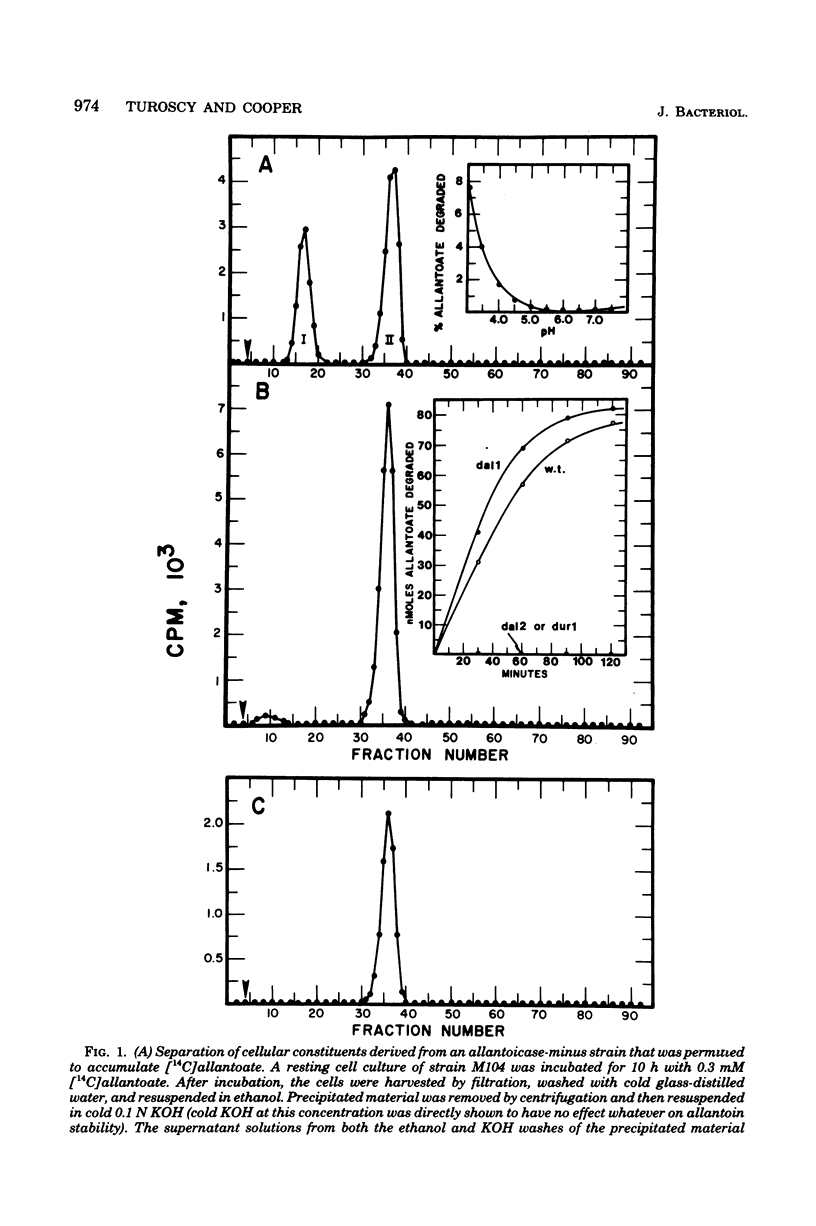
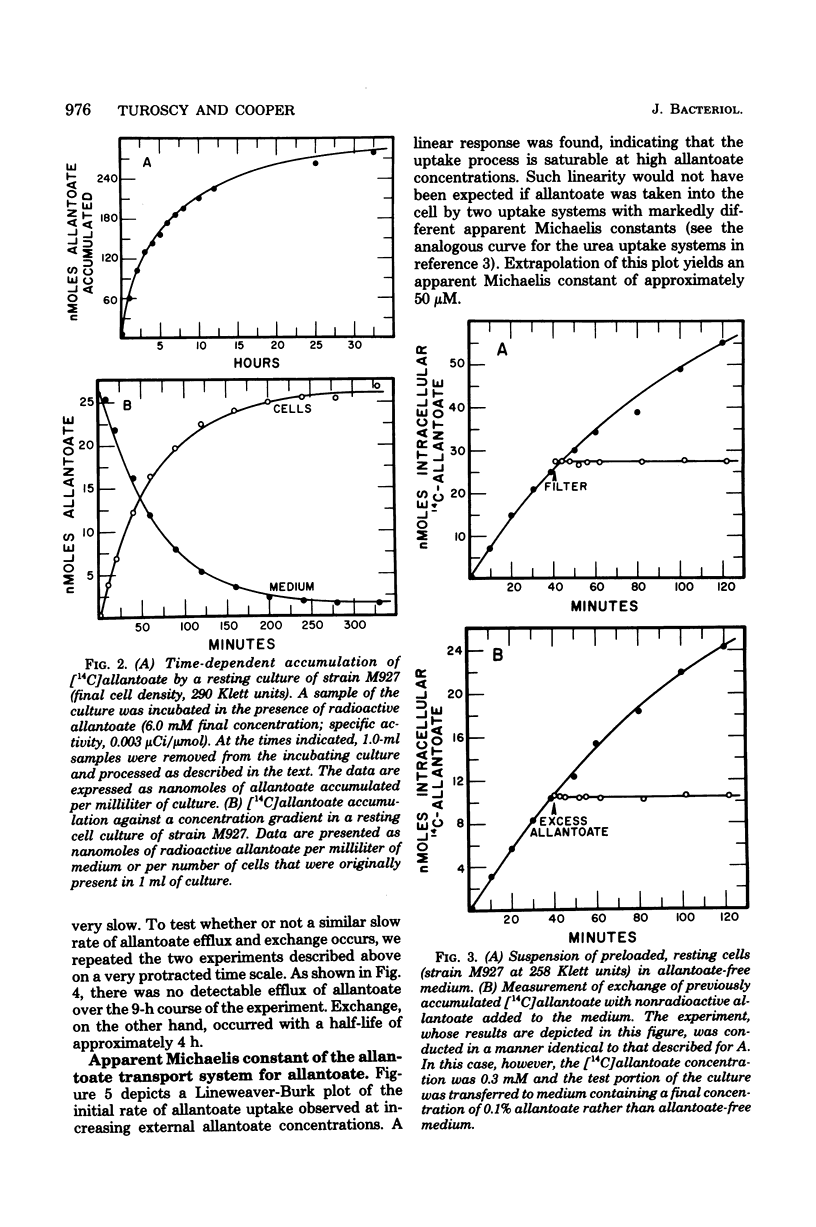
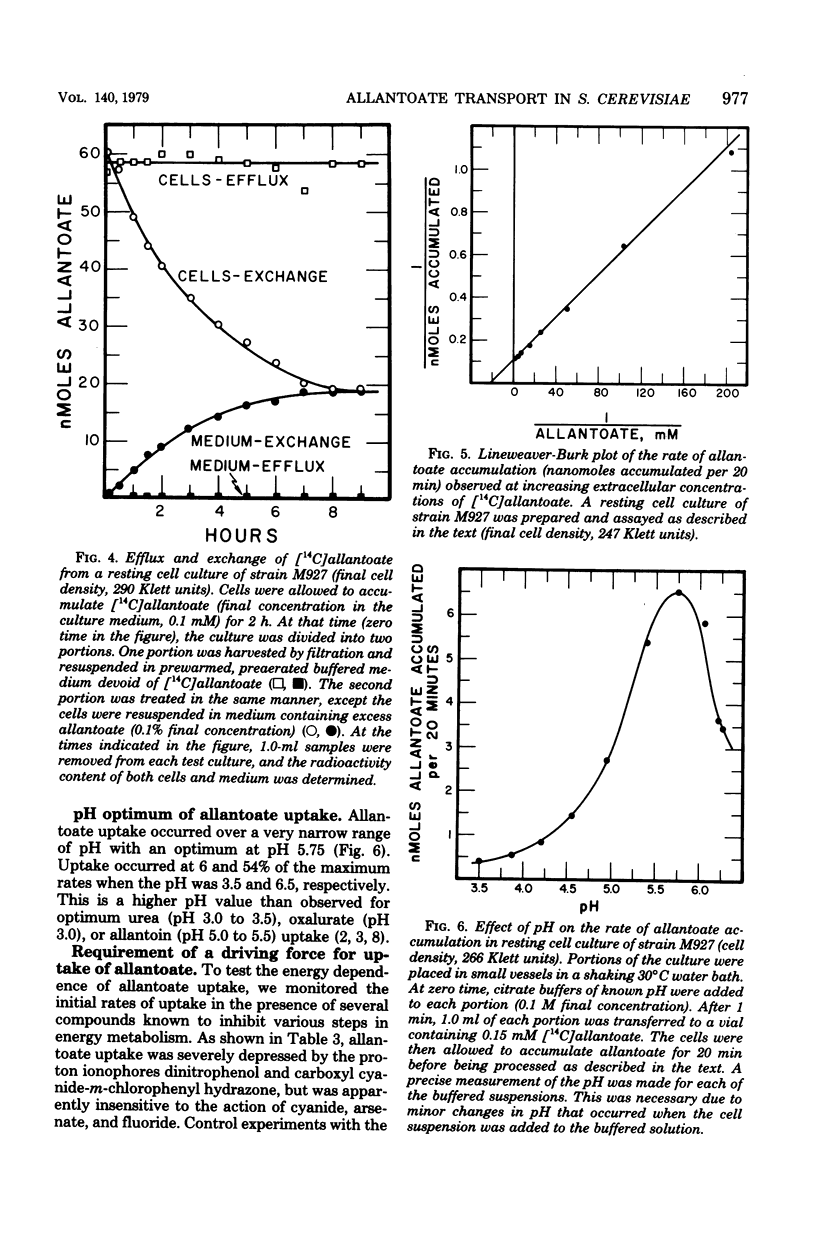
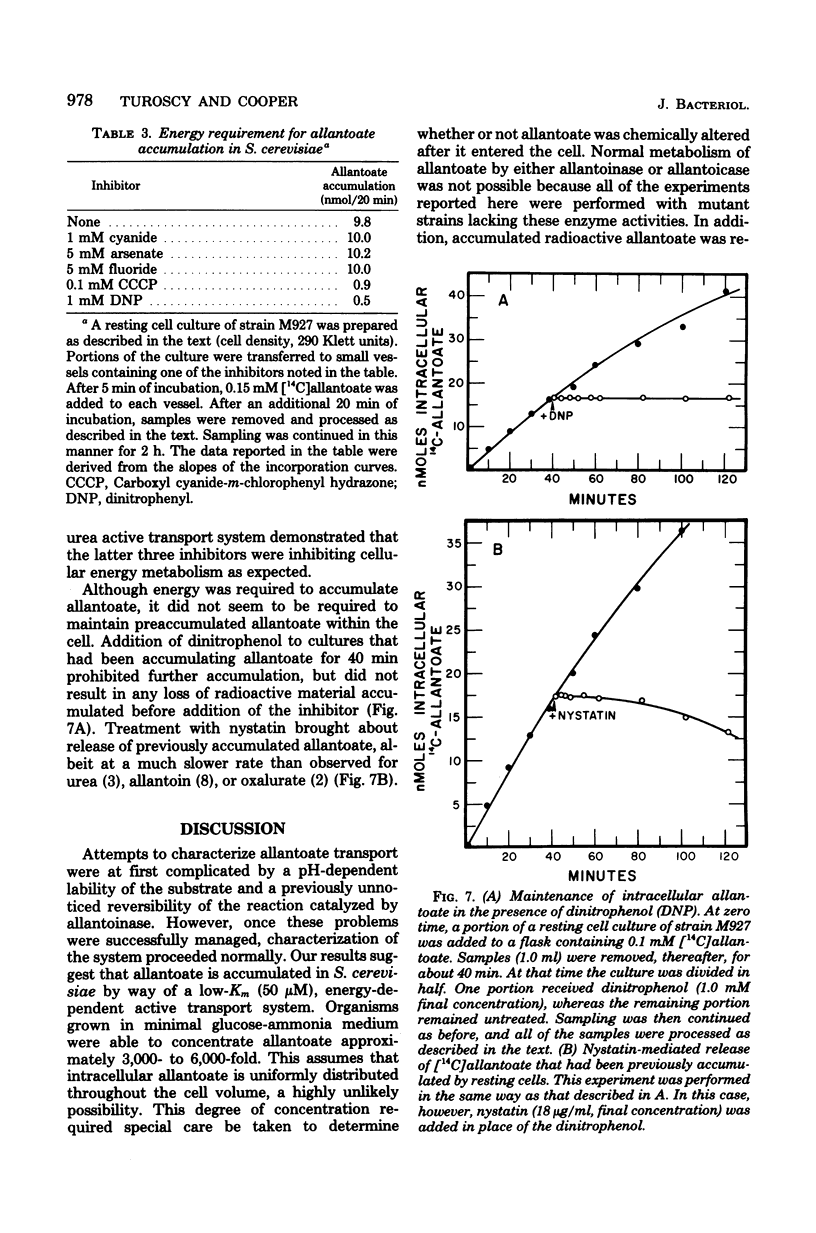
Abstract
Allantoate uptake appears to be mediated by an energy-dependent active transport system with an apparent Michaelis constant of about 50 microM. Cells were able to accumulate allantoate to greater than 3,000 times the extracellular concentration. The rate of accumulation was maximum at pH 5.7 to 5.8. The energy source for allantoate uptake is probably different from that for uptake of the other allantoin pathway intermediates. The latter systems are inhibited by arsenate, fluoride, dinitrophenol, and carboxyl cyanide-m-chlorophenyl hydrazone, whereas allantoate accumulation was sensitive to only dinitrophenol and carboxyl cyanide-m-chlorophenyl hydrazone. Efflux of preloaded allanotate did not occur at detectable levels. However, exchange of intra- and extracellular allantoate was found to occur very slowly. The latter two characteristics are shared with the allantoin uptake system and may result from the sequestering of intracellular allantoate within the cell vacuole. During the course of these studies, we found that, contrary to earlier reports, the reaction catalyzed by allantoinase is freely reversible.