Abstract
During cryopreservation, the immature oocyte is subjected to anisosmotic conditions potentially impairing subsequent nuclear and cytoplasmic maturation in vitro. In preparation for cryopreservation protocols and to characterize osmotic tolerance, cat cumulus–oocyte complexes (COC) at the germinal vesicle (GV) stage were exposed for 15 min to sucrose solutions ranging from 100 to 2,000 mOsm and then examined for structural integrity and developmental competence in vitro. Osmolarities ≥200 and ≤750 mOsm had no effect on incidence of oocyte nuclear maturation, fertilization success, and blastocyst formation compared to control COC (exposed to 290 mOsm). This relatively high osmotic tolerance of the immature cat oocyte appeared to arise from a remarkable stability of the GV chromatin structure as well as plasticity in mitochondrial distribution, membrane integrity, and ability to maintain cumulus–oocyte communications. Osmolarities < 200 mOsm only damaged cumulus cell membrane integrity, which contributed to poor nuclear maturation but ultimately had no adverse effect on blastocyst formation in vitro. Osmolarities >750 mOsm compromised nuclear maturation and blastocyst formation in vitro via disruption of cumulus–oocyte communications, an effect that could be mitigated through 1,500 mOsm by adding cytochalasin B to the hyperosmotic solutions. These results (1) demonstrate, for the first time, the expansive osmotic tolerance of the immature cat oocyte, (2) characterize the fundamental role of cumulus–oocyte communications when tolerance limits are exceeded, and (3) reveal an interesting hyperosmotic tolerance of the immature oocyte that can be increased two-fold by supplementation with cytochalasin B.
Keywords: immature oocyte, domestic cat, osmotic stress, sucrose, in vitro culture, cell communications
INTRODUCTION
Oocyte cryopreservation has significant potential as a tool for preserving the female genome to assist in genetic management of populations of valuable laboratory models or endangered species (Wildt, 2000). However, the processes of cooling, freezing and thawing harshly affect the survival and quality of oocytes in a host of mammalian species, including the domestic cat (Murakami et al., 2004; Oktay et al., 2006; Pukazhenthi et al., 2006; Luvoni, 2006). The majority of such studies has been conducted with oocytes that are at the metaphase II (MII) stage. This is interesting since cumulus–oocyte complexes (COC) at the germinal vesicle stage (GV) can be considered to be more resistant to freezing and thawing than their MII counterparts, largely because (1) GV chromatin is protected by a nuclear envelope, (2) microtubules are depolymerized, and (3) cumulus cells protect against rapid influx or efflux of cryoprotectant (Van der Elst et al., 1992; Fabbri et al., 2001; LaRosa and Downs, 2006). There also is a practical justification for studying immature oocytes as these cells are readily available in significant numbers in ovaries without preemptive hormonal treatment to provoke oocyte maturation. The reaction of the immature cat oocyte to procedures associated with cryopreservation has received minimal research attention, the exception being two studies related to cryoprotectant toxicity (Luvoni and Pellizzari, 2000; Comizzoli et al., 2004). Another important prerequisite to developing a practical freezing protocol is understanding oocyte tolerance to radical changes in osmolarity that occur during adding and removing cryoprotectant and exposing the gamete to low temperatures (Critser et al., 1997). The resulting changes in cell volume have been shown to have significant effects on oocyte developmental competence in the mouse (Litkouhi et al., 1997), human (Newton et al., 1999; Mullen et al., 2004), cow (Agca et al., 2000), and rhesus monkey (Songsasen et al., 2002). However, no information is available on the impact of osmotic stress on the immature cat oocyte.
Besides exploring the developmental competence of the immature cat oocyte after anisosmotic exposures, understanding how such perturbations affect both structural and functional integrity of COC is critical. Structure of the GV chromatin strongly influences successful nuclear and cytoplasmic maturation in vitro as demonstrated in mouse (Miyara et al., 2003), mare (Choi et al., 2006), and cow (Lodde et al., 2006) studies. Likewise, cytoplasmic mitochondrial distribution within the immature oocyte (also reflecting the microfilament organization) plays a critical role in driving later embryo development (Wang et al., 2000; Stojkovic et al., 2001; Rho et al., 2002; Van Blerkom et al., 2002; Velilla et al., 2006). Because GV chromatin structure and mitochondrial distribution may both be influenced by non uniform shrinking or swelling of an oocyte exposed to an anisosmotic solution, these two targets of interest were chosen as indicators of developmental competence. It also was reasonable to explore the influence of anisosmotic solutions on membrane integrity and cumulus–oocyte communications. High or low osmotic pressures are well known to damage or rupture cell membranes (Adams et al., 2003; Paynter, 2005). Furthermore, as shown in other species, the immature cat oocyte is surrounded by several layers of cumulus cells that communicate with each other and with the oocyte through gap junctions (Luvoni et al., 2001, 2006; Albertini and Barrett, 2003; Colleoni et al., 2004; Luciano et al., 2004). Cumulus–oocyte communications permit the transfer of small molecules, such as nutrients and signaling molecules, for oocyte growth in vivo as well as nuclear and cytoplasmic maturation in vitro after FSH stimulation (Comizzoli et al., 2003; Luciano et al., 2004; Luvoni et al., 2006). Steps to cryopreservation can be detrimental to the functionality of cumulus–oocyte communications in mouse (Ruppert-Lingham et al., 2003, 2006), cow (Modina et al., 2004), and porcine oocytes (Wu et al., 2006). Interestingly, it has been demonstrated that cytochalasin B (CB) increases cytoskeletal flexibility and preserves the coupling between the oocyte and its cumulus cells, especially during times of hyperosmotic exposures (Le Gal et al., 1994; Isachenko et al., 1998; Rho et al., 2002).
Therefore, in preparation for forthcoming cryopreservation trials, the objective of this study was to evaluate and characterize the impact of osmotic stress on structural and functional integrity of COC at the GV stage in the domestic cat. Specifically, we examined the effect of exposures to hypo- and hyperosmotic sucrose solutions on developmental competence of cat oocytes and then characterize the impact of detrimental exposures on GV chromatin structure, mitochondrial distribution, membrane integrity, and cumulus–oocyte communications in the absence or presence of CB.
