Abstract
The in vitro neutralization of the killing activity of cloacin DF13 by incubation with its purified receptor protein was shown to be the result of the formation of a direct and specific equimolar complex of both proteins. The binding of cloacin DF13 to its receptor protein did not result in a fragmentation of the cloacin molecules nor in the expulsion of immunity protein from the bacteriocin. The rate of the cloacin DF13-receptor interaction in vitro was found to be enhanced significantly in the presence of peptidoglycan, but lysozyme-treated peptidoglycan did not affect this interaction. Incubation of the cloacin DF13 as well as its receptor protein with peptidoglycan showed that the receptor protein but not the cloacin DF13 was able to bind to the peptidoglycan.
Full text
PDF


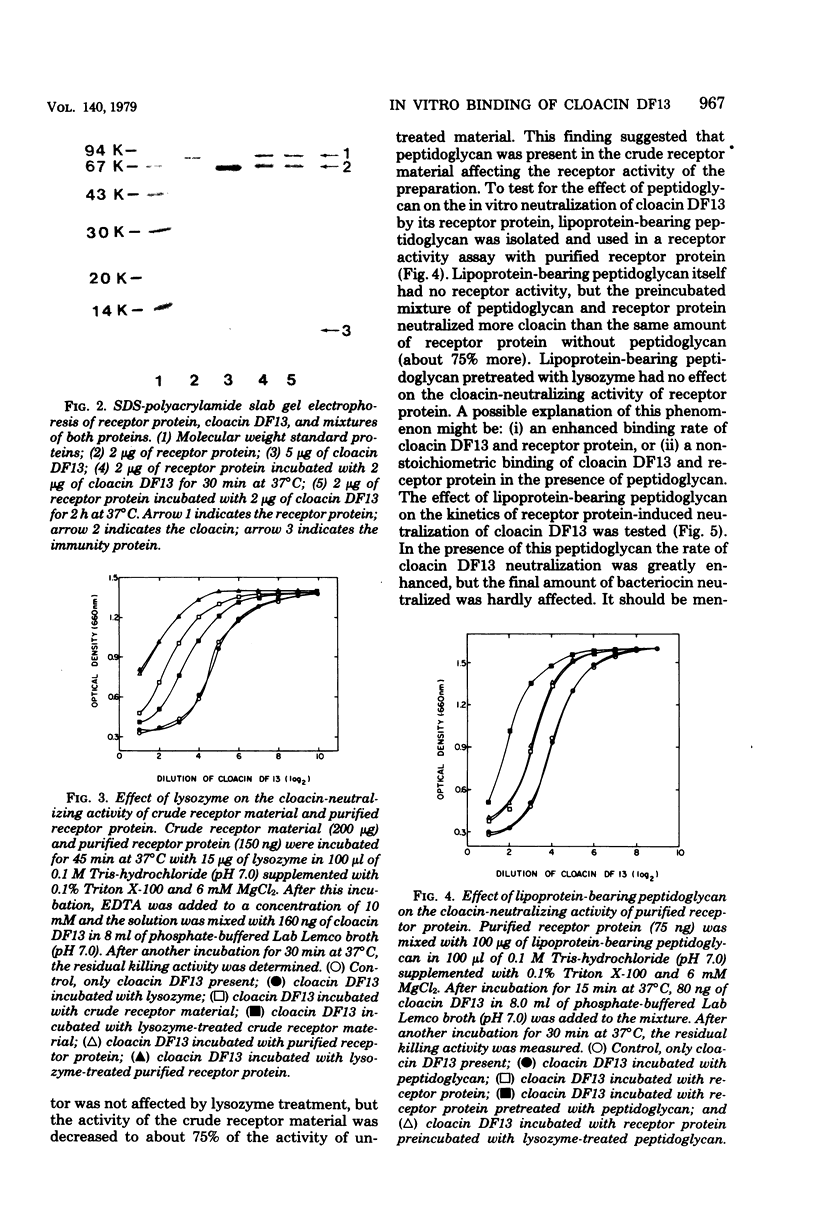



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boon T. Inactivation of ribosomes in vitro by colicin E 3 and its mechanism of action. Proc Natl Acad Sci U S A. 1972 Mar;69(3):549–552. doi: 10.1073/pnas.69.3.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Dahlberg J. E., Ikemura T., Konisky J., Nomura M. Specific inactivation of 16S ribosomal RNA induced by colicin E3 in vivo. Proc Natl Acad Sci U S A. 1971 May;68(5):964–968. doi: 10.1073/pnas.68.5.964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman C. M., Sidikaro J., Nomura M. Specific inactivation of ribosomes by colicin E3 in vitro and mechanism of immunity in colicinogenic cells. Nat New Biol. 1971 Dec 1;234(48):133–137. doi: 10.1038/newbio234133a0. [DOI] [PubMed] [Google Scholar]
- Cavard D., Lazdunski C. Interaction of colicin E4 with specific receptor sites mediates its cleavage into two fragments inactive towards whole cells. Eur J Biochem. 1979 Jun 1;96(3):525–533. doi: 10.1111/j.1432-1033.1979.tb13066.x. [DOI] [PubMed] [Google Scholar]
- Chandrarajan J., Klein L. Lowry assay of dilute protein solutions containing high concentrations of Triton X-100. Anal Biochem. 1975 Dec;69(2):632–636. doi: 10.1016/0003-2697(75)90169-4. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K., Klaasen-Boor P. Purification and characterization of a complex between cloacin and its immunity protein isolated from Enterobacter cloacae (Clo DF13). Dissociation and reconstitution of the complex. Eur J Biochem. 1977 Feb 15;73(1):107–114. doi: 10.1111/j.1432-1033.1977.tb11296.x. [DOI] [PubMed] [Google Scholar]