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bossinger J., Cooper T. G. Sequence of molecular events involved in induction of allophanate hydrolase. J Bacteriol. 1976 Apr;126(1):198–204. doi: 10.1128/jb.126.1.198-204.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., McKelvey J., Sumrada R. Oxalurate transport in Saccharomyces cerevisiae. J Bacteriol. 1979 Sep;139(3):917–923. doi: 10.1128/jb.139.3.917-923.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper T. G., Sumrada R. Urea transport in Saccharomyces cerevisiae. J Bacteriol. 1975 Feb;121(2):571–576. doi: 10.1128/jb.121.2.571-576.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabeel M., Grenson M. Regulation of histidine uptake by specific feedback inhibition of two histidine permeases in Saccharomyces cerevisiae. Eur J Biochem. 1970 May 1;14(1):197–204. doi: 10.1111/j.1432-1033.1970.tb00278.x. [DOI] [PubMed] [Google Scholar]
- Greth M. L., Chevallier M. R., Lacroute F. Ureidosuccinic acid permeation in Saccharomyces cerevisiae. Biochim Biophys Acta. 1977 Feb 14;465(1):138–151. doi: 10.1016/0005-2736(77)90362-5. [DOI] [PubMed] [Google Scholar]
- Kotyk A., Ríhová L. Transport of -aminoisobutyric acid in Saccharomyces cerevisiae. Biochim Biophys Acta. 1972 Nov 2;288(2):380–389. doi: 10.1016/0005-2736(72)90259-3. [DOI] [PubMed] [Google Scholar]
- Lawther R. P., Riemer E., Chojnacki B., Cooper T. G. Clustering of the genes for allantoin degradation in Saccharomyces cerevisiae. J Bacteriol. 1974 Aug;119(2):461–468. doi: 10.1128/jb.119.2.461-468.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R., Cooper T. G. Allantoin transport in Saccharomyces cerevisiae. J Bacteriol. 1977 Sep;131(3):839–847. doi: 10.1128/jb.131.3.839-847.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R., Cooper T. G. Control of vacuole permeability and protein degradation by the cell cycle arrest signal in Saccharomyces cerevisiae. J Bacteriol. 1978 Oct;136(1):234–246. doi: 10.1128/jb.136.1.234-246.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R., Gorski M., Cooper T. Urea transport-defective strains of Saccharomyces cerevisiae. J Bacteriol. 1976 Mar;125(3):1048–1056. doi: 10.1128/jb.125.3.1048-1056.