MATERIALS AND METHODS
Immature Oocyte Collection and Exposure to Anisosmotic Solutions
Ovaries from adult domestic cats were collected from local veterinary clinics and transported to the laboratory in PBS at 4°C within 6 hr of routine ovariectomy. Immature oocytes were recovered by slicing the ovaries with a scalpel blade in Hepes-buffered Minimum Essential Medium (H-MEM; Gibco Laboratories, Grand Island, NY) supplemented with 1 mM pyruvate, 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 4 mg/ml bovine serum albumin (BSA; Sigma Chemical Co., St. Louis, MO). Only grade I immature oocytes (with homogenous dark cytoplasm, surrounded by several layers of compacted cumulus; Wood and Wildt, 1997) were selected and pooled from different ovaries collected on a given day. Hyperosmotic solutions (500, 750, 1,000, 1,500 and 2,000 mOsm) were prepared by adding different concentrations of sucrose (range, 0.2–1.4 M) to isotonic PBS (290 mOsm). Hypoosmotic solutions (100 and 200 mOsm) were prepared by adding de-ionized water to PBS. Osmolarity of each solution was adjusted using a vapor pressure osmometer (Wescor, Inc., Logan, UT) at room temperature (22 ± 2°C). In Experiment 1, groups of immature oocytes (see details in Experimental Design) were exposed for 15 min at room temperature to one of the seven anisosmotic solutions or to isotonic PBS. In Experiment 2, groups of immature oocytes were (1) exposed to 100, 290, 1,000, 1,500, or 2,000 mOsm for 15 min at room temperature or (2) pre-incubated for 10 min in 7.5 μg/ml CB (Sigma) before exposure to 100, 290, 1,000, 1,500, or 2,000 mOsm solutions containing 7.5 μg/ml CB for 15 min at room temperature. After exposure, immature oocytes were transferred stepwise through droplets with decreasing or increasing osmolarities (1,500, 1,000, 750, 500, or 200 mOsm; 5 min in each solution) to return to isosmotic conditions. In both experiments, immature oocytes then were washed 3 times (5 min each) in isotonic PBS before fixation, staining or culture in vitro.
In Vitro Culture of Oocytes
In vitro maturation (IVM) was conducted under standard conditions as previously described for our laboratory (Comizzoli et al., 2003) followed by in vitro fertilization (IVF) using a protocol described by Spindler and Wildt (1999). Briefly, frozen-thawed motile spermatozoa from a single sperm donor (three different males were alternatively used) were selected by swim-up processing in Ham’s F-10 medium (Irvine Scientific, Santa Ana, CA) supplemented with 25 mM Hepes, 1 mM pyruvate, 2 mM L-glutamine, 100 IU/ml penicillin, 100 μg/ml streptomycin, and 5% fetal calf serum (complete Ham’s with Hepes). Oocytes were inseminated with 5 × 105 motile spermatozoa/ml in 50-μl microdrops of complete Ham’s without Hepes under equilibrated mineral oil at 38.5°C in air with 5% CO2. A part of oocytes (~10% in each treatment group) was incubated without semen to assess the incidence of spontaneous activation (parthenogenesis). At 18 hours post-insemination (hpi), oocytes were cleaned by gentle pipetting. Presumptive zygotes then were cultured in vitro for 7 days in complete Ham’s F-10 (38.5°C, in air with 5% CO2). Embryos were observed for early cleavage at 48 hpi and after 7 days of in vitro development.
Assessment of Oocyte Nuclear Status and Embryo Stages
Oocytes post-IVM (28 hr), uncleaved oocytes at 48 hpi, or embryos without a visible blastocoele after 7 days of culture were fixed by air drying and exposure to 100% ethanol (Comizzoli et al., 2003). Chromatin was stained with a PBS solution containing 10 μg/ml Hoechst 33341 and then examined under a microscope fitted with epifluorescence (Olympus BX 41; Olympus Corporation, Melville, NY). Oocytes at the telophase I (TI) or at the MII stage were considered as matured (Comizzoli et al., 2003). Oocytes arrested at the germinal vesicle breakdown (GVBD) or only progressing to metaphase I (MI) were designated as immature. Oocytes with fragmented cytoplasm and abnormal chromatin patterns were considered as degenerated (Comizzoli et al., 2003). In Experiment 2, GV chromatin structure was recorded and area was measured at the end of anisosmotic or isosmotic exposure using SPOT software 3.5.9 (Diagnostic Instruments, Inc., Sterling Heights, MI). The fixation technique was the same as described above except that staining was performed with Hoechst solutions of the same osmolarity as the exposure (thereby preserving cell volume). In both experiments, oocytes were considered successfully fertilized when two pronuclei were observed in the cytoplasm. Oocytes were considered as activated in the parthenogenetic control if at least one pronucleus was present. Embryos with a visible blastocoele were defined as blastocysts and were subjected to differential staining to evaluate the percentage of inner cell mass (Comizzoli et al., 2004). Other embryo stages were determined by counting the number of blastomere nuclei after ethanol fixation and Hoechst staining. Embryos composed of 25–45 blastomeres without a blastocoele were designated as morulae.
Assessment of Mitochondrial Distribution
In Experiment 2, part of the oocytes was denuded of cumulus cells (by repeatedly pipetting the oocyte in a narrow glass pipette) after exposure to anisosmotic solution and return to an isosmotic condition. These oocytes were incubated for 15 min in 400 nM Mito-tracker green (Molecular Probes, Inc., Eugene, OR), washed in PBS for 10 min, and then mounted on a Teflon slide for evaluation of mitochondrial distribution by epifluorescence microscopy. Distributions were classified and recorded for each oocyte as normal (homogenous distribution throughout the cytoplasm) or abnormal (centered or heterogeneous distribution in the cytoplasm; Rho et al., 2002; Fulka et al., 2004).
Assessment of Cytoplasmic Membrane Integrity
After exposure to anisosmotic solution and return to isosmotic conditions, part of the COC in Experiment 2 was stained with a live/dead stain (Molecular Probes, Inc.) containing two nucleic acid dyes. Specifically, the oocytes were first incubated in 0.02 mM SYBR14 (in PBS) for 10 min at room temperature and counter-stained with 2.4 mM propidium iodide for an additional 5 min before mounting on a Teflon slide for evaluation with fluorescence microscopy. Presence of intact cells (stained green with vital staining SYBR14) and membrane disrupted cells (stained red with propidium iodide) was recorded for each COC. A COC received a score of 1 if 76–100% of the surface of the oocyte was covered by cumulus cells with intact membranes. A score of 2 was given to a COC if 51–75% of the oocyte was covered by cumulus cells with intact membranes. A score of 3 was assigned if the oocyte was covered by 26–50% intact cumulus cells, and a score of 4 if 0–25% of the oocyte was surrounded by intact cumulus cells (Ruppert-Lingham et al., 2006).
Assessment of Cumulus–Oocyte Communications
In Experiment 2, intercellular communications between cumulus cells and the oocyte were assessed after exposure to iso- or anisosmotic solutions and return to isosmotic conditions (as described above) by Lucifer yellow (Sigma) microinjection under an inverted microscope (Olympus BX 41, Olympus Corporation) equipped with micromanipulators (Narishige, Sterling, VA) and micro-tools (Humagen Fertility Diagnostics, Inc., Charlottesville, VA). Immature oocytes were placed into HMEM drop covered with mineral oil. Using the holding pipette, each COC was held and the injection pipette inserted into the middle of the oocyte. Ooplasm was aspirated into the injection pipette until it was observed that the oolemma had been broken. A solution of 3% Lucifer yellow then was injected into the oocyte (maximum of 1% of the total volume). Microinjected oocytes were mounted on a Teflon slide and then observed under fluorescence 10 min later. Extent of diffusion of Lucifer yellow enabled classifying and recording, for each COC, cumulus–oocyte communications as open (diffusion in the entire cumulus), partially open (<50% of the circumference of the cumulus with dye diffusion) or closed (no dye diffusion; Luvoni et al., 2006).