- De Graaf F. K. Mode of action of colicin E2, colicin E3 and cloacin DF13. Zentralbl Bakteriol Orig A. 1979 Jun;244(1):121–134. [PubMed] [Google Scholar]
- De Graaf F. K., Planta R. J., Stouthamer A. H. Effect of a bacteriocin produced by Enterobacter cloacae on protein biosynthesis. Biochim Biophys Acta. 1971 Jun 17;240(1):123–136. [PubMed] [Google Scholar]
- DiRienzo J. M., Nakamura K., Inouye M. The outer membrane proteins of Gram-negative bacteria: biosynthesis, assembly, and functions. Annu Rev Biochem. 1978;47:481–532. doi: 10.1146/annurev.bi.47.070178.002405. [DOI] [PubMed] [Google Scholar]
- Gaastra W., Oudega B., de Graaf F. K. The use of mutants in the study of structure-function relationships in cloacin DF13. Biochim Biophys Acta. 1978 May 3;540(2):301–312. doi: 10.1016/0304-4165(78)90143-5. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y., Yamada H., Mizushima S. Interactions of outer membrane proteins O-8 and O-9 with peptidoglycan sacculus of Escherichia coli K-12. J Biochem. 1976 Dec;80(6):1401–1409. doi: 10.1093/oxfordjournals.jbchem.a131413. [DOI] [PubMed] [Google Scholar]
- Herschman H. R., Helinski D. R. Purification and characterization of colicin E2 and colicin E3. J Biol Chem. 1967 Nov 25;242(22):5360–5368. [PubMed] [Google Scholar]
- Lugtenberg B., Bronstein H., van Selm N., Peters R. Peptidoglycan-associated outer membrane proteins in gammegatine bacteria. Biochim Biophys Acta. 1977 Mar 17;465(3):571–578. doi: 10.1016/0005-2736(77)90274-7. [DOI] [PubMed] [Google Scholar]
- Lugtenberg B., Meijers J., Peters R., van der Hoek P., van Alphen L. Electrophoretic resolution of the "major outer membrane protein" of Escherichia coli K12 into four bands. FEBS Lett. 1975 Oct 15;58(1):254–258. doi: 10.1016/0014-5793(75)80272-9. [DOI] [PubMed] [Google Scholar]
- Mooi F. R., De Graaf F. K. Effect of limited proteolysis on bacteriocin activity in vivo and in vitro. FEBS Lett. 1976 Mar 1;62(3):304–308. doi: 10.1016/0014-5793(76)80081-6. [DOI] [PubMed] [Google Scholar]
- Oudega B., Klaasen-Boor P., De Graaf F. K. Mode of action of the cloacin DF13-immunity protein. Biochim Biophys Acta. 1975 May 5;392(1):184–195. [PubMed] [Google Scholar]