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumrada R., Zacharski C. A., Turoscy V., Cooper T. G. Induction and inhibition of the allantoin permease in Saccharomyces cerevisiae. J Bacteriol. 1978 Aug;135(2):498–510. doi: 10.1128/jb.135.2.498-510.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Requirement for HCO3- by ATP: urea amido-lyase in yeast. Biochem Biophys Res Commun. 1970 Aug 24;40(4):814–819. doi: 10.1016/0006-291x(70)90975-7. [DOI] [PubMed] [Google Scholar]
- Whitney P. A., Cooper T. G. Urea carboxylase and allophanate hydrolase. Two components of adenosine triphosphate:urea amido-lyase in Saccharomyces cerevisiae. J Biol Chem. 1972 Mar 10;247(5):1349–1353. [PubMed] [Google Scholar]
- Wickerham L. J. A Critical Evaluation of the Nitrogen Assimilation Tests Commonly Used in the Classification of Yeasts. J Bacteriol. 1946 Sep;52(3):293–301. [PMC free article] [PubMed] [Google Scholar]
- Zacharski C. A., Cooper T. G. Metabolite compartmentation in Saccharomyces cerevisiae. J Bacteriol. 1978 Aug;135(2):490–497. doi: 10.1128/jb.135.2.490-497.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]