Experimental Design and Statistical Analysis
Experiment 1 evaluated the influence of different hypo- versus hyperosmotic exposures on oocyte developmental competence in vitro. Groups of immature oocytes were randomly allocated to one of the eight exposure conditions and then cultured in vitro (n = 21 oocytes/exposure condition/replicate; 15 replicates; n = 2,520 total oocytes). After IVM, a portion of the oocytes (n = 10 oocytes/exposure condition/replicate) was fixed to determine the incidence of nuclear maturation (number of TI + MII oocytes relative to number of fixed oocytes). Remaining oocytes were inseminated and cultured in vitro (including n = 1 oocyte/exposure condition/replicate for parthenogenetic control). At 48 hpi, percentages of cleaved oocytes (number of cleaved oocytes relative to total number of cultured oocytes) were recorded, and uncleaved oocytes were fixed to assess their nuclear status. This enabled determining the fertilization success ([number of fertilized oocytes + cleaved oocytes] relative to total number of cultured oocytes) after each exposure condition. After 7 days of embryo culture, proportion of different embryo stages (relative to total number of cleaved oocytes) and blastocyst quality were determined. Experiment 2 was designed according to Experiment 1 results and was devised to characterize the impact of detrimental exposures on structural and functional integrity of COC. Groups of immature oocytes were randomly distributed to one of the 10 exposure conditions (n = 25 oocytes/exposure condition/replicate; 15 replicates; n = 3,750 total oocytes). At the end of the exposure, a part of oocytes was directly assessed for GV chromatin structure and area (n = 2 oocytes/exposure condition/ replicate). After exposure and return to isosmotic condition, another part of oocytes was assessed for mitochondrial distribution (n = 2 oocytes/exposure condition/replicate), membrane integrity (n = 2 oocytes/ exposure condition/replicate), and status of cumulus–oocyte communications (n = 2 oocytes/exposure condition/replicate). Remaining oocytes were cultured in vitro and assessed as in Experiment 1 (n = 8 oocytes/exposure condition/replicate fixed after IVM, n = 9 oocytes/exposure condition/replicate for IVF and embryo development in vitro). In Experiments 1 and 2, percentages were calculated by pooling the different replicates and compared pair-wise using the Chi-square test. Means were compared using the Student’s t-test (SigmaStat; SPSS, Chicago, IL).
RESULTS
Impact of Anisosmotic Exposures on COC Developmental Competence In Vitro
Regardless of the exposure condition, there were no differences (P >0.05) in the proportions of GVBD (range, 2.6–6.7%) or degenerate (range, 5.6–9.3%) oocytes after IVM. Percentages of oocytes at the MI stagewerehigher (P < 0.05) afterexposures to 100 mOsm and osmolarities ≥1,500 mOsm compared to other exposure conditions, including the control (290 mOsm, Table 1). As a result, percentages of oocytes reaching MII stage were lower (P < 0.05) after exposures to 100 mOsm and osmolarities ≥1,500 mOsm but not affected (P > 0.05) after exposures to osmolarities ≥200 mOsm and ≤1,000 mOsm (Table 1). This effect was sustained through fertilization and early cleavage. At 48 hpi, percentages of fertilized and cleaved oocytes were not affected (P > 0.05) by exposures of immature oocytes to osmolarities ≥200 and ≤1,000 mOsm but were higher (P < 0.05) than after exposure of immature oocytes to 100 mOsm and osmolarities ≥1,500 mOsm (Table 1). Fertilized oocytes that did not cleave were never polyspermic since they always contained two pronuclei. Control groups of oocytes that were incubated without sperm did not show any spontaneous activation regardless of the immature oocyte exposure. By analyzing the nuclear status of unfertilized oocytes, it was possible to calculate the proportion of matured oocytes that were subsequently fertilized (range, 82.9–93.7%), which did not differ (P > 0.05) regardless of the treatment group. Likewise, the proportion of fertilized oocytes completing a first cell cycle and then cleaving (range, 86.1–96.5%) was similar (P > 0.05) regardless of the exposure condition. After exposure of immature oocytes to osmolarities ≥1,000 mOsm, proportions of embryos arrested before the 8-cell stage or at the 8–16-cell stage were higher (P < 0.05) and proportions of morulae and blastocysts were lower (P < 0.05) compared to exposures of lower osmolarities (Table 2). Blastomere number per blastocyst was negatively affected (P < 0.05) by exposure of immature oocytes to 100 mOsm and was in turn even lower (P < 0.05) after oocyte exposure to osmolarities ≥ 1,000 mOsm (Table 2). However, proportions of inner cell mass in blastocysts (range, 22–28%) were similar (P > 0.05) regardless of the exposure conditions.
TABLE 1.
Incidences of Nuclear Maturation, Fertilization Success and Embryo Cleavage After Exposure of Immature Oocytes to Anisosmotic versus Isosmotic (290 mOsm) Solutions
| Exposure (mOsm) | Metaphase I (%)a | Metaphase II (%)a | Fertilized (%)b | Cleaved (%)b |
|---|---|---|---|---|
| 100 | 23.3 b | 66.0 b | 54.0 b | 48.7 b |
| 200 | 8.7 a | 80.7 a | 70.0 a | 66.0 a |
| 290 | 8.0 a | 82.0 a | 76.0 a | 73.3 a |
| 500 | 9.3 a | 78.0 a | 73.3 a | 70.7 a |
| 750 | 10.0 a | 78.7 a | 74.0 a | 70.0 a |
| 1,000 | 11.3 a | 79.3 a | 72.0 a | 66.7 a |
| 1,500 | 26.0 b | 62.7 b | 52.7 b | 45.3 b |
| 2,000 | 30.0 b | 56.0 b | 49.3 b | 44.0 b |
Within each column values with different letters differ (P < 0.05).
Percentage relative to 150 total oocytes/exposure condition fixed after IVM.
Percentage relative to 150 total oocytes/exposure condition observed at 48 hpi.
TABLE 2.
Proportions of Different Embryo Stages After 7 days of In Vitro Culture (Relative to Number of Cleaved Oocytes) Following Exposure of Immature Oocytes to Anisosmotic versus Isosmotic (290 mOsm) Solutions
| Exposure (mOsm) | Cleaved oocytes (n) | <8 cells (%) | 8–16 cells (%) | Morulae (%) | Blastocysts (%) | Blastomere number per blastocyst (mean 3SD) |
|---|---|---|---|---|---|---|
| 100 | 73 | 16.4 a | 24.7 ab | 27.4 ab | 31.5 a | 72.4 ± 3.2 b |
| 200 | 99 | 12.1 a | 25.3 ab | 30.3 a | 32.3 a | 79.3 ± 3.7 a |
| 290 | 110 | 12.7 a | 20.0 a | 30.0 a | 37.3 a | 82.5 ± 4.7 a |
| 500 | 106 | 16.0 a | 19.8 a | 30.2 a | 34.0 a | 82.1 ± 3.8 a |
| 750 | 105 | 14.3 a | 28.6 ab | 26.7 ab | 30.5 a | 78.3 ± 2.9 a |
| 1,000 | 100 | 27.0 b | 34.0 b | 23.0 ab | 16.0 b | 64.7 ± 3.6 c |
| 1,500 | 68 | 38.2 b | 35.3 b | 14.7 b | 11.8 b | 61.4 ± 2.7 c |
| 2,000 | 66 | 42.4 b | 34.8 b | 15.2 b | 7.6 b | 57.8 ± 6.7 c |
Within each column values with different letters differ (P < 0.05).
