Abstract
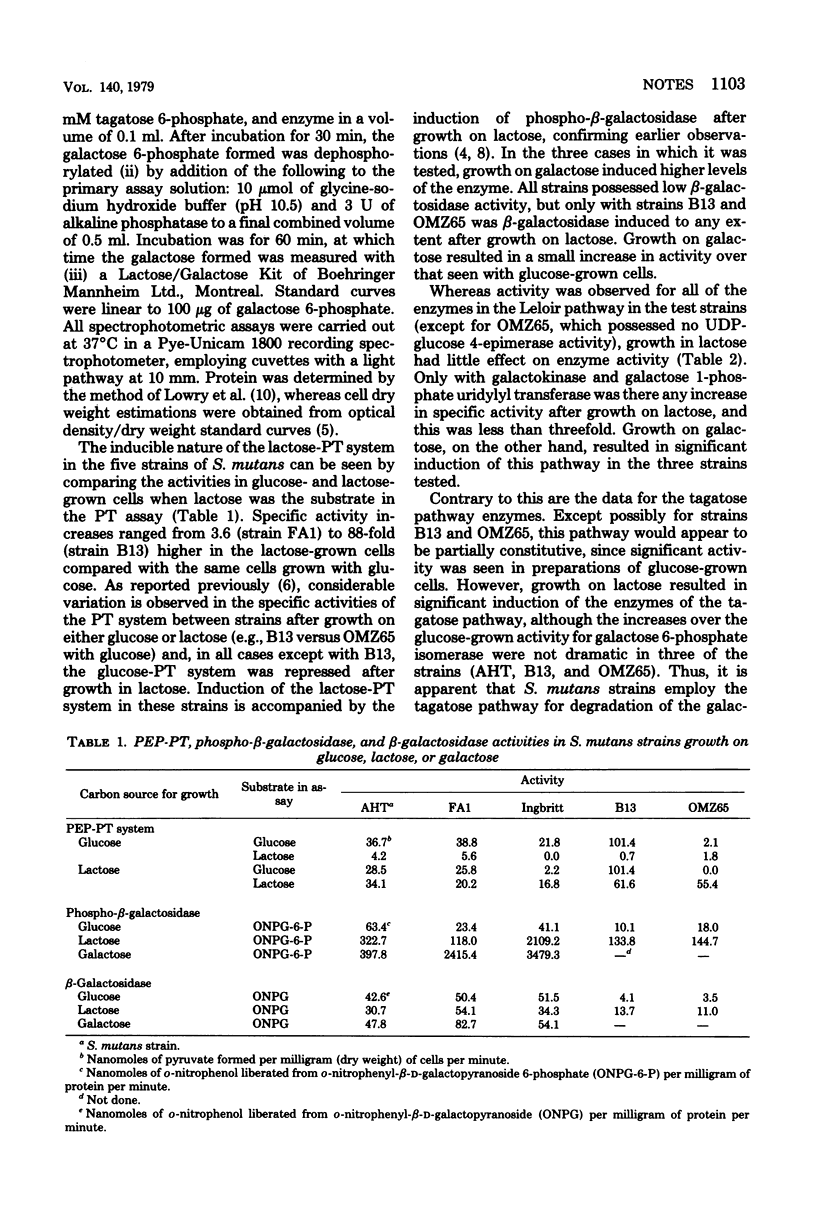
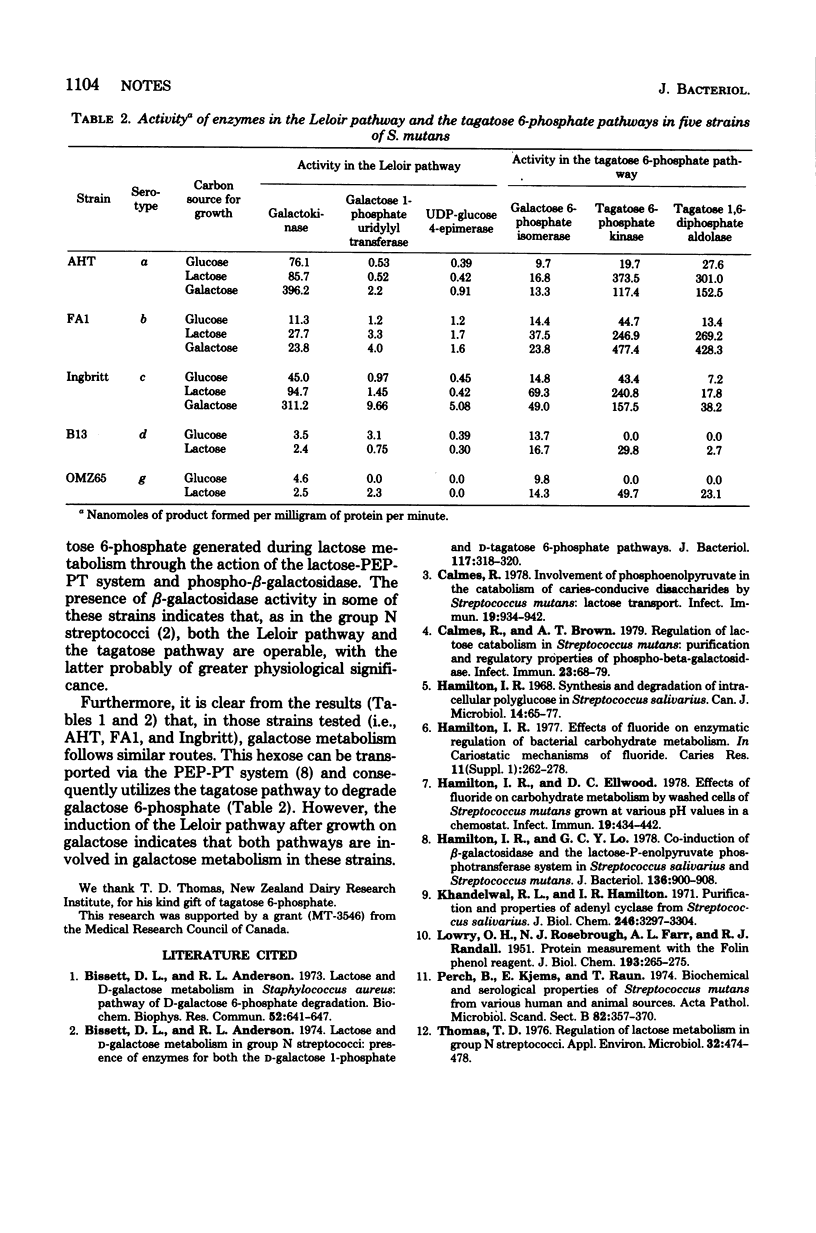
Growth on lactose by strains of Streptococcus mutans resulted in the induction of the lactose-phosphoenolpyruvate-phosphotransferase system, phospho-beta-galactosidase, and the enzymes of the tagatose 6-phosphate pathway.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in group N streptococci: presence of enzymes for both the D-galactose 1-phosphate and D-tagatose 6-phosphate pathways. J Bacteriol. 1974 Jan;117(1):318–320. doi: 10.1128/jb.117.1.318-320.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D0galactose metabolism in Staphylococcus aureus: pathway of D-galactose 6-phosphate degradation. Biochem Biophys Res Commun. 1973 May 15;52(2):641–647. doi: 10.1016/0006-291x(73)90761-4. [DOI] [PubMed] [Google Scholar]
- Calmes R., Brown A. T. Regulation of lactose catabolism in Streptococcus mutans: purification and regulatory properties of phospho-beta-galactosidase. Infect Immun. 1979 Jan;23(1):68–79. doi: 10.1128/iai.23.1.68-79.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calmes R. Involvement of phosphoenolpyruvate in the catabolism of caries-conducive disaccharides by Streptococcus mutans: lactose transport. Infect Immun. 1978 Mar;19(3):934–942. doi: 10.1128/iai.19.3.934-942.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R. Effects of fluoride on enzymatic regulation of bacterial carbohydrate metabolism. Caries Res. 1977;11 (Suppl 1):262–291. doi: 10.1159/000260304. [DOI] [PubMed] [Google Scholar]
