Abstract
Alterations in the peripheral immune system are associated with dementia and the neuropathology of Alzheimer’s disease, but have yet to be studied early in the disease process. To test the hypothesis that the balance of immune cell phenotypes is disrupted in the early progression of memory deterioration, patients with mild cognitive impairment (MCI) and healthy elderly controls were examined for the distribution of subpopulations of leukocytes (lymphocytes, granulocytes, and monocytes) and lymphocyte subtypes (helper/inducer and suppressor/cytotoxic T lymphocytes and B lymphocytes) in blood. MCI subjects had a significantly higher percentage of total lymphocytes and a lower percentage of granulocytes compared to elderly controls. Furthermore, the expression of cell surface amyloid precursor protein (APP) and intracellular amyloid-β peptide (Aβ) in lymphocytes and monocytes were determined. We found lymphocyte APP expression to be significantly increased in MCI subjects compared to controls. Our data indicate that changes in immunological parameters may be detected early in MCI, and an alteration of the immune response may precede clinical AD.
Keywords: Alzheimer’s disease, Lymphocytes, Monocytes, Amyloid precursor protein, Amyloid-β, Flow cytometry
1. Introduction
Age-associated neural and circulatory alterations in the immune system have been reported in Alzheimer’s disease (AD), the most common form of dementia in the elderly (McGeer &McGeer, 1998;Richartz et al., 2005). Inflammatory cytokines, activated microglia, and immigration of certain T lymphocytes are found in association with AD neuropathologies, including amyloid plaques and neurofibrillary tangles (McGeer et al., 1989;Griffin et al., 1995;Singh, 1997; Neuroinflammation Working Group, 2000), and alterations in peripheral immune cells are also reported in patients with AD. The proportion of both CD4+ (Lombardi et al,. 1999) and CD8+ lymphocytes have been shown to be decreased in AD relative to healthy aged-matched controls (Pirttila et al., 1992). Furthermore, Shalit et al. (1995)have demonstrated that in moderately severe, but not mild AD patients, there was a significant decrease in lymphocyte proliferation compared to controls. A decrease in proliferation in peripheral blood mononuclear cells (PBMCs) of AD patients has also been found (Lombardi et al., 1999). However, other reports indicate no differences in either CD4/CD8 ratios (Ikeda et al., 1991), total lymphocytes (Dysken et al., 1992) or the percentage of CD3+ lymphocytes (total T lymphocytes) (Lombardi et al,. 1999) between AD and controls. Differences in methodology or heterogeneity in the selection of patients with respect to the severity of dementia may partly account for these differences.
Changes in functional responses by peripheral leukocytes have been linked to pathological processes that occur in the AD brain. Trieb et al. (1996)have shown that lymphocyte proliferation is stimulated by amyloid precursor protein (APP), the processing of which produces amyloid-β (Aβ) peptide, the major component of amyloid plaques (Mott et al., 2005), in control but not AD patients. Leukocytes also express APP mRNA and protein (Allen et al., 1991;Schlossmacher et al., 1992;Bullido et al., 1996), and APP expression on lymphocytes and monocytes can be increased with activating signals (Li et al., 1999;Bullido et al., 1996). Moreover, APP mRNA in PBMCs and APP protein in lymphocytes and monocytes have been found to be elevated in AD patients compared to controls (Jiang et al., 2003). Functional changes in APP production and processing in peripheral immune cells may reflect dysregulation of APP metabolism in the brain (Pallister et al., 1997;Jung et al., 1999). Recently Migliore et al. (2005)demonstrated increased DNA damage in peripheral leukocytes of subjects with mild cognitive impairment (MCI) and AD patients compared to controls, suggesting the possibility that alterations in peripheral blood cells may be an early event in AD. MCI is a pre-clinical stage of AD representing a transition state between normal aging and early AD (Petersen, 2004). However, no study has examined peripheral blood cells in subjects with MCI even though a significant proportion of these patients eventually transition into mild AD.
Development of early biomarkers for diagnosis and potential intervention in patients with MCI has promise for providing a definite diagnosis of people at risk for developing AD. Thus, the main hypothesis addressed by the present study is that dysregulation of peripheral immune responses are early events in AD pathogenesis and can be detected in subjects with MCI. The approach was to determine if alterations in leukocyte immunophenotypes and peripheral blood cell expression of APP and Aβ, critical markers for AD, occur in MCI subjects.