Characterization of Impact of Detrimental Anisosmotic Conditions on COC Structural and Functional Integrity
Regardless of anisosmotic conditions and the absence or presence of CB, there was no variation (P > 0.05) in the area (range, 1,480–1,796 μm2) and round shape of the GV chromatin during shrinking or swelling of the cytoplasm. Likewise, filamentous nuclear chromatin as well as nucleoli were consistently observed within the GV during all exposure conditions (Fig. 1A,D,G). As observed in controls, 100% of oocytes had normal mitochondrial distributions (Fig. 1B,E,H) after all exposure conditions. After isosmotic and hyperosmotic exposures, 100% of COC were scored as 1 (vast majority of cumulus cells with intact membranes; Fig. 1F,I). In contrast, hypoosmotic exposures (100 mOsm) resulted in 100% of COC being scored as 4 (including all cumulus cells located at the COC periphery with damaged membranes; Fig. 1C) regardless of CB absence or presence. Under all conditions, oocyte membranes remained intact (as illustrated by the consistent green staining of the oocyte; Fig. 1C,F,I).
Fig. 1.
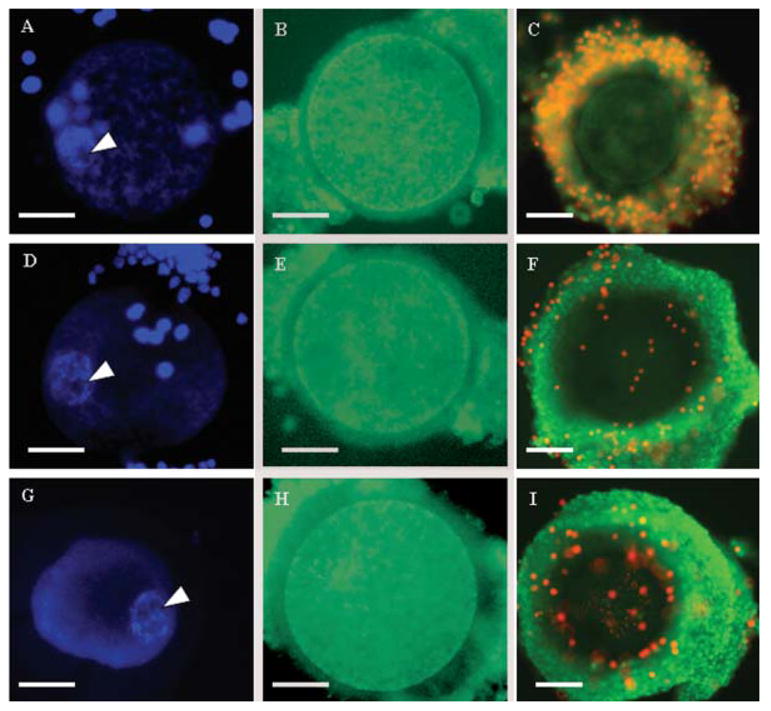
Structural integrity of grade I immature oocyte during or after exposure to hypo- (100 mOsm; A–C), iso- (290 mOsm; D–F), and hyperosmotic (2,000 mOsm; G–I) solutions. A,D,G: Germinal vesicle chromatin stained with Hoechst 33341 containing nucleolus (white arrow) during exposure. B,E,H: Mitochondrial distribution assessed with Mitotracker green after exposure. C,F,I: Membrane integrity of cumulus cells (intact cells stained with green SYBR14, non-intact cells stained with propidium iodide) after exposure. Bar = 50 μm. [See color version online at www.interscience.wiley.com.]
Data on cumulus–oocyte communication status are presented in Table 3. In the absence of CB, proportion of COC: (1) with open communications (Fig. 2A,B) was higher (P < 0.05) after exposures to 100 and 290 mOsm than after other treatments; (2) with partially open communications (Fig. 2C,D) was higher (P < 0.05) after exposure to 1,000 mOsm than after other treatments; and (3) with closed communications (Fig. 2E,F) was higher (P < 0.05) after exposures to 1,500 and 2,000 mOsm than counterpart treatments. In the presence of CB: (1) proportions of COC with open communications (Fig. 2E,F) after exposures to 1,000 mOsm were higher (P < 0.05) and proportions of COC with partially open communications were lower (P < 0.05) than in the absence of CB; (2) proportions of COC with open communications after exposures to 1,500 mOsm were higher (P < 0.05) and proportions of COC with closed communications were lower (P < 0.05) than in the absence of CB; and (3) proportions of COC with partially open communications after exposure to 2,000 mOsm were higher (P < 0.05) and proportions of COC with closed communications were lower (P < 0.05) than in the absence of CB. In the absence of CB, the incidence of nuclear maturation, fertilization success, and embryo development in vitro were consistent with the findings from Experiment 1. In the absence of CB, extremes at each end of the osmolarity scale (100, 1,500 or 2,000 mOsm) compromised each of these events (Tables 4 and 5). Even though presence of CB during immature oocyte exposure to 100 mOsm had no effect (P > 0.05), presence of CB during exposures to 1,500 or 2,000 mOsm improved (P < 0.05) the incidence of nuclear maturation, fertilization success and embryo cleavage (Table 4). As in Experiment 1, no polyspermy or spontaneous activation was observed. Supplementation with CB during exposures to 1,000, 1,500, or 2,000 mOsm also improved (P < 0.05) embryo development in vitro (Table 5). This effect was especially profound for oocytes initially exposed to 1,000 or 1,500 mOsm. Even at 2,000 mOsm, presence of CB increased (P < 0.05) the proportions of resulting morulae, although this effect was not sustained (P > 0.05) for blastocyst formation and blastomere numbers per blastocyst. The proportion of inner cell mass in blastocysts was similar (P > 0.05) across exposure conditions (range, 23–28%).
TABLE 3.
Proportions of Cumulus Oocyte Complexes With Open, Partially Open, or Closed Communications After Exposure to Anisosmotic versus Isosmotic (290 mOsm) Solutions in the Presence or Absence of Cytochalasin B (CB)*
| Exposure (mOsm) | Open (%) | Partially open (%) | Closed (%) |
|---|---|---|---|
| 100 | 76.7 a | 23.3 a | 0 a |
| 100 + CB | 73.3 a | 26.7 a | 0 a |
| 290 | 83.3 a | 16.7 a | 0 a |
| 290 + CB | 80.0 a | 20.0 a | 0 a |
| 1,000 | 16.7 b | 83.3 b | 0 a |
| 1,000 + CB | 83.3 a | 16.7 a | 0 a |
| 1,500 | 0 b | 16.7 a | 83.3 b |
| 1,500 + CB | 70.0 a | 30.0 a | 0 a |
| 2,000 | 0 b | 13.3 a | 86.7 b |
| 2,000 + CB | 0 b | 83.3 b | 16.7 a |
Within each column values with different letters differ (P < 0.05).