- Oudega B., Klaasen-Boor P., Sneeuwloper G., De Graaf F. K. Interaction of the complex between cloacin and its immunity protein and of cloacin with the outer and cytoplasmic membranes of sensitive cells. Eur J Biochem. 1977 Sep;78(2):445–453. doi: 10.1111/j.1432-1033.1977.tb11757.x. [DOI] [PubMed] [Google Scholar]
- Oudega B., Oldenziel-Werner W. J., Klaasen-Boor P., Rezee A., Glas J., de Graaf F. K. Purification and characterization of cloacin DF13 receptor from Enterobacter cloacae and its interaction with cloacin DF13 in vitro. J Bacteriol. 1979 Apr;138(1):7–16. doi: 10.1128/jb.138.1.7-16.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oudega B., de Graaf F. K. Enzymatic properties of cloacin DF13 and kinetics of ribosome inactivation. Biochim Biophys Acta. 1976 Mar 17;425(3):296–304. doi: 10.1016/0005-2787(76)90256-2. [DOI] [PubMed] [Google Scholar]
- Rice R. H., Means G. E. Radioactive labeling of proteins in vitro. J Biol Chem. 1971 Feb 10;246(3):831–832. [PubMed] [Google Scholar]
- Rosenbusch J. P. Characterization of the major envelope protein from Escherichia coli. Regular arrangement on the peptidoglycan and unusual dodecyl sulfate binding. J Biol Chem. 1974 Dec 25;249(24):8019–8029. [PubMed] [Google Scholar]
- Tieze G. A., Stouthamer A. H., Jansz H. S., Zandberg J., van Bruggen E. F. A bacteriocinogenic factor of Enterobacter cloacae. Mol Gen Genet. 1969;106(1):48–65. [PubMed] [Google Scholar]
- Watson D. H., Sherratt D. J. An homologous region of colicins Ia and A [proceedings]. Biochem Soc Trans. 1979 Aug;7(4):706–708. doi: 10.1042/bst0070706. [DOI] [PubMed] [Google Scholar]
- Watson D. H., Sherratt D. J. In vivo proteolytic cleavage of colicins requires specific receptor binding. Nature. 1979 Mar 22;278(5702):362–364. doi: 10.1038/278362a0. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K. Effects of cloacin DF13 on the functioning of the cytoplasmic membrane. Antonie Van Leeuwenhoek. 1973;39(1):109–119. doi: 10.1007/BF02578846. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Niekus H. G., Klootwijk J. Inactivation of bacterial ribosomes in vivo and in vitro by cloacin DF13. FEBS Lett. 1973 Sep 1;35(1):161–165. doi: 10.1016/0014-5793(73)80601-5. [DOI] [PubMed] [Google Scholar]
- de Graaf F. K., Spanjaerdt Speckman E. A., Stouthamer A. H. Mode of action of a bacteriocin produced by Enterobacter cloacae DF13. Antonie Van Leeuwenhoek. 1969;35(3):287–306. doi: 10.1007/BF02219150. [DOI] [PubMed] [Google Scholar]




