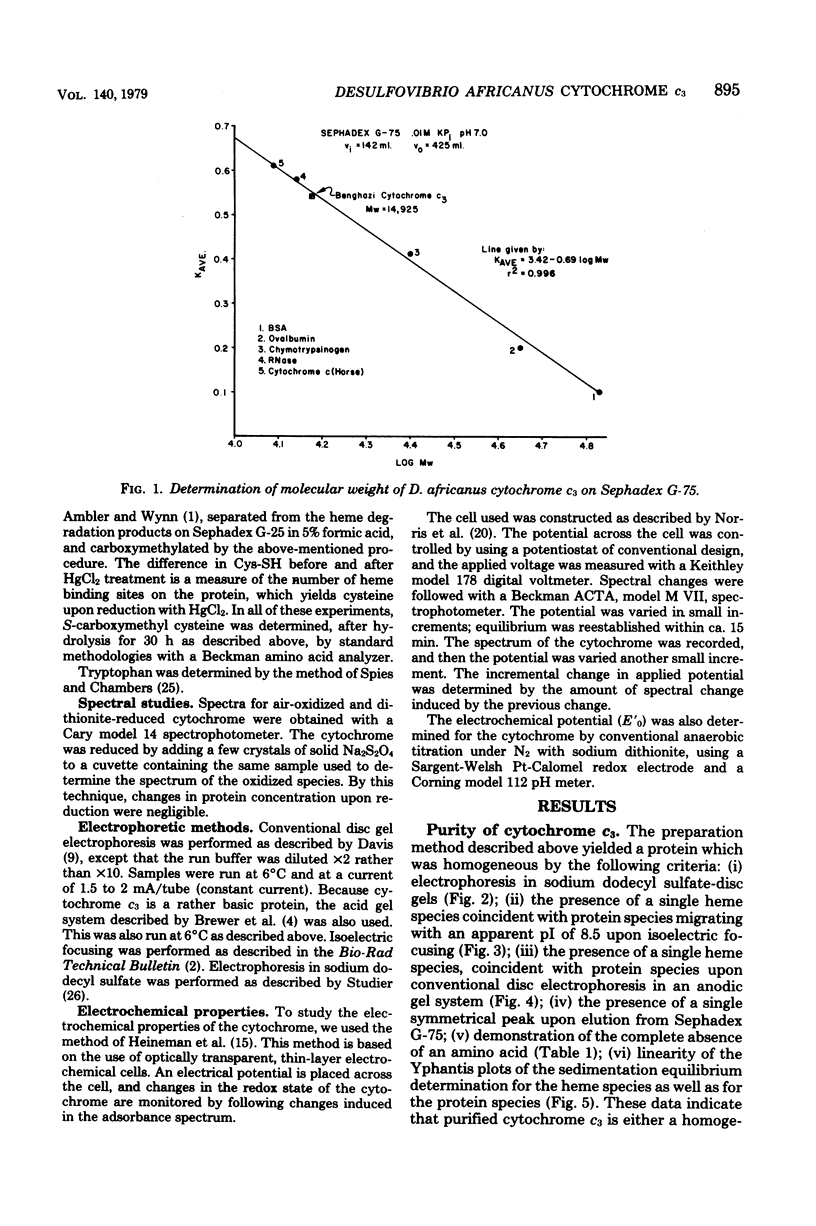
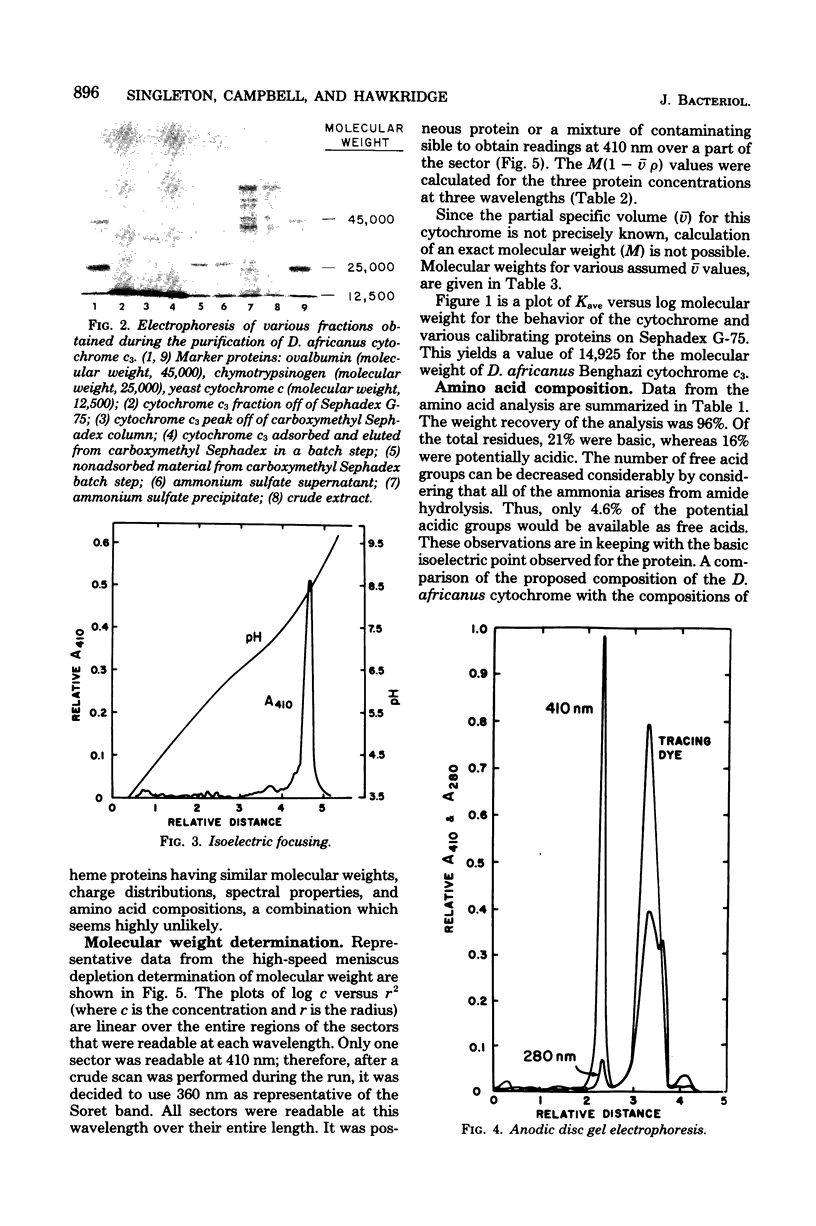
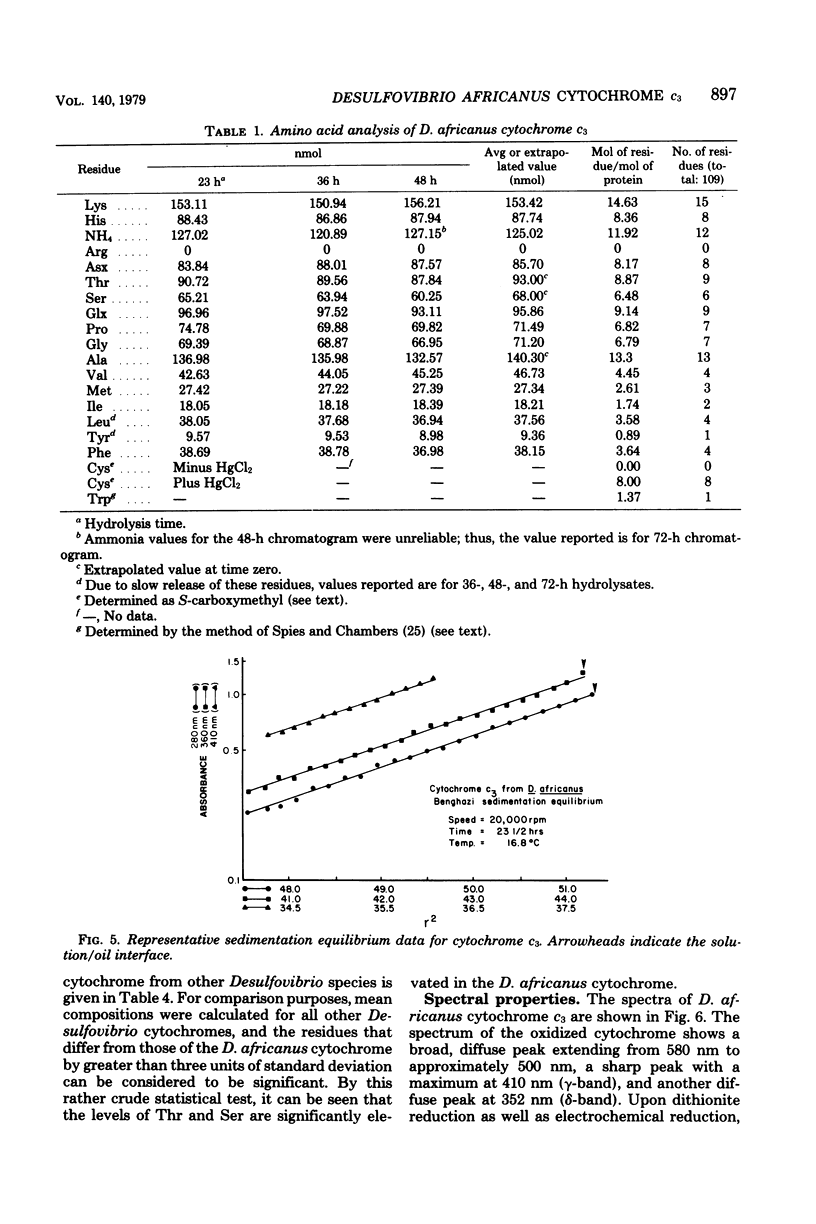
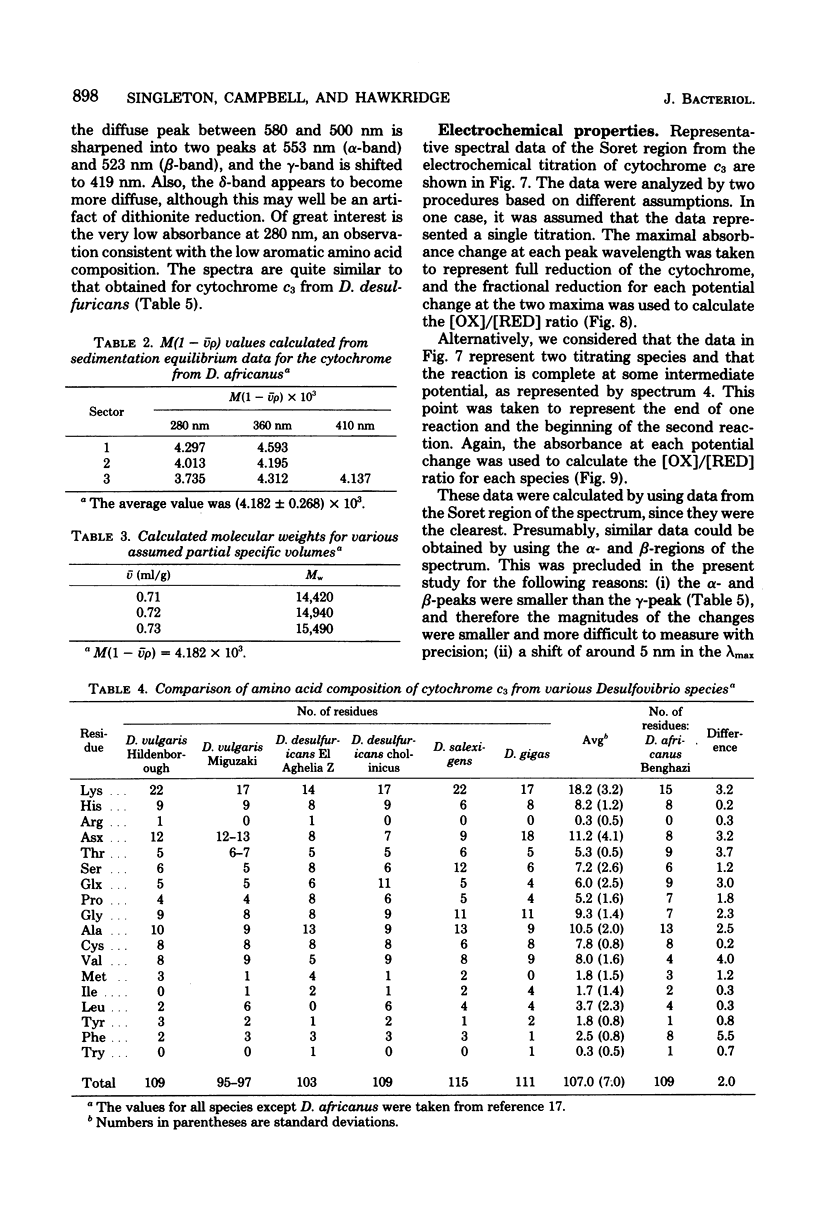
Cytochrome c3 was purified from Desulfovibrio africanus Benghazi by extraction with alkaline deoxyribonuclease, fractionation with ammonium sulfate, batch elution from carboxymethyl Sephadex followed by chromatography on the same resin, and gel filtration on Sephadex G-75. The preparation was judge homogeneous by a variety of criteria. The molecular weight was determined in an analytical ultracentrifuge, and values between 14,400 and 15,490 were obtained, depending upon the presumed value of partial specific volume. Gel filtration on a calibrated column of Sephadex G-75 gave a value of 14,900 daltons. The amino acid composition was very similar to that observed for the cytochrome from other species of Desulfovibrio, with the exception of increased levels of ThR and PhE. S-Carboxymethylation of the protein before and after heme removal by HgCl2 demonstrated eight Cys molecules involved in heme binding or four heme sites per molecule. Titration with sodium dithionite under N2 gave an electrochemical potential (E' 0) of -276 mV relative to the normal hydrogen electrode. Electrochemical titration of the cytochrome gave a Nernst plot with two linear