Percentage relative to 30 total oocytes/exposure condition.
Fig. 2.
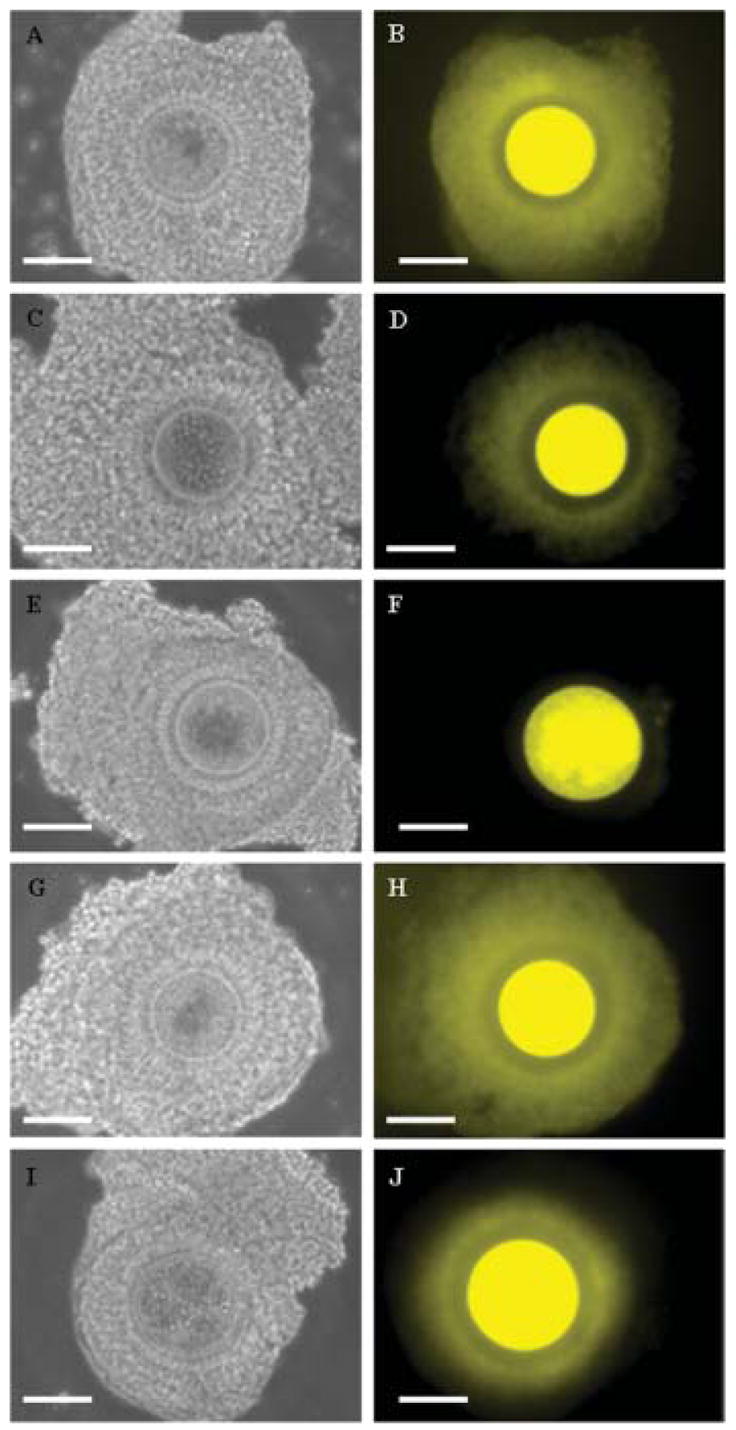
Structural integrity of grade I immature oocytes (bright field microscopy; A,C,E,G,I) and cumulus–oocyte communications (micro-injection and diffusion of fluorescent Luciferin Yellow; B,D,F,H,J) after exposure to anisosmotic and isosmotic solutions. A,B: Cumulus–oocyte complex (COC) with open communications after exposure to hypo- or isosmotic condition. C,D: COC with partially open communications after exposure to 1,000 mOsm. E,F: COC with closed communications after exposure to 2,000 mOsm. G,H: COC with open communications after treatment with cytochalasin B and exposure to 1,000 mOsm. I,J: COC with partially open communications after treatment with cytochalasin B and exposure to 2,000 mOsm. Bar = 100 μm. [See color version online at www.interscience.wiley.com.]
TABLE 4.
Incidences of Nuclear Maturation, Success of Fertilization, and Embryo Cleavage After Exposure of Immature Oocytes to Anisosmotic versus Isosmotic (290 mOsm) Solutions in the Presence or Absence of Cytochalasin B (CB)
| Exposure (mOsm) | Metaphase I (%)a | Metaphase II (%)a | Fertilized (%)b | Cleaved (%)b |
|---|---|---|---|---|
| 100 | 25.0 b | 62.5 b | 56.7 b | 51.7 b |
| 100 + CB | 25.0 b | 63.3 b | 54.2 b | 50.8 b |
| 290 | 8.3 a | 85.8 a | 75.0 a | 72.5 a |
| 290 + CB | 7.5 a | 84.2 a | 73.3 a | 73.3 a |
| 1,000 | 10.0 a | 80.8 a | 72.5 a | 70.8 a |
| 1,000 + CB | 9.2 a | 80.0 a | 71.7 a | 70.0 a |
| 1,500 | 29.2 b | 57.5 b | 53.3 b | 50.0 b |
| 1,500 + CB | 11.7 a | 79.2 a | 74.2 a | 70.0 a |
| 2,000 | 30.8 b | 55.8 b | 50.8 b | 44.2 b |
| 2,000 + CB | 12.5 a | 78.3 a | 72.5 a | 69.2 a |
Within each column values with different letters differ (P < 0.05).
Percentage relative to 120 total oocytes/exposure condition fixed after IVM.
Percentage relative to 120 total oocytes/exposure condition observed at 48 hpi.
TABLE 5.