2. Materials and methods
2.1. Subjects
In this study, we investigated 48 subjects that met criteria described below for MCI (mean age 77.3 ± 5.2, male/female = 28/20, years of education = 14.3 ± 2.3) and 24 healthy aged controls (mean age 73.6 ± 7.2, male/female = 9/15, years of education = 13.7 ± 2.7). MCI and controls subjects were recruited by referrals from neurologists, internists, geriatricians, and family practitioners and through advertisement in the local media in the Southern California area. The investigation was carried out in accordance with the Declaration of Helsinki, informed consent was obtained from all subjects, and protocols for collecting blood samples were approved by the Institutional Review Board of Loma Linda University School of Medicine. Following initial screening with the mini-mental state examination (MMSE) and Logical Memory I and II tests, all subjects selected for inclusion in the study underwent a full neuropsychological battery of tests including clinical dementia rating (CDR), North American Reading Test, Verbal Fluency: Phonological and Semantic tests, Wisconsin Card Sorting Test, Trailmaking Test A & B, Boston Naming Test, Depressive Features Battery Version II and the Geriatric Depression Scale. Physical evaluations were also performed including blood tests and MRI. MCI was diagnosed following the guidelines byGrundman et al. (2004). MCI was defined as either abnormal memory determined by scoring below the education adjusted cutoff on the delayed paragraph recall Logical Memory II subtest of the Wechsler Memory Scale – Revised (Wechsler, 1987) (maximum score = 25, cutoff scores: less than or equal to 8 for 16 or more years of education, less than or equal to 4 for 8-15 years of education, less than or equal to 2 for 0-7 years of education) or a CDR memory score of 0.5-1.0 which is based on both performance and informant data. All MCI cases were subtyped as amnestic MCI. In brief, all MCI subjects have memory complaints confirmed by either logical memory or CDR informant scoring (CDR = 0.5), global CDR of 0.5, abnormal memory for age, normal activities of daily living, normal general cognitive function, and no dementia. All control subjects were without objective memory impairment, have a CDR memory component of 0, global CDR of 0, and were within normal ranges in neuropsychological tests. All subjects had normal thyroid function and vitamin B12 levels. Subjects were excluded from the study when clinical symptoms of other dementias, neurological diseases, history of major head trauma, or DSM-IV major depression were present within the preceding 12-month period. Individuals with an unstable medical condition, history of schizophrenia, and history of alcohol or substance abuse, medicated with neuroleptics, chronic anxiolytics, or sedative hypnotics were also excluded. There was no evidence of acute illness at the time of clinical evaluation or blood drawing and no significant difference in the incidence of medical comorbidities, including hypertension, diabetes and coronary heart disease (CHD) between controls and MCI subjects (percentage of subjects previously diagnosed with hypertension: 17% control and 25% MCI; diabetes: 0% control and 4% MCI; and CHD: 4% control and 6% MCI).
2.2. Immunophenotyping
Blood was drawn from each subject into ACD Vacutainer tubes (8.5ml; containing 1.5 mL of trisodium citrate [22.0g / L], citric acid [8.0 g / L], and dextrose [24.5 g / L]) between 8 am and 12 pm in the morning to avoid the influence of circadian rhythm on lymphocyte subpopulations. The immunophenotyping panel consisted of the following labeled monoclonal antibody (mAb) combinations: IgG1-FITC/IgG1-PE/CD45-Cy5PE (leukocytes), CD45-FITC/CD14-PE/CD3-Cy5PE (total T lymphocytes), CD3-FITC/CD4-PE/CD45-Cy5PE (helper/inducer T lymphocytes), CD3-FITC/CD8-PE/CD45-Cy5PE (suppressor/cytotoxic T lymphocytes), and CD3-FITC/CD19-PE/CD45-Cy5PE (B lymphocytes). A 0.1 ml aliquot of blood was incubated with each mAb combination for 20 min in the dark at room temperature (RT). Erythrocytes were lysed with 2 ml of 1× VitaLyse (BioE, St. Paul, MN) for 60 min in the dark at RT, and centrifuged at 1000 ×g for 1 min. Lysate was decanted, cells were vortexed vigorously to resuspend the pellet, and then washed with 2 mL of 1× PBS, repeating the centrifugation and decanting. Cells were resuspended in 0.5 ml 1× PBS and immediately analyzed on a FACSort flow cytometer. CellQuest software (Becton Dickinson, Sunnyvale, CA) was used for data analysis. Electronic gates were set to define total leukocytes: forward scatter (FS, relative cell size) /side scatter (SS, cell granularity) and SS/CD45. Regions within the SS vs. CD14 gates determined the subpopulations of granulocytes, monocytes, and lymphocytes: CD14neg/SSlow (lymphocytes), CD14bright/ SSlow (monocytes), and CD14pos/SShigh (granulocytes). Lymphocyte subsets were determined using the FS/SS (total leukocyte) gate along with a CD45pos/SSlow gate. The instrument was calibrated with CaliBrite beads (Becton Dickinson) using FACSComp software (Becton Dickinson) before each analysis. Cells were identified by mouse monoclonal antibodies directly conjugated to either fluorescein isothiocyanate (FITC) or R-phycoerythrin (PE), purchased from BD Biosciences, or Cy5-phycoerythrin (Cy5PE), acquired from Caltag Laboratories (Burlingame, CA). Non-specific staining was determined using the isotype controls, mouse mIgG1-FITC and mIgG1-PE, and 5000 lymphocyte events were analyzed per sample.
