Abstract
Evidence for reductive carboxylation of succinate to synthesize alpha-ketoglutarate was sought in anaerobic heterotrophs from the rumen and from other anaerobic habitats. Cultures were grown in media containing unlabeled energy substrates plus [14C]succinate, and synthesis of cellular glutamate with a much higher specific activity than that of cellular asparate was taken as evidence for alpha-ketoglutarate synthase activity. Our results indicate alpha-ketoglutarate synthase functions in Selenomonas ruminantium, Veillonella alcalescens, Bacteroides fragilis, Bacteroides vulgatus, Bacteroides uniformis, Bacteroides distasonis, and Bacteroides multiacidus. Evidence for this carboxylation was not found in strains representative of 10 other species.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison M. J., Littledike E. T., James L. F. Changes in ruminal oxalate degradation rates associated with adaptation to oxalate ingestion. J Anim Sci. 1977 Nov;45(5):1173–1179. doi: 10.2527/jas1977.4551173x. [DOI] [PubMed] [Google Scholar]
- Allison M. J., Robinson I. M., Baetz A. L. Tryptophan biosynthesis from indole-3-acetic acid by anaerobic bacteria from the rumen. J Bacteriol. 1974 Jan;117(1):175–180. doi: 10.1128/jb.117.1.175-180.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
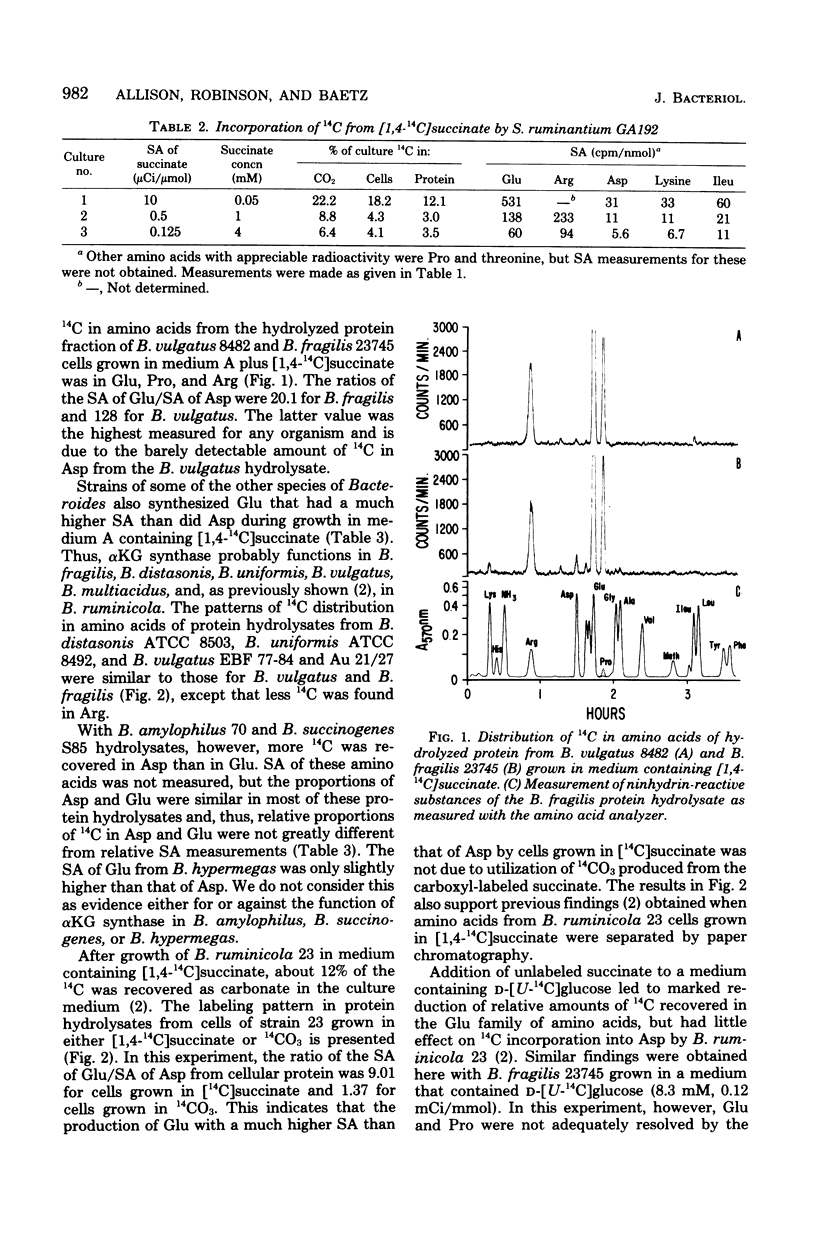
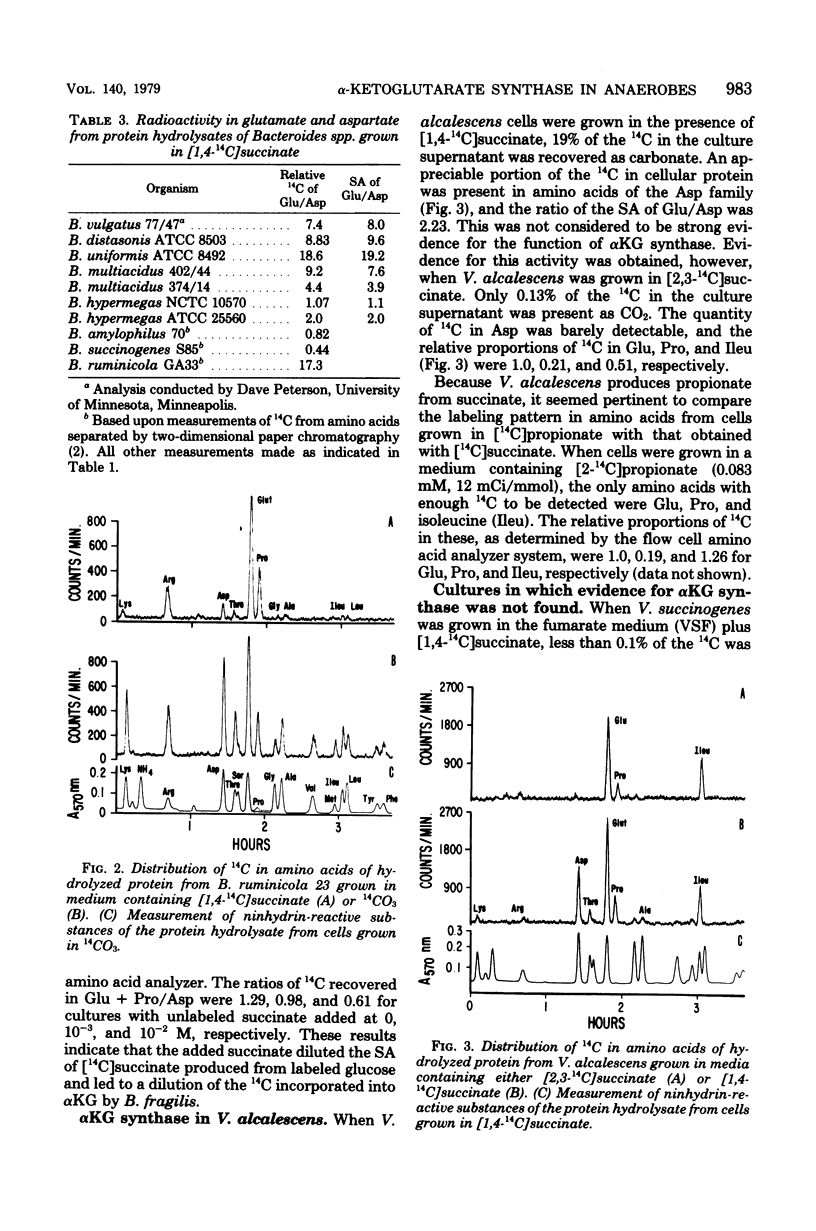
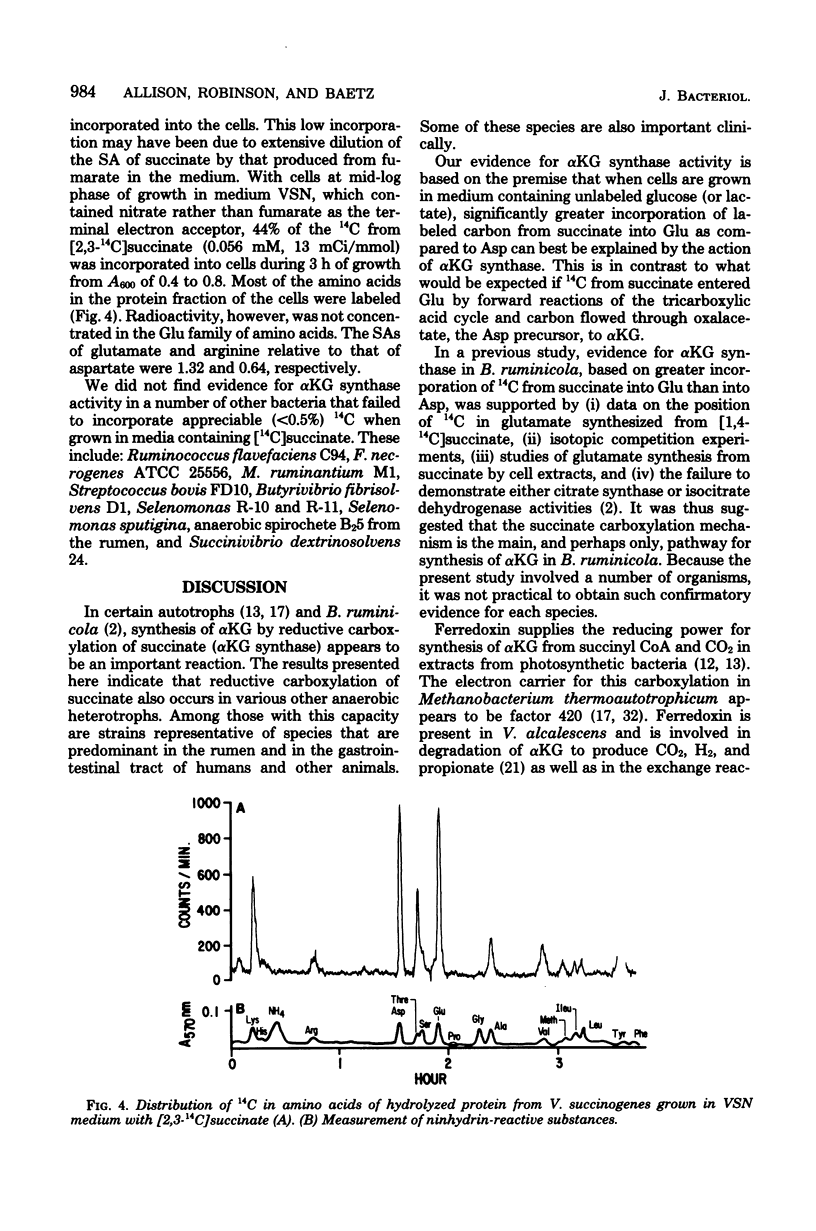
- Allison M. J., Robinson I. M. Biosynthesis of alpha-ketoglutarate by the reductive carboxylation of succinate in Bacteroides ruminicola. J Bacteriol. 1970 Oct;104(1):50–56. doi: 10.1128/jb.104.1.50-56.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson R. E., Synder F. Quantitative collection of 14CO2 in the presence of labeled short-chain acids. Anal Biochem. 1969 Feb;27(2):311–314. doi: 10.1016/0003-2697(69)90038-4. [DOI] [PubMed] [Google Scholar]