Proportions of Different Embryo Stages After 7 Days of In Vitro Culture (Relative to Number of Cleaved Oocytes) Following Exposure of Immature Oocytes to Anisosmotic versus Isosmotic (290 mOsm) Solutions in the Presence or Absence of Cytochalasin B (CB)
| Exposure (mOsm) | Cleaved oocytes (n) | <8 cells (%) | 8–16 cells (%) | Morulae (%) | Blastocysts (%) | Blastomere number per blastocyst (mean ±SD) |
|---|---|---|---|---|---|---|
| 100 | 62 | 21.0 ab | 25.8 ab | 22.6 ab | 30.6 ab | 71.3 ± 4.1 b |
| 100 + CB | 61 | 19.7 ab | 29.5 ab | 21.3 ab | 29.5 ab | 72.7 ± 2.9 b |
| 290 | 87 | 17.2 ab | 21.8 a | 27.6 a | 33.3 a | 82.2 ± 3.9 a |
| 290 + CB | 88 | 15.9 a | 22.7 ab | 28.4 a | 33.0 a | 80.2 ± 5.7 a |
| 1,000 | 85 | 30.6 bc | 29.4 ab | 24.7 ab | 15.3 c | 62.7 ± 4.3 c |
| 1,000 + CB | 84 | 17.9 ab | 25.0 ab | 27.4 a | 29.8 ab | 77.8 ± 3.1 ab |
| 1,500 | 60 | 38.3 c | 36.7 b | 13.3 b | 11.7 c | 59.7 ± 5.8 c |
| 1,500 + CB | 84 | 19.0 ab | 21.4 a | 29.8 a | 29.8 ab | 79.6 ± 6.0 ab |
| 2,000 | 53 | 43.4 c | 35.8 ab | 13.2 b | 7.5 c | 55.8 ± 7.6 c |
| 2,000 + CB | 83 | 19.3 ab | 32.5 ab | 28.9 a | 18.1 bc | 66.8 ± 3.7 bc |
Within each column values with different letters differ (P < 0.05).
DISCUSSION
This study revealed the expansive osmotic tolerance limits of the immature cat oocyte. Compared to similar studies conducted in the cow (Agca et al., 2000), we discovered that the cat COC at the GV stage had the capacity to withstand the inherent stressors associated with a wide range of anisosmotic conditions (from 200 to 750 mOsm) without affecting subsequent structural or functional integrity, including blastocyst formation in vitro and quality. These two tolerated extremes represented 70% and 300%, respectively, of the normal isotonic environment experienced by the cat oocyte. Therefore, these cells appeared especially resistant to unusually high hyperosmotic conditions. Osmolarities exceeding 1,000 mOsm were detrimental to oocyte developmental competence, but the impact was not exerted on the GV itself or on mitochondrial distribution. Rather, the cat oocyte revealed a remarkably stable chromatin structure and cytoplasmic plasticity. However, oocytes under hyperosmotic conditions eventually succumbed to impaired communications with the cumulus cells, likely due to extreme shrinkage of oocyte. In contrast, consequences of hypoosmotic stress were expressed in a different fashion, specifically from a loss of cumulus cell membrane integrity. Importantly, the eventual failure of oocytes exposed to extreme osmolarities was mitigated through 1,500 mOsm by exposure to CB that could preserve villi extensions between cumulus cells and the oocyte.
It was important that the nuclear maturation and developmental competence of immature oocytes used in the present study and exposed to 200–750 mOsm for 15 min was comparable to in vitro culture success routinely achieved in our laboratory under isosmotic conditions (Comizzoli et al., 2003, 2004). This further affirmed that the immature cat oocyte appeared unusually resistant to osmolarity fluctuations. This has not been the case with developmental competence of cat oocytes matured in vitro that was severely impacted by exposure to 0.5 M sucrose (~800 mOsm) for 5 min only (Murakami et al., 2004). We suspect that metaphase plate and spindle of cat oocytes likely are highly sensitive to anisosmotic conditions as it has clearly been shown in mature human oocytes (Mullen et al., 2004). Interestingly, exposure of immature oocytes to dehydrating sucrose solutions for 15 min, wherein oocytes did not regain their initial volume unless returned to isotonic conditions, appeared to be more detrimental than exposures to permeating cryoprotectant solutions of similar osmolarity (e.g. 1.5 M propylene glycol; ~1,600 mOsm; Comizzoli et al., 2004). When exposed to permeating cryoprotectant, an oocyte re-expands after an initial shrinking that lasts <5 min (Critser et al., 1997), which is probably too short to disrupt cumulus–oocyte communications. At the same time, sucrose as an agent for regulating osmolarity offers an advantage over sodium chloride because it promotes hydrogen bond formation with phospholipids, thereby stabilizing membranes (Stachecki et al., 1998; Agca et al., 2000; Fabbri et al., 2001). This also could explain the differences in osmotic tolerance between the cat and cow oocyte, since studies of the latter relied on sodium chloride to alter culture conditions (Agca et al., 2000). An examination of the proportion of MII oocytes that were fertilized and the proportion of the latter that cleaved revealed no difference across the entire range of anisosmotic conditions. These findings indicated that even the extremes in osmolarity had no affect on sperm binding to the zona pellucida or sperm–oocyte interactions that ended in fertilization. This contrasted to observations involving the immature bovine oocyte where anisosmotic exposures contributed to significantly higher incidences of polyspermy (Agca et al., 2000).
That the anisosmotic exposure had no impact on the GV chromatin structure (especially during extreme shrinking of the cytoplasm). Furthermore, the incidence of GVBD was the first indication that osmotic stress had no, or a negligible, effect on the initial steps of meiotic resumption, including on regulating cell kinases as shown in epithelial cells (Barnes et al., 2002; Mao et al., 2004). If indeed this is the case, then the cat would be in stark contrast to the mouse oocyte where osmotic stress induces nuclear maturation (LaRosa and Downs, 2006). Nonetheless, the immature cat oocyte exposed to anisosmotic environments may well be vulnerable to DNA fragmentation as observed in kidney cells exposed to high concentration of sodium chloride (Kultz and Chakravarty, 2001). Even so, more evidence for the cat oocyte uniqueness was revealed through its consistently homogeneous mitochondrial distribution regardless of the anisosmotic exposure and in opposition to same stage cow oocytes. When the latter cells are placed in anisosmotic cryoprotectant solutions, mitochondrial distribution is altered (Rho et al., 2002). Taken together our observations support the notion for flexibility of the microfilament structure in the immature cat oocyte compared to the porcine oocytes (Wu et al., 2006). Even in the absence of distribution differences, of course mitochondrial function in the cat oocyte could have been compromised (Stojkovic et al., 2001), an issue that likely deserves study. Although biological membranes might be damaged and become leaky when exposed to high osmolarities (Adams et al., 2003), there was no evidence of compromised membrane integrity in such conditions. Regardless, all structural data together in this study, including consistent findings on the proportion of degenerated oocytes, revealed an impressive resistance of cat oocyte membranes to a wide range of anisosmotic environments.