2.3. Amyloid precursor protein (APP) and amyloid-β peptide (Aβ)
Surface expression of APP and intracellular expression of Aβ in lymphocytes and monocytes were determined using a mouse IgG1 monoclonal antibody directed against the epitope within amino acids L18-R288 or P365-R411 of APP (R&D Systems, Minneapolis, MN), and a mouse IgG1 monoclonal antibody recognizing an epitope within amino acid residues 1-12 of the Aβ peptide (clone BAM-10, Sigma, Saint Louis, MO). FITC labeled goat anti-mouse IgG antibody (GAM-FITC; Pierce, Rockford, IL) was used as the secondary fluorescent label for both surface APP and intracellular Aβ. For surface APP staining, whole blood (0.1 ml) was incubated with primary antibody (0.5 mg/ml) for 20 min. Then cells were washed twice with 2 ml of 1× PBS, followed by incubation with GAM-FITC (1:50 dilution) for 20 min in the dark at RT. Erythrocytes were lysed with 2 ml of 1× VitaLyse for 60 min in the dark at RT and centrifuged at 1000×g for 1 min. Lysate was decanted, cells were vortexed vigorously to resuspend the pellet, and then washed with 2 ml of 1× PBS, repeating the centrifugation and decanting. Cells were resuspended in 0.5 ml 1× PBS and immediately analyzed by flow cytometry. For staining of intracellular Aβ peptide, following erythrocyte lysis, cells were fixed and permeabilized using 0.1 ml of 1× PermaCyte-FP (BioE) for 2 h. After fixation, cells were centrifuged (1000 xg for 1 min) and supernatant decanted. Cells were incubated with the primary antibody for 20 min, washed twice with 2 ml of 1× PermaCyte-FP, and incubated with the secondary, GAM-FITC, for 20 min. Cells were washed twice with 2 ml of 1× PermaCyte, centrifuged, resuspended in 0.5 ml 1× PBS, and analyzed by flow cytometry. Electronic gates (FS/SS) were used for analysis of lymphocytes and monocytes, and a minimum of 1000 monocyte events were required for analysis.
2.4. Statistics
Data for immunophenotypes were evaluated with the non-parametric Mann-Whitney U test since cell markers are known to have a non-Gaussian distribution (Lombardi et al., 1999). The flow cytometry data for APP and Aβ expression were analyzed by both comparing the percentage of cells of a certain population expressing the marker of interest and comparing the mean fluorescence units of each population. Since lymphocyte APP expression is known to have a normal distribution (Ledoux et a., 1993;Pallister et al., 1997), comparison of APP and Aβ expression between groups was done using the student’s t test. Data are presented as means ± SEM, and a P value of < 0.05 was chosen to indicate statistical significance. There was no significant difference in gender distribution as determined by chi square analysis or in age or education as determined by the student’s t test between MCI subjects and controls. All statistics were performed using the SPSS 12.0.1 software.
3. Results
3.1. Immunophenotyping
We analyzed the relative percentage of leukocyte subpopulations in circulation, i.e., lymphocytes, monocytes, and granulocytes, as well as lymphocyte subsets, including T helper/inducer, T suppressor/cytotoxic, and B lymphocytes in subjects with MCI. Compared to healthy elderly controls, the percentage of total lymphocytes was significantly lower in MCI subjects (p = 0.02, Table 1). By contrast, the proportion of granulocytes was significantly higher in MCI patients versus controls (p = 0.04). No difference was seen in the percentage of monocytes between the groups. The relative proportion of lymphocyte subsets (T and B cells) indicated a trend that approached statistical significance toward lower percentages of CD3+ cells and CD3+CD4+ cells in MCI subjects compared to controls. Similarly, the percentage of CD8+ cells and the ratio of CD4+/CD8+ cells did not significantly differ between the two groups. No correlation between the proportion of lymphocytes and granulocytes and patient age was seen. Thus, the percentage of lymphocytes and granulocytes, but not lymphocyte subtypes studied, were altered in MCI subjects compared to that in controls.
Table 1.