regions with E' 0 values of -0.376 and -0.534 V. The spectra produced at various potentials exhibited shifts in isosbestic points upon reduction, suggesting changes in conformation during the reaction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Wynn M. The amino acid sequences of cytochromes c-551 from three species of Pseudomonas. Biochem J. 1973 Mar;131(3):485–498. doi: 10.1042/bj1310485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianco P., Haladjian J. Study of cytochromes c3 from Desulfovibrio vulgaris (Hildenborough) and Desulfovibrio desulfuricans (Norway) by differential pulse polarography and spectroelectrochemical method. Biochim Biophys Acta. 1979 Jan 11;545(1):86–93. doi: 10.1016/0005-2728(79)90116-6. [DOI] [PubMed] [Google Scholar]
- CRESTFIELD A. M., MOORE S., STEIN W. H. The preparation and enzymatic hydrolysis of reduced and S-carboxymethylated proteins. J Biol Chem. 1963 Feb;238:622–627. [PubMed] [Google Scholar]
- Campbell L. L., Postgate J. R. Classification of the spore-forming sulfate-reducing bacteria. Bacteriol Rev. 1965 Sep;29(3):359–363. doi: 10.1128/br.29.3.359-363.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Der Vartanian D. V., LeGall J. A monomolecular electron transfer chain: structure and function of cytochrome C3. Biochim Biophys Acta. 1974 Apr 30;346(1):79–99. doi: 10.1016/0304-4173(74)90012-3. [DOI] [PubMed] [Google Scholar]