Cumulus–oocyte communications recently have been evaluated in the immature cat oocyte by Lucifer Yellow injection (Luvoni et al., 2006), which is the primary reason we applied this useful tool to the present study. Clearly, exposing these cells to 1,000 mOsm partially disrupted communication that, in turn, had no apparent negative influence on the oocyte ability to reach MII, be fertilized or even advance to a morulae stage in vitro. However, an effect was expressed in a greater incidence of developmental arrest at <8 cells as well as reduced overall percentage of blastocysts and blastomeres per blastocyst. The developmental stage most sensitive to this initial hyperosmotic condition was near the 8-cell stage, an interval associated with activation of the embryonic genome (Hoffert et al., 1997). Exposures to osmolarities ≥1,000 mOsm impacted the embryo development first (50–80% decrease in the proportion of blastocysts) and then nuclear maturation (25% decrease in MII). We suspect that the cumulus cells of oocytes exposed to this extreme environment retained the ability to bind FSH that allowed triggering meiotic resumption. However, it also was likely that the subsequent transit of glutathione required to ensure proper blastocyst formation in vitro (Luciano et al., 2004; Luvoni et al., 2006) was inadequate. Even though the loss of blastocyst quality was reflected by the decrease in blastomere numbers, embryo ploidy as well as their developmental potential after transfer into recipient females is currently being studied to better evaluate this quality. Testing the influence of presence/ absence of CB provided the opportunity to clearly demonstrate the impact of hyperosmotic conditions on cumulus–oocyte communication integrity and COC developmental competence. CB increased cytoskeletal flexibility and enabled preserving the villi extensions through the zona pellucida between cumulus cells and the oocyte (Le Gal et al., 1994; Isachenko et al., 1998; Rho et al., 2002; Wu et al., 2006). This compound had a profound impact on the immature cat oocyte by expanding the range of osmotic tolerance up to 1,500 mOsm, which represented more than 500% of the isosmotic condition. Exposures to 2,000 mOsm resulted in >85% of COC with closed communications, and CB treatment resulted in >80% of COC with partially opened communications. Although not always comparable to results achieved under less extreme conditions, there was rather striking success in these oocytes having the capacity to mature, fertilize and advance to blastocyst stages in vitro. Interestingly, exposure of cat oocytes to the excessive 2,000 mOsm treatment was not as detrimental to COC functionality as denuding all cumulus cells or artificially disrupting communications with the oocyte by other artificial means (Vozzi et al., 2001; Atef et al., 2005; Luciano et al., 2005). This shows that a minimum of communications (not detectable by Lucifer Yellow diffusion) were retained within the COC exposed to 2,000 mOsm which allowed producing viable appearing embryos.
In contrast to findings from hyperosmotic treatments, there was no link between the hypoosmotic exposure, cumulus–oocyte communication metrics, and developmental competence in vitro. Supplementation with CB also failed to improve the oocyte nuclear maturation in vitro. Under these conditions, the disruptive mechanism appeared to be the loss of membrane integrity in the peripheral cumulus cells. Physiologically, the latter cells are expected to tolerate anisosmotic conditions due to normal variations in osmotic pressure exerted during follicular fluid formation (Clarke et al., 2006). However, in the case of the immature cat oocyte, a culture of <200 mOsm resulted in extensive membrane injury in surrounding cumulus cells. Based on earlier work, we suspect that this damage likely impaired the FSH ligand–receptor interaction and the release of a second messenger (Webb et al., 2002; Comizzoli et al., 2003) that, in turn, could well explain a reduced incidence of nuclear maturation in vitro.
In summary, in characterizing the immature cat oocyte reaction to anisosmotic conditions in vitro, we discovered an unusual resistance to osmotic stress. Nonetheless, extremes at the hyper- or hypoosmotic level caused severe damage, but by different mechanisms. In the former environment, nuclear maturation in vitro was negatively affected by an apparent compromise in membrane integrity of peripheral cumulus cell. In contrast, immature oocytes exposed to hyperosmotic conditions experienced loss in cumulus–oocyte communications. This reduction was reversible in many cases by increasing membrane plasticity through the supplementation of CB. Integrated together these findings were relevant for two primary reasons. Our ultimate goal is to develop reliable methods for cryopreserving immature felid oocytes, which requires a thorough understanding of cellular sensitivities, especially given that cooling, freezing and thawing causes extreme osmotic stresses. Determining that immature cat oocytes have an expansive osmotic tolerance is valuable for predicting optimal cryoprotocols. Likewise, important is considering that the sucrose used in our experiments may have exerted a membrane stabilizing effect. This could be especially important in overcoming expected hypoosmotic challenges, as perhaps step-wise dilutions using a sucrose buffer may be beneficial post-oocyte thawing. This approach has been shown to minimize osmotic shock while controlling water afflux intracellularly in the human oocyte limiting swelling/rupture risks as the cryoprotectant exits the cell (Fabbri et al., 2001). Studies are ongoing in our laboratory to assess membrane permeability to water and cryoprotectant in cat immature oocytes. Combined with the osmotic tolerance information from the present study, we will be able to mathematically model optimal oocyte cryopreservation protocols that will improve the loading and off-loading of cryoprotectant. Such applications hold considerable promise for assisting in the genetic management of small populations of rare domestic cat models, used in the study of human diseases, and for endangered species.
Acknowledgments
Grant sponsor: National Center for Research Resources; Grant number: K01 RR020045; Grant sponsor: National Institutes of Health.
The project described was supported by Grant Number K01 RR020045 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH) and its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. Funding also was received from the Friends of the National Zoo. Authors thank Dr. Michael Cranfield and Dr. Brent Whitaker (Maryland Line Animal Rescue) and Dr. Darby Thornburgh (Petworth Animal Hospital) for providing domestic cat ovaries.