Peripheral blood leukocyte subpopulations expressed as a percentage of total leukocytes (SD) and lymphocyte subpopulations (T lymphocyte subsets and B lymphocytes) expressed as a percentage of total lymphocytes (SD) in MCI subjects (n = 48) and elderly controls (n =24).
| MCI subjects Mean (SD) | Elderly controls Mean (SD) | P values | |
|---|---|---|---|
| CD45bright/SSlow (Lymphocytes) | 25.06 (9.05)* | 28.87(7.97) | p= 0.02 |
| CD45dim/SShigh (Granulocytes) | 67.28(9.61)* | 64.07(8.23) | p= 0.04 |
| CD45bright/CD14+ (Monocytes) | 7.33(2.01) | 6.72 (1.86) | ns |
| CD3 (T lymphocytes) | 64.64(10.14) | 68.64(8.00) | p= 0.06 |
| CD3CD4 (Helper/inducer T lymphocytes) | 42.12(14.26) | 48.81(9.79) | p= 0.06 |
| CD3CD8 (Suppressor/cytotoxic T lymphocytes) | 24.55(17.77) | 24.31(11.74) | ns |
| CD4/CD8 ratio (Helper/Cytotoxic T lymphocytes) | 2.25(1.58) | 2.53(1.39) | ns |
| CD19(B lymphocytes) | 11.26(9.58) | 10.26(4.60) | ns |
statistically significant, p < 0.05 vs. controls; ns, not significant.
3.2. APP and Aβ
We also analyzed APP and Aβ expression on lymphocytes and monocytes from subjects with MCI compared to elderly controls. Although a small proportion of lymphocytes were positive for surface APP expression, both the percentage (9.42% ± 0.79 in controls versus 12.83% ± 1.37 in MCI, p = 0.01) and mean fluorescence (115.9 ± 5.0 in controls versus 138.9 ± 7.3 in MCI, p = 0.001) of lymphocytes expressing surface APP were significantly higher in MCI subjects compared to controls (Figure 1). By contrast, Aβ expression in lymphocytes did not differ between the two groups. Monocytes represented approximately 4-11% of PBMCs in both MCI subjects and controls, and approximately 50% of the monocytes gated showed expression of APP while nearly 100% had Aβ expression in both subject groups. However, there were no significant differences in the expression of APP or Aβ peptide in monocytes between MCI subjects and elderly controls. Thus, APP expression was increased in lymphocytes but not monocytes in MCI relative to controls, and Aβ expression did not differ between the two patient groups in either cell type.
Figure 1.
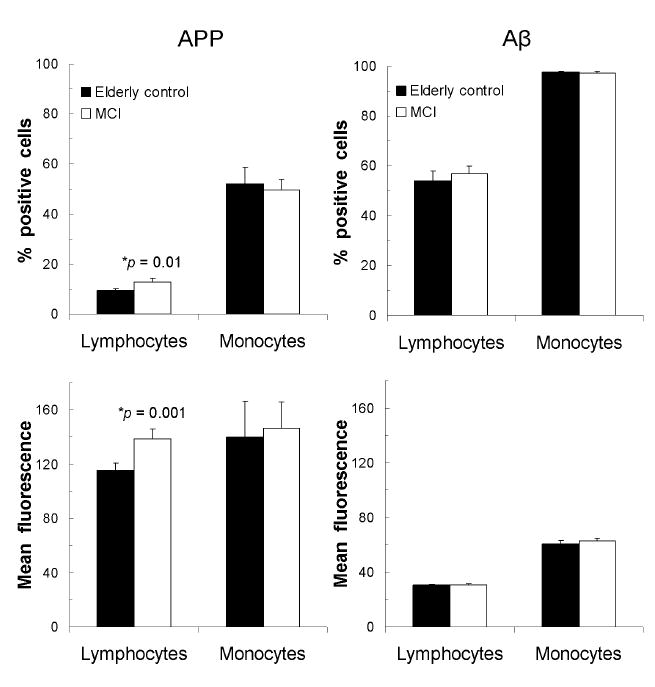
Cell surface amyloid precursor protein (APP) and intracellular amyloid beta peptide (Aβ) expression in lymphocytes and monocytes as percentage of positively-labeled cells or as mean fluorescence levels in MCI subjects (n = 48) and healthy elderly controls (n =24). Data are shown as mean (± SEM). * statistically significant, p < 0.05 compared to controls.