- Drucker H., Campbell L. L. Electrophoretic and immunological differences between the cytochrome c3 of Desulfovibrio desulfuricans and that of Desulfovibrio vulgaris. J Bacteriol. 1969 Oct;100(1):358–364. doi: 10.1128/jb.100.1.358-364.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drucker H., Trousil E. B., Campbell L. L., Barlow G. H., Margoliash E. Amino acid composition, heme content, and molecular weight of cytochrome c3 of Desulfovibrio desulfuricans and Desulfovibrio vulgaris. Biochemistry. 1970 Mar 31;9(7):1515–1518. doi: 10.1021/bi00809a006. [DOI] [PubMed] [Google Scholar]
- Drucker H., Trousil E. B., Campbell L. L. Purification and properties of cytochrome C 3 of Desulfovibrio salexigens. Biochemistry. 1970 Aug 18;9(17):3395–3400. doi: 10.1021/bi00819a016. [DOI] [PubMed] [Google Scholar]
- Heineman W. R., Norris B. J., Goelz J. F. Measurement of enzyme E'values by optically transparent thin layer electrochemical cells. Anal Chem. 1975 Jan;47(1):79–84. doi: 10.1021/ac60351a001. [DOI] [PubMed] [Google Scholar]
- LEGALL J., MAZZA G., DRAGONI N. LE CYTOCHROME C3 DE DESULFOVIBRIO GIGAS. Biochim Biophys Acta. 1965 May 18;99:385–387. [PubMed] [Google Scholar]