- BLACKBURN T. H., HUNGATE R. E. Succinic acid turnover and propionate production in the bovine rumen. Appl Microbiol. 1963 Mar;11:132–135. doi: 10.1128/am.11.2.132-135.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes E. M., Impey C. S. Anaerobic gram negative nonsporing bacteria from the caeca of poultry. J Appl Bacteriol. 1968 Dec;31(4):530–541. doi: 10.1111/j.1365-2672.1968.tb00402.x. [DOI] [PubMed] [Google Scholar]
- Bryant M. P., Robinson I. M. Some Nutritional Requirements of the Genus Ruminococcus. Appl Microbiol. 1961 Mar;9(2):91–95. doi: 10.1128/am.9.2.91-95.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Arnon D. I. Ferredoxins: chemistry and function in photosynthesis, nitrogen fixation, and fermentative metabolism. Adv Enzymol Relat Areas Mol Biol. 1970;33:119–176. doi: 10.1002/9780470122785.ch3. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B., Evans M. C. The synthesis of alpha-ketoglutarate from succinate and carbon dioxide by a subcellular preparation of a photosynthetic bacterium. Proc Natl Acad Sci U S A. 1965 Oct;54(4):1212–1218. doi: 10.1073/pnas.54.4.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B. Role of ferredoxin in the synthesis of alpha-ketobutyrate from propionyl coenzyme A and carbon dioxide by enzymes from photosynthetic and nonphotosynthetic bacteria. J Biol Chem. 1969 Aug 10;244(15):4218–4223. [PubMed] [Google Scholar]
- Bush R. S., Sauer F. D. Enzymes of 2-oxo acid degradation and biosynthesis in cell-free extracts of mixed rumen micro-organisms. Biochem J. 1976 Aug 1;157(2):325–331. doi: 10.1042/bj1570325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bush R. S., Sauer F. D. This isolation of a new, low-potential, iron-containing electron-transfer protein from rumen bacteria. Biochem Biophys Res Commun. 1976 Jul 12;71(1):53–59. doi: 10.1016/0006-291x(76)90248-5. [DOI] [PubMed] [Google Scholar]
