Abstract
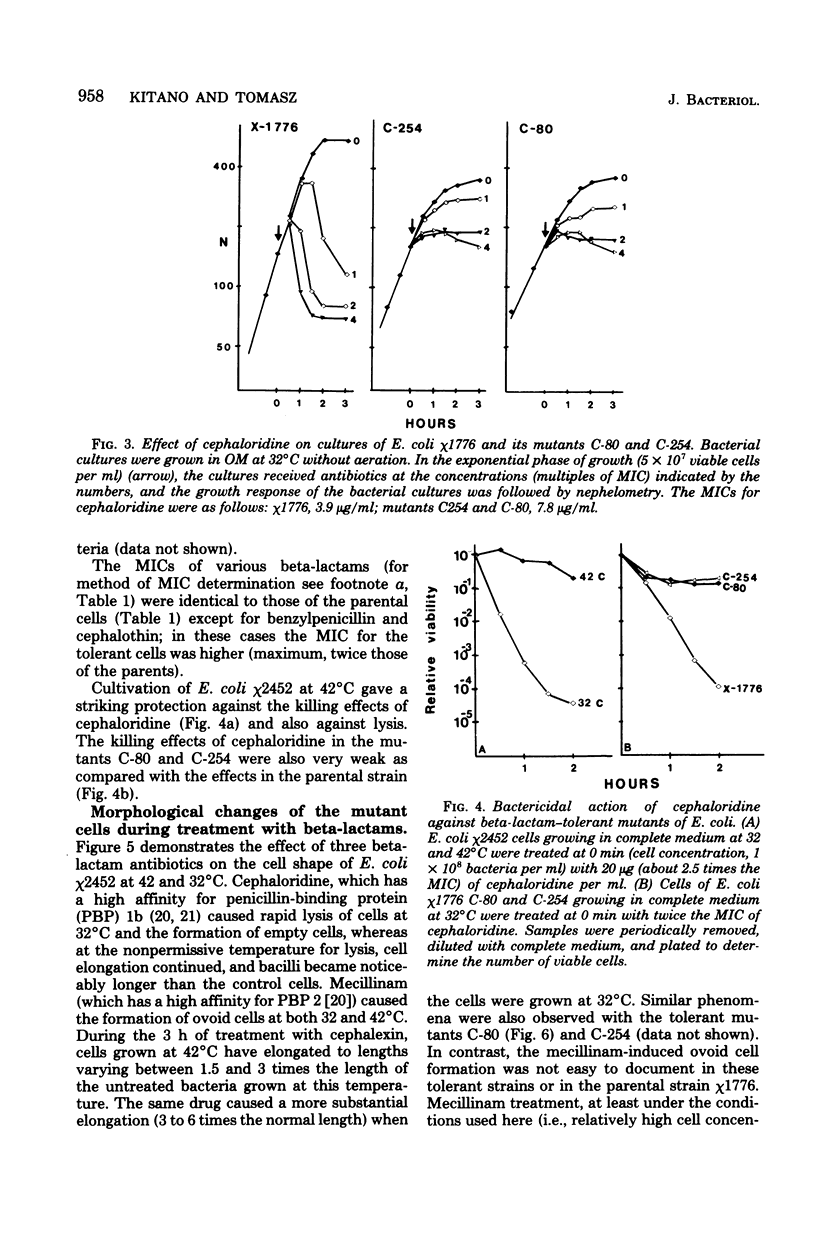
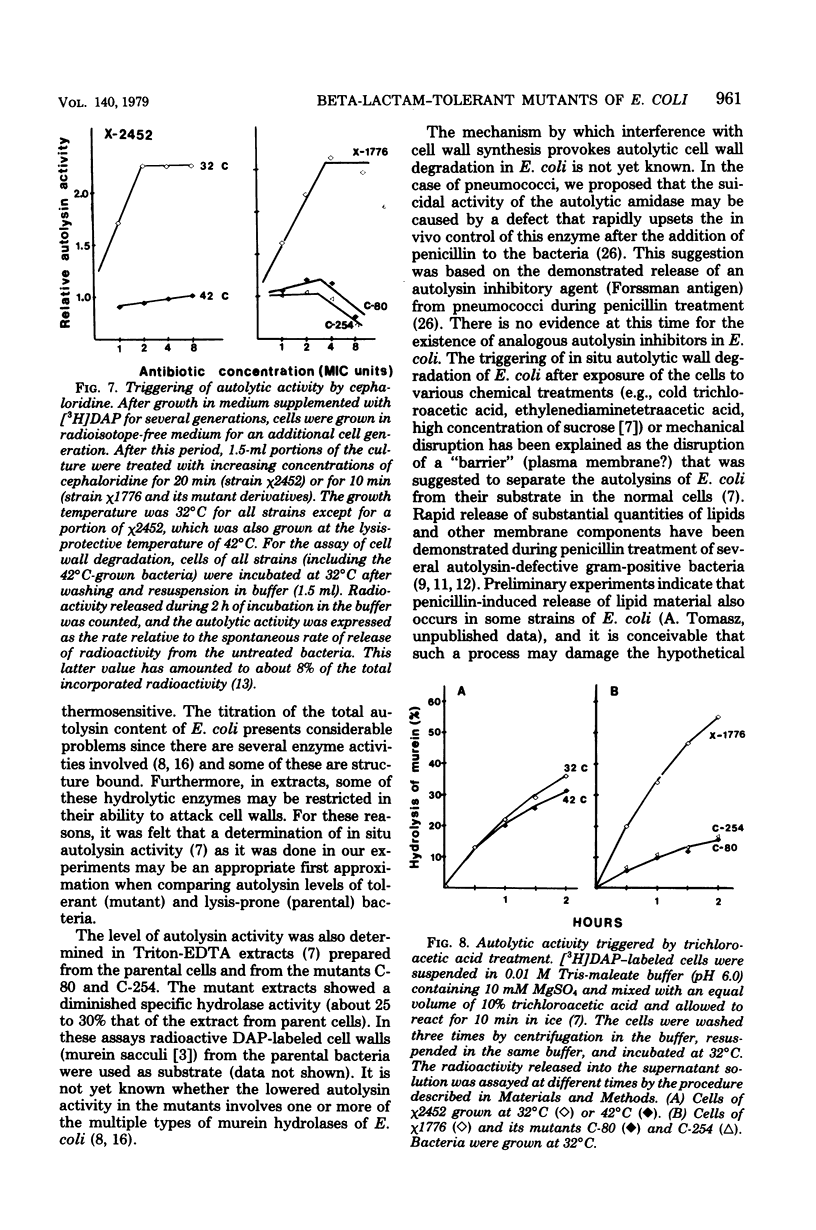
Two types of Escherichia coli mutants tolerant to beta-lactam antibiotics were isolated. One is E. coli χ2452, which showed a tolerant response against beta-lactam antibiotics when grown at 42°C, and the others are the mutants C-80 and C-254, selected from mutagenized E. coli χ1776 by cycles of exposure to ampicillin, cephaloridine, and starvation of the nutritionally required diaminopimelic acid. Beta-lactam antibiotics caused rapid loss of viability and lysis in cultures of χ1776 or in χ2452 grown at 32°C. In contrast, the same antibiotics caused only a reversible inhibition of growth in mutants C-80 and C-254 or in cultures of χ2452 grown at 42°C. Beta-lactam antibiotics that show high affinity for penicillin-binding proteins 2 or 3 (mecillinam and cephalexin, respectively) induced similar morphological effects (ovoid cell formation and filament formation) in both parent and mutant strains. In contrast, beta-lactam antibiotics which have a high affinity for penicillin-binding protein 1 (e.g., cephaloridine or cefoxitin), which cause rapid lysis in the parental strains, caused cell elongation in the tolerant bacteria. In contrast to the parental cells, autolytic cell wall degradation was not triggered by beta-lactam treatment of χ2452 cells grown at 42°C or in mutants C-80 and C-254. The total autolytic activity of mutants C-80 and C-254 was less than 30% that of the parent strain. However, virtually identical autolytic activities were found in cells of χ2452 grown either at 42 or 32°C. Possible mechanisms for the penicillin tolerance of E. coli are considered on the basis of these findings.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayusawa D., Yoneda Y., Yamane K., Maruo B. Pleiotropic phenomena in autolytic enzyme(s) content, flagellation, and simultaneous hyperproduction of extracellular alpha-amylase and protease in a Bacillus subtilis mutant. J Bacteriol. 1975 Oct;124(1):459–469. doi: 10.1128/jb.124.1.459-469.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Biological containment and cloning vector transmissibility. J Infect Dis. 1978 May;137(5):668–675. doi: 10.1093/infdis/137.5.668. [DOI] [PubMed] [Google Scholar]