4. Discussion
The purpose of this study was to characterize leukocyte phenotypes and determine differences in the expression of APP and Aβ in peripheral blood cells from MCI subjects relative to elderly controls. We have found a decrease in the percentage of total lymphocytes and a concomitant increase in granulocytes, with no change in monocytes, in MCI subjects compared to controls. The findings support the hypothesis that changes in certain peripheral immunophenotypes occur in MCI patients. The decrease in lymphocytes in MCI subjects is consistent with reports of a decrease in the proliferative ability of lymphocytes from AD patients compared to controls (Shalit et al., 1995). Furthermore, our results of increased granulocytes in MCI subjects are in agreement with the findings of Licastro et al. (1994)who found granulocyte activity to be increased in AD but not in Parkinson’s disease patients, suggesting an inflammatory state specific to AD. An increase in oxidative stress and the expression of CD11b, important in transendothelial migration, in neutrophils from AD patients compared to controls has also been reported (Scali et al., 2002;Vitte et al., 2004). We do not know if the increase in granulocytes we found in our MCI subjects was accompanied by an increase in their activity since we did not measure granulocyte activity, but our findings of increased granulocytes give evidence for an early inflammatory state in AD. Therefore, the decrease in lymphocytes and elevation in granulocytes in MCI subjects may indicate changes in the relative balance of immune responses in MCI that could be predictive of clinical manifestations of the progression to AD.
In another important aspect, the distribution of lymphocyte subpopulations was similar between MCI and elderly controls. MCI subjects tended to have a lower percentage of CD3+ cells (total T lymphocytes) and CD3+CD4+ cells (helper/inducer T lymphocytes) compared to controls but these did not reach significance. Moreover, although MCI subjects had a slightly lower proportion of CD4+ cells, we saw no differences in the percentage of CD8+ cells and the ratio of CD4+/CD8+ cells between MCI and controls. These results are in agreement with other investigators who have found no differences in the distribution of CD4+ and CD8+ cells and CD4+/CD8+ ratios in AD patients compared to controls (Richartz-Salzburger et al., 2006;Ikeda et al., 1991).
Findings that leukocytes express APP have led to studies of amyloid metabolism in AD patients. Increased expression of APP mRNA and protein in lymphocytes and monocytes has been demonstrated in AD patients, suggesting that the same alterations in APP expression leading to amyloid deposition in the brain may be reflected in peripheral immune cells (Pallister et al., 1997;Jung et al., 1999;Jiang et a., 2003) and/or potentially be a source for abnormal amyloid deposited in the brain (Allen et al., 1991;Reale et al., 2005). We investigated APP and Aβ expression in lymphocytes and monocytes from subjects with MCI to determine if similar alterations could be observed in a preclinical stage of AD. Compared to controls, we found significantly higher lymphocyte APP expression in MCI subjects. However, the expression of APP and Aβ in monocytes did not differ between the two groups. Only a small proportion of lymphocytes were positive for surface APP for both patient groups which is consistent with findings by Bullido et al. (1996)who demonstrated that B lymphocytes express surface APP while no APP expression is observed in T lymphocytes. Thus, the lymphocytes staining for APP in our study are likely to be B lymphocytes, but since we examined total lymphocyte staining for APP, the determination of the subpopulation of lymphocytes expressing APP must be confirmed by costaining for cell specific markers. Increased APP could be a source for circulating Aβ that may be linked to Aβ deposited in CNS vasculature. In addition to APP, we also examined intracellular Aβ as increasing evidence suggests that intracellular Aβ accumulation precedes plaque and neurofibrillary tangle formation and is an early event in the disease process (Wilson et al., 1999;Gouras et al., 2000;Gouras et al., 2005). Kienlen-Campard et al. (2002)have shown that long term expression of human APP in rat cortical neurons induce neuronal apoptosis that is dependent on intracellular but not extracellular Aβ. In this study, no differences in intracellular Aβ expression in lymphocytes or monocytes were found. Increased APP without a concomitant elevation in Aβ could indicate changes in the processing of APP or an increase in the secreted form of Aβ although this would need to be confirmed as we did not measure secreted Aβ. Moreover, APP and Aβ were also measured in granulocytes, as this population showed an increase in MCI, but no differences were seen between controls and MCI subjects (data not shown). It remains unclear whether increased APP expression in lymphocytes from MCI subjects reflects an emerging dysfunction in APP metabolism seen in APP expressing cells in general, including neurons, in AD or is a causative factor in the disease. Either way, alterations in peripheral blood cells may prove to be an accessible biomarker for diagnosis and study as to the mechanisms underlying AD (Jung et al., 1999).