- Emmanuel B., Milligan L. P. Enzymes of the conversion of succinate to glutamate in extracts of rumen microorganisms. Can J Biochem. 1972 Jan;50(1):1–8. doi: 10.1139/o72-001. [DOI] [PubMed] [Google Scholar]
- Fuchs G., Stupperich E., Thauer R. K. Acetate assimilation and the synthesis of alanine, aspartate and glutamate in Methanobacterium thermoautotrophicum. Arch Microbiol. 1978 Apr 27;117(1):61–66. doi: 10.1007/BF00689352. [DOI] [PubMed] [Google Scholar]
- HUNGATE R. E. The anaerobic mesophilic cellulolytic bacteria. Bacteriol Rev. 1950 Mar;14(1):1–49. doi: 10.1128/br.14.1.1-49.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris M. A., Reddy C. A. Hydrogenase activity and the H2-fumarate electron transport system in Bacteroides fragilis. J Bacteriol. 1977 Sep;131(3):922–928. doi: 10.1128/jb.131.3.922-928.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kafkewitz D. Improved growth media for Vibrio succinogenes. Appl Microbiol. 1975 Jan;29(1):121–122. doi: 10.1128/am.29.1.121-122.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindley R. W., Delwiche E. A. Degradiation of alpha-ketoglutarate by Veillonella alcalescens. J Bacteriol. 1969 Apr;98(1):315–316. doi: 10.1128/jb.98.1.315-316.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macy J. M., Ljungdahl L. G., Gottschalk G. Pathway of succinate and propionate formation in Bacteroides fragilis. J Bacteriol. 1978 Apr;134(1):84–91. doi: 10.1128/jb.134.1.84-91.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan L. P. Carbon dioxide fixing pathways of glutamic acid synthesis in the rumen. Can J Biochem. 1970 Apr;48(4):463–468. doi: 10.1139/o70-075. [DOI] [PubMed] [Google Scholar]