- Moura J. J., Xavier A. V., Hatchikian E. C., Le Gall J. Structural control of the redox potentials and of the physiological activity by oligomerization of ferredoxin. FEBS Lett. 1978 May 1;89(1):177–179. doi: 10.1016/0014-5793(78)80549-3. [DOI] [PubMed] [Google Scholar]
- Norris B. J., Meckstroth M. L., Heineman W. R. Optically transparent thin layer electrode for anaerobic measurements on redox enzymes. Anal Chem. 1976 Mar;48(3):630–632. doi: 10.1021/ac60367a052. [DOI] [PubMed] [Google Scholar]
- POSTGATE J. R. Cytochrome c3 and desulphoviridin; pigments of the anaerobe Desulphovibrio desulphuricans. J Gen Microbiol. 1956 Jul;14(3):545–572. doi: 10.1099/00221287-14-3-545. [DOI] [PubMed] [Google Scholar]
- Postgate J. R., Campbell L. L. Classification of Desulfovibrio species, the nonsporulating sulfate-reducing bacteria. Bacteriol Rev. 1966 Dec;30(4):732–738. doi: 10.1128/br.30.4.732-738.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postgate J. R. Media for sulphur bacteria. Lab Pract. 1966 Nov;15(11):1239–1244. [PubMed] [Google Scholar]
- Postgate J. R. Media for sulphur bacteria: some amendments. Lab Pract. 1969 Mar;18(3):286–286. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Vosjan J. H. ATP generation by electron transport in Desulfovibrio desulfuricans. Antonie Van Leeuwenhoek. 1970;36(4):584–586. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]