A limitation to this study is the presence of confounding factors such as hypertension, diabetes and CHD which have been associated with both dementia (Qiu et al., 2005; Bergmann & Sano, 2006) and peripheral immune dysregulation (Aukrust et al., 2005;Kempf et al., 2006;Zuliani et al., 2006) although no differences in their incidence between controls and MCI subjects were found. Furthermore, the changes in the proportions of lymphocytes and granulocytes, and lymphocyte APP expression are relatively small and may not have immediately apparent clinical consequences in MCI. However, a chronic imbalance in granulocytes and lymphocytes may predispose to increased inflammatory responses, and overexpression of APP in lymphocytes, which may reflect similar processes in the brain, could lead to increased deposition of Aβ and promote the development of AD pathology. The detection of these changes at a pre-clinical stage of AD could also aid in the development of biomarkers for AD. However, the second limitation to this study is the heterogeneity of the MCI subjects as not all of them will transition into AD. Progression rates from MCI to AD of 10-15% per year have been reported in several studies (Grundman et al., 2004) but other groups have reported varying progression rates, such as 5% (Thal et al., 2005). Future studies will be necessary to determine the specificity of these immune changes in MCI subjects that progress to AD versus those who remain as MCI.
In conclusion, our results show an alteration in the peripheral immune response in MCI. Decreased lymphocytes and increased granulocytes in MCI patients compared to elderly controls may indicate a disturbance in the balance of the immune response in the preclinical stages of AD. Furthermore, we detected increases in peripheral APP expression in MCI, a change previously reported in AD. Whether both alterations are a cause or consequence of disease progression remains to be investigated. These results have implications in elucidating the role of peripheral immune factors in the pathogenesis of AD and allowing for monitoring of disease progression and response to therapeutics.
References
- Allen JS, Murphy GM, Jr, Eng LF, Stultz KE, Davies HD, Pickford LB, Tinklenberg JR. Alzheimer’s disease: beta-amyloid precursor protein mRNA expression in mononuclear blood cells. Neuroscience Letters. 1991;132:109–112. doi: 10.1016/0304-3940(91)90445-y. [DOI] [PubMed] [Google Scholar]
- Aukrust P, Yndestad A, Waehre T, Gullestad L, Halvorsen B, Damas JK. Inflammation in coronary artery disease: potential role for immunomodulatory therapy. Expert Review of Cardiovascular Therapy. 2005;3:1111–1124. doi: 10.1586/14779072.3.6.1111. [DOI] [PubMed] [Google Scholar]
- Bergmann C, Sano M. Cardiac risk factors and potential treatments in Alzheimer’s disease. Neurological Research. 2006;28:595–604. doi: 10.1179/016164106X130498. [DOI] [PubMed] [Google Scholar]
- Bullido MJ, Munoz-Fernandez MA, Recuero M, Fresno M, Valdivieso F. Alzheimer’s amyloid precursor protein is expressed on the surface of hematopoietic cells upon activation. Biochimica et Biophysica Acta. 1996;1313:54–62. doi: 10.1016/0167-4889(96)00015-8. [DOI] [PubMed] [Google Scholar]
- Dysken MW, Minichiello MD, Hill JL, Skare S, Little JT, Molchan SE, Sunderland T. Distribution of peripheral lymphocytes in Alzheimer patients and controls. Journal of Psychiatric Research. 1992;26:213–218. doi: 10.1016/0022-3956(92)90024-i. [DOI] [PubMed] [Google Scholar]
- Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer’s disease: significance in plaque evolution. Journal of Neuropathology and Experimental Neurology. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Gouras GK, Tsai J, Naslund J, Vincent B, Edgar M, Checler F, Greenfield JP, Haroutunian V, Buxbaum JD, Xu H, Greengard P, Relkin NR. Intraneuronal Abeta42 accumulation in human brain. American Journal of Pathology. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouras GK, Almeida CG, Takahashi RH. Intraneuronal Abeta accumulation and origin of plaques in Alzheimer’s disease. Neurobiology of Aging. 2005;26:1235–1244. doi: 10.1016/j.neurobiolaging.2005.05.022. [DOI] [PubMed] [Google Scholar]