- Niederman R. A., Wolin M. J. Requirement of succinate for the growth of Vibrio succinogenes. J Bacteriol. 1972 Feb;109(2):546–549. doi: 10.1128/jb.109.2.546-549.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paynter M. J., Elsden S. R. Mechanism of propionate formation by Selenomonas ruminantium, a rumen micro-organism. J Gen Microbiol. 1970 Apr;61(1):1–7. doi: 10.1099/00221287-61-1-1. [DOI] [PubMed] [Google Scholar]
- Sauer F. D., Erfle J. D., Mahadevan S. Amino acid biosynthesis in mixed rumen cultures. Biochem J. 1975 Sep;150(3):357–372. doi: 10.1042/bj1500357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITELEY H. R., McCORMICK N. G. Degradation of pyruvate by Micrococcus lactilyticus. III. Properties and cofactor requirements of the carbon dioxide-exchange reaction. J Bacteriol. 1963 Feb;85:382–393. doi: 10.1128/jb.85.2.382-393.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLIN M. J., WOLIN E. A., JACOBS N. J. Cytochrome-producing anaerobic Vibrio succinogenes, sp. n. J Bacteriol. 1961 Jun;81:911–917. doi: 10.1128/jb.81.6.911-917.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallnöfer P., Baldwin R. L. Pathway of propionate formation in Bacteroides ruminicola. J Bacteriol. 1967 Jan;93(1):504–505. doi: 10.1128/jb.93.1.504-505.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley H. R. The Mechanism of Propionic Acid Formation by Succinate Decarboxylation: II. The Formation and Decarboxylation of Succinyl-CoA. Proc Natl Acad Sci U S A. 1953 Aug;39(8):779–785. doi: 10.1073/pnas.39.8.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeikus J. G., Fuchs G., Kenealy W., Thauer R. K. Oxidoreductases involved in cell carbon synthesis of Methanobacterium thermoautotrophicum. J Bacteriol. 1977 Nov;132(2):604–613. doi: 10.1128/jb.132.2.604-613.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]