- Fontana R., Satta G., Romanzi C. A. Penicillins activate autolysins extracted from both Escherichia coli and Klebsiella pneumoniae envelopes. Antimicrob Agents Chemother. 1977 Dec;12(6):745–747. doi: 10.1128/aac.12.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Bock-Hennig S. B., Schwarz U. Murein hydrolases in the envelope of Escherichia coli. Properties in situ and solubilization from the envelope. Eur J Biochem. 1974 Jan 3;41(1):203–208. doi: 10.1111/j.1432-1033.1974.tb03261.x. [DOI] [PubMed] [Google Scholar]
- Horne D., Hakenbeck R., Tomasz A. Secretion of lipids induced by inhibition of peptidoglycan synthesis in streptococci. J Bacteriol. 1977 Nov;132(2):704–717. doi: 10.1128/jb.132.2.704-717.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Release of lipoteichoic acid from Streptococcus sanguis: stimulation of release during penicillin treatment. J Bacteriol. 1979 Mar;137(3):1180–1184. doi: 10.1128/jb.137.3.1180-1184.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne D., Tomasz A. Tolerant response of Streptococcus sanguis to beta-lactams and other cell wall inhibitors. Antimicrob Agents Chemother. 1977 May;11(5):888–896. doi: 10.1128/aac.11.5.888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Höltje J. V., Mirelman D., Sharon N., Schwarz U. Novel type of murein transglycosylase in Escherichia coli. J Bacteriol. 1975 Dec;124(3):1067–1076. doi: 10.1128/jb.124.3.1067-1076.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitano K., Tomasz A. Triggering of autolytic cell wall degradation in Escherichia coli by beta-lactam antibiotics. Antimicrob Agents Chemother. 1979 Dec;16(6):838–848. doi: 10.1128/aac.16.6.838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oka T. Mode of action of penicillins in vivo and in vitro in Bacillus megaterium. Antimicrob Agents Chemother. 1976 Oct;10(4):579–591. doi: 10.1128/aac.10.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PELZER H. MUCOPEPTIDHYDROLASEN IN ESCHERICHIA COLI B. I. NACHWEIS UND WIRKUNGSSPEZIFITAET. Z Naturforsch B. 1963 Nov;18:950–956. [PubMed] [Google Scholar]