- Grundman M, Petersen RC, Ferris SH, Thomas RG, Aisen PS, Bennett DA, Foster NL, Jack CR, Jr, Galasko DR, Doody R, Kaye J, Sano M, Mohs R, Gauthier S, Kim HT, Jin S, Schultz AN, Schafer K, Mulnard R, van Dyck CH, Mintzer J, Zamrini EY, Cahn-Weiner D, Thal LJ. Alzheimer’s Disease Cooperative Study. Mild cognitive impairment can be distinguished from Alzheimer disease and normal aging for clinical trials. Archives of Neurology. 2004;61:59–66. doi: 10.1001/archneur.61.1.59. [DOI] [PubMed] [Google Scholar]
- Ikeda T, Yamamoto K, Takahashi K, Yamada M. Immune system-associated antigens on the surface of peripheral blood lymphocytes in patients with Alzheimer’s disease. Acta Psychiatrica Scandinavica. 1991;83:444–448. doi: 10.1111/j.1600-0447.1991.tb05573.x. [DOI] [PubMed] [Google Scholar]
- Jiang S, Zhang M, Ren D, Tang G, Lin S, Qian Y, Zhang Y, Jiang K, Li F, Wang D. Enhanced production of amyloid precursor protein mRNA by peripheral mononuclear blood cell in Alzheimer’s disease. American Journal of Medical Genetics Part B-Neuropsychiatric Genetics. 2003;118:99–102. doi: 10.1002/ajmg.b.10067. [DOI] [PubMed] [Google Scholar]
- Jung SS, Gauthier S, Cashman NR. Beta-amyloid precursor protein is detectable on monocytes and is increased in Alzheimer’s disease. Neurobiology of Aging. 1999;20:249–257. doi: 10.1016/s0197-4580(99)00051-2. [DOI] [PubMed] [Google Scholar]
- Kempf K, Rose B, Herder C, Kleophas U, Martin S, Kolb H. Inflammation in metabolic syndrome and type 2 diabetes: impact of dietary glucose. Annals of the New York Academy of Sciences. 2006;1084:30–48. doi: 10.1196/annals.1372.012. [DOI] [PubMed] [Google Scholar]
- Kienlen-Campard P, Miolet S, Tasiaux B, Octave JN. Intracellular amyloid-beta 1-42, but not extracellular soluble amyloid-beta peptides, induces neuronal apoptosis. Journal of Biological Chemistry. 2002;277:15666–15670. doi: 10.1074/jbc.M200887200. [DOI] [PubMed] [Google Scholar]
- Ledoux S, Rebai N, Dagenais A, Shaw IT, Nalbantoglu J, Sekaly RP, Cashman NR. Amyloid precursor protein in peripheral mononuclear cells is up-regulated with cell activation. Journal of Immunology. 1993;150:5566–5575. [PubMed] [Google Scholar]
- Li QX, Fuller SJ, Beyreuther K, Masters CL. The amyloid precursor protein of Alzheimer disease in human brain and blood. Journal of Leukocyte Biology. 1999;66:567–574. doi: 10.1002/jlb.66.4.567. [DOI] [PubMed] [Google Scholar]
- Licastro F, Morini MC, Davis LJ, Malpassi P, Cucinotta D, Parente R, Melotti C, Savorani G. Increased chemiluminescence response of neutrophils from the peripheral blood of patients with senile dementia of the Alzheimer’s type. Journal of Neuroimmunology. 1994;51:21–26. doi: 10.1016/0165-5728(94)90124-4. [DOI] [PubMed] [Google Scholar]
- Lombardi VR, Garcia M, Rey L, Cacabelos R. Characterization of cytokine production, screening of lymphocyte subset patterns and in vitro apoptosis in healthy and Alzheimer’s Disease (AD) individuals. Journal of Neuroimmunology. 1999;97:163–171. doi: 10.1016/s0165-5728(99)00046-6. [DOI] [PubMed] [Google Scholar]
- McGeer PL, Akiyama H, Itagaki S, McGeer EG. Immune system response in Alzheimer’s disease. Canadian Journal of Neurological Sciences. 1989;16:516–27. doi: 10.1017/s0317167100029863. [DOI] [PubMed] [Google Scholar]
- McGeer EG, McGeer PL. The importance of inflammatory mechanisms in Alzheimer disease. Experimental Gerontology. 1998;33:371–378. doi: 10.1016/s0531-5565(98)00013-8. [DOI] [PubMed] [Google Scholar]
- Migliore L, Fontana I, Trippi F, Colognato R, Coppede F, Tognoni G, Nucciarone B, Siciliano G. Oxidative DNA damage in peripheral leukocytes of mild cognitive impairment and AD patients. Neurobiology of Aging. 2005;26:567–573. doi: 10.1016/j.neurobiolaging.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Mott RT, Hulette CM. Neuropathology of Alzheimer’s disease. Neuroimaging Clinics of North America. 2005;15:755–765. doi: 10.1016/j.nic.2005.09.003. [DOI] [PubMed] [Google Scholar]