- Hamilton I. R., Ellwood D. C. Effects of fluoride on carbohydrate metabolism by washed cells of Streptococcus mutans grown at various pH values in a chemostat. Infect Immun. 1978 Feb;19(2):434–442. doi: 10.1128/iai.19.2.434-442.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R., Lo G. C. Co-induction of beta-galactosidase and the lactose-P-enolpyruvate phosphotransferase system in Streptococcus salivarius and Streptococcus mutans. J Bacteriol. 1978 Dec;136(3):900–908. doi: 10.1128/jb.136.3.900-908.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton I. R. Synthesis and degradiation of intracellular polyglucose in Streptococcus salivarius. Can J Microbiol. 1968 Jan;14(1):65–77. doi: 10.1139/m68-011. [DOI] [PubMed] [Google Scholar]
- Khandelwal R. L., Hamilton I. R. Purification and properties of adenyl cyclase from Streptococcus salivarius. J Biol Chem. 1971 May 25;246(10):3297–3304. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Perch B., Kjems E., Ravn T. Biochemical and serological properties of Streptococcus mutans from various human and animal sources. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Jun;82(3):357–370. doi: 10.1111/j.1699-0463.1974.tb02338.x. [DOI] [PubMed] [Google Scholar]
- Thomas T. D. Regulation of lactose fermentation in group N streptococci. Appl Environ Microbiol. 1976 Oct;32(4):474–478. doi: 10.1128/aem.32.4.474-478.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]