- SCHWARZ U., WEIDEL W. ZUM WIRKUNGSMECHANISMUS VON PENICILLIN. I. ISOLIERUNG UND CHARAKTERISIERUNG 2,6-DIAMINOPIMELINSAEURE ENTHALTENDER NIEDERMOLEKULARER PEPTIDE AUS PENICILLINSPHAEROPLASTEN VON ESCHERICHIA COLI B. Z Naturforsch B. 1965 Feb;20:147–153. [PubMed] [Google Scholar]
- Sabath L. D., Wheeler N., Laverdiere M., Blazevic D., Wilkinson B. J. A new type of penicillin resistance of Staphylococcus aureus. Lancet. 1977 Feb 26;1(8009):443–447. doi: 10.1016/s0140-6736(77)91941-9. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Distinct penicillin binding proteins involved in the division, elongation, and shape of Escherichia coli K12. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2999–3003. doi: 10.1073/pnas.72.8.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spratt B. G. Properties of the penicillin-binding proteins of Escherichia coli K12,. Eur J Biochem. 1977 Jan;72(2):341–352. doi: 10.1111/j.1432-1033.1977.tb11258.x. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Nishimura Y., Hirota Y. On the process of cellular division in Escherichia coli: a series of mutants of E. coli altered in the penicillin-binding proteins. Proc Natl Acad Sci U S A. 1978 Feb;75(2):664–668. doi: 10.1073/pnas.75.2.664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaki S., Nakajima S., Matsuhashi M. Thermosensitive mutation in Escherichia coli simultaneously causing defects in penicillin-binding protein-1Bs and in enzyme activity for peptidoglycan synthesis in vitro. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5472–5476. doi: 10.1073/pnas.74.12.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasz A., Albino A., Zanati E. Multiple antibiotic resistance in a bacterium with suppressed autolytic system. Nature. 1970 Jul 11;227(5254):138–140. doi: 10.1038/227138a0. [DOI] [PubMed] [Google Scholar]
- Tomasz A. The mechanism of the irreversible antimicrobial effects of penicillins: how the beta-lactam antibiotics kill and lyse bacteria. Annu Rev Microbiol. 1979;33:113–137. doi: 10.1146/annurev.mi.33.100179.000553. [DOI] [PubMed] [Google Scholar]
- Tomasz A., Waks S. Mechanism of action of penicillin: triggering of the pneumococcal autolytic enzyme by inhibitors of cell wall synthesis. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4162–4166. doi: 10.1073/pnas.72.10.4162. [DOI] [PMC free article] [PubMed] [Google Scholar]