- Neuroinflammation Working Group. Inflammation and Alzheimer’s disease. Neurobiology of Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallister C, Jung SS, Shaw I, Nalbantoglu J, Gauthier S, Cashman NR. Lymphocyte content of amyloid precursor protein is increased in Down’s syndrome and aging. Neurobiology of Aging. 1997;18:97–103. doi: 10.1016/s0197-4580(96)00207-2. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. Journal of Internal Medicine. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Pirttila T, Mattinen S, Frey H. The decrease of CD8-positive lymphocytes in Alzheimer’s disease. Journal of the Neurological Sciences. 1992;107:160–165. doi: 10.1016/0022-510x(92)90284-r. [DOI] [PubMed] [Google Scholar]
- Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. Lancet Neurology. 2005;4:487–499. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- Reale M, Iarlori C, Gambi F, Lucci I, Salvatore M, Gambi D. Acetylcholinesterase inhibitors effects on oncostatin-M, interleukin-1 beta and interleukin-6 release from lymphocytes of Alzheimer’s disease patients. Experimental Gerontology. 2005;40:165–171. doi: 10.1016/j.exger.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Richartz E, Batra A, Simon P, Wormstall H, Bartels M, Buchkremer G, Schott K. Diminished production of proinflammatory cytokines in patients with Alzheimer’s disease. Dementia and Geriatric Cognitive Disorders. 2005;19:184–188. doi: 10.1159/000083497. [DOI] [PubMed] [Google Scholar]
- Richartz-Salzburger E, Batra A, Stransky E, Laske C, Kohler N, Bartels M, Buchkremer G, Schott K. Altered lymphocyte distribution in Alzheimer’s disease. Journal of Psychiatric Research. 2006 Mar;1 doi: 10.1016/j.jpsychires.2006.01.010. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Scali C, Prosperi C, Bracco L, Piccini C, Baronti R, Ginestroni A, Sorbi S, Pepeu G, Casamenti F. Neutrophils CD11b and fibroblasts PGE(2) are elevated in Alzheimer’s disease. Neurobiology of Aging. 2002;23:523–530. doi: 10.1016/s0197-4580(01)00346-3. [DOI] [PubMed] [Google Scholar]
- Schlossmacher MG, Ostaszewski BL, Hecker LI, Celi A, Haass C, Chin D, Lieberburg I, Furie BC, Furie B, Selkoe DJ. Detection of distinct isoform patterns of the beta-amyloid precursor protein in human platelets and lymphocytes. Neurobiology of Aging. 1992;13:421–434. doi: 10.1016/0197-4580(92)90117-g. [DOI] [PubMed] [Google Scholar]
- Shalit F, Sredni B, Brodie C, Kott E, Huberman M. T lymphocyte subpopulations and activation markers correlate with severity of Alzheimer’s disease. Clinical Immunology and Immunopathology. 1995;75:246–250. doi: 10.1006/clin.1995.1078. [DOI] [PubMed] [Google Scholar]
- Singh VK. Neuroautoimmunity: pathogenic implications for Alzheimer’s disease. Gerontology. 1997;43:79–94. doi: 10.1159/000213837. [DOI] [PubMed] [Google Scholar]
- Thal LJ, Ferris SH, Kirby L, Block GA, Lines CR, Yuen E, Assaid C, Nessly ML, Norman BA, Baranak CC, Reines SA Rofecoxib Protocol 078 study group. A randomized, double-blind, study of rofecoxib in patients with mild cognitive impairment. Neuropsychopharmacology. 2005;30:1204–1215. doi: 10.1038/sj.npp.1300690. [DOI] [PubMed] [Google Scholar]
- Trieb K, Ransmayr G, Sgonc R, Lassmann H, Grubeck-Loebenstein B. APP peptides stimulate lymphocyte proliferation in normals, but not in patients with Alzheimer’s disease. Neurobiology of Aging. 1996;17:541–547. doi: 10.1016/0197-4580(96)00068-1. [DOI] [PubMed] [Google Scholar]
- Vitte J, Michel BF, Bongrand P, Gastaut JL. Oxidative stress level in circulating neutrophils is linked to neurodegenerative diseases. Journal of Clinical Immunology. 2004;24:683–692. doi: 10.1007/s10875-004-6243-4. [DOI] [PubMed] [Google Scholar]
- Wechsler D. WMS-R Wechsler Memory Scale-Revised Manual. New York: Harcourt Brace Jovanovich; 1987. [Google Scholar]
- Wilson CA, Doms RW, Lee VM. Intracellular APP processing and A beta production in Alzheimer disease. Journal of Neuropathology and Experimental Neurology. 1999;58:787–794. doi: 10.1097/00005072-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Zuliani G, Ranzini M, Guerra G, Rossi L, Munari MR, Zurlo A, Volpato S, Atti AR, Ble A, Fellin R. Plasma cytokines profile in older subjects with late onset Alzheimer’s disease or vascular dementia. Journal of Psychiatric Research. 2006 Apr 3; doi: 10.1016/j.jpsychires.2006.02.008. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]