References
- Adams SL, Kleinhans FW, Mladenov PV, Hessian PA. Membrane permeability characteristics and osmotic tolerance limits of sea urchin (Evechinus chloroticus) eggs. Cryobiology. 2003;47:1–13. doi: 10.1016/s0011-2240(03)00063-4. [DOI] [PubMed] [Google Scholar]
- Agca Y, Liu J, Rutledge JJ, Critser ES, Critser JK. Effect of osmotic stress on the developmental competence of germinal vesicle and metaphase II stage bovine cumulus oocyte complexes and its relevance to cryopreservation. Mol Reprod Dev. 2000;55:212–219. doi: 10.1002/(SICI)1098-2795(200002)55:2<212::AID-MRD11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Albertini DF, Barrett SL. Oocyte–somatic cell communication. Reprod Suppl. 2003;61:49–54. [PubMed] [Google Scholar]
- Atef A, Francois P, Christian V, Marc-Andre S. The potential role of gap junction communication between cumulus cells and bovine oocytes during in vitro maturation. Mol Reprod Dev. 2005;71:358–367. doi: 10.1002/mrd.20281. [DOI] [PubMed] [Google Scholar]
- Barnes K, Ingram J, Porras O, Barros F, Hudson E, Fryer L, Foufelle F, Carling D, Hardie D, Baldwin S. Activation of GLUT1 by metabolic and osmotic stress: Potential involvement of AMP-activated protein kinase (AMPK) J Cell Sci. 2002;115:2433–2442. doi: 10.1242/jcs.115.11.2433. [DOI] [PubMed] [Google Scholar]
- Choi YH, Love LB, Varner DD, Hinrichs K. Holding immature equine oocytes in the absence of meiotic inhibitors: Effect on germinal vesicle chromatin and blastocyst development after intracytoplasmic sperm injection. Theriogenology. 2006;66:955–963. doi: 10.1016/j.theriogenology.2006.01.064. [DOI] [PubMed] [Google Scholar]
- Clarke HG, Hope SA, Byers S, Rodgers RJ. Formation of ovarian follicular fluid may be due to the osmotic potential of large glycosaminoglycans and proteoglycans. Reproduction. 2006;132:119–131. doi: 10.1530/rep.1.00960. [DOI] [PubMed] [Google Scholar]
- Colleoni S, Luciano AM, Gandolfi F. Cumulus–oocyte communications in the horse: Role of the breeding season and of the maturation medium. Reprod Domest Anim. 2004;39:70–75. doi: 10.1046/j.1439-0531.2003.00479.x. [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE, Pukazhenthi BS. Overcoming poor in vitro nuclear maturation and developmental competence of domestic cat oocytes during the non-breeding season. Reproduction. 2003;126:809–816. [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE, Pukazhenthi BS. Effect of 1,2-propanediol versus 1,2-ethanediol on subsequent oocyte maturation, spindle integrity, fertilization, and embryo development in vitro in the domestic cat. Biol Reprod. 2004;71:598–604. doi: 10.1095/biolreprod.104.027920. [DOI] [PubMed] [Google Scholar]
- Critser JK, Agca Y, Gunasena KT. The cryobiology of mammalian oocytes. In: Karow A, Critser JK, editors. Reproductive tissue banking: Scientific principle. Academic Press; 1997. pp. 329–357. [Google Scholar]
- Fabbri R, Porcu E, Marsella T, Rocchetta G, Venturoli S, Flamigni C. Human oocyte cryopreservation: New perspectives regarding oocyte survival. Hum Reprod. 2001;16:411–416. doi: 10.1093/humrep/16.3.411. [DOI] [PubMed] [Google Scholar]
- Fulka H, Mrazek M, Tepla O, Fulka J., Jr DNA methylation pattern in human zygotes and developing embryos. Reproduction. 2004;128:703–708. doi: 10.1530/rep.1.00217. [DOI] [PubMed] [Google Scholar]
- Hoffert KA, Anderson GB, Wildt DE, Roth TL. Transition from maternal to embryonic control of development in IVM/IVF domestic cat embryos. Mol Reprod Dev. 1997;48:208–215. doi: 10.1002/(SICI)1098-2795(199710)48:2<208::AID-MRD8>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Isachenko V, Soler C, Isachenko E, Perez-Sanchez F, Grishchenko V. Vitrification of immature porcine oocytes: Effects of lipid droplets, temperature, cytoskeleton, and addition and removal of cryoprotectant. Cryobiology. 1998;36:250–253. doi: 10.1006/cryo.1998.2079. [DOI] [PubMed] [Google Scholar]
- Kultz D, Chakravarty D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc Natl Acad Sci USA. 2001;98:1999–2004. doi: 10.1073/pnas.98.4.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaRosa C, Downs SM. Stress stimulates AMP-activated protein kinase and meiotic resumption in mouse oocytes. Biol Reprod. 2006;74:585–592. doi: 10.1095/biolreprod.105.046524. [DOI] [PubMed] [Google Scholar]
- Le Gal F, Gasqui P, Renard JP. Differential osmotic behavior of mammalian oocytes before and after maturation: A quantitative analysis using goat oocytes as a model. Cryobiology. 1994;31:154–170. doi: 10.1006/cryo.1994.1019. [DOI] [PubMed] [Google Scholar]
- Litkouhi B, Marlow D, McGrath JJ, Fuller B. The influence of cryopreservation on murine oocyte water permeability and osmotically inactive volume. Cryobiology. 1997;34:23–35. doi: 10.1006/cryo.1996.1984. [DOI] [PubMed] [Google Scholar]
- Lodde V, Modina S, Galbusera C, Franciosi F, Luciano AM. Large-scale chromatin remodeling in germinal vesicle bovine oocytes: Interplay with gap junction functionality and developmental competence. Mol Reprod Dev. 2006;74:740–749. doi: 10.1002/mrd.20639. [DOI] [PubMed] [Google Scholar]
- Luciano AM, Modina S, Vassena R, Milanesi E, Lauria A, Gandolfi F. Role of intracellular cyclic adenosine 3′,5′-monophosphate concentration and oocyte–cumulus cells communications on the acquisition of the developmental competence during in vitro maturation of bovine oocyte. Biol Reprod. 2004;70:465–472. doi: 10.1095/biolreprod.103.020644. [DOI] [PubMed] [Google Scholar]
- Luciano AM, Lodde V, Beretta MS, Colleoni S, Lauria A, Modina S. Developmental capability of denuded bovine oocyte in a co-culture system with intact cumulus–oocyte complexes: Role of cumulus cells, cyclic adenosine 3′,5′-monophosphate, and glutathione. Mol Reprod Dev. 2005;71:389–397. doi: 10.1002/mrd.20304. [DOI] [PubMed] [Google Scholar]
- Luvoni GC. Gamete cryopreservation in the domestic cat. Theriogenology. 2006;66:101–111. doi: 10.1016/j.theriogenology.2006.03.012. [DOI] [PubMed] [Google Scholar]
- Luvoni GC, Pellizzari P. Embryo development in vitro of cat oocytes cryopreserved at different maturation stages. Theriogenology. 2000;53:1529–1540. doi: 10.1016/S0093-691X(00)00295-8. [DOI] [PubMed] [Google Scholar]
- Luvoni GC, Luciano AM, Modina S, Gandolfi F. Influence of different stages of the oestrous cycle on cumulus–oocyte communications in canine oocytes: Effects on the efficiency of in vitro maturation. J Reprod Fert Suppl. 2001;57:141–146. [PubMed] [Google Scholar]
- Luvoni GC, Chigioni S, Perego L, Lodde V, Modina S, Luciano AM. Effect of gonadotropins during in vitro maturation of feline oocytes on oocyte–cumulus cells functional coupling and intracellular concentration of glutathione. Anim Reprod Sci. 2006;96:66–78. doi: 10.1016/j.anireprosci.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Mao X, Bravo I, Cheng H, Alonso A. Multiple independent kinase cascades are targeted by hyperosmotic stress but only one activates stress kinase p38. Exp Cell Res. 2004;292:304–311. doi: 10.1016/j.yexcr.2003.09.012. [DOI] [PubMed] [Google Scholar]
- Miyara F, Migne C, Dumont-Hassan M, Le Meur A, Cohen-Bacrie P, Aubriot FX, Glissant A, Nathan C, Douard S, Stanovici A, Debey P. Chromatin configuration and transcriptional control in human and mouse oocytes. Mol Reprod Dev. 2003;64:458–470. doi: 10.1002/mrd.10233. [DOI] [PubMed] [Google Scholar]
- Modina S, Beretta M, Lodde V, Lauria A, Luciano AM. Cytoplasmic changes and developmental competence of bovine oocytes cryopreserved without cumulus cells. Eur J Histochem. 2004;48:337–346. [PubMed] [Google Scholar]
- Mullen SF, Agca Y, Broermann DC, Jenkins CL, Johnson CA, Critser JK. The effect of osmotic stress on the metaphase II spindle of human oocytes, and the relevance to cryopreservation. Hum Reprod. 2004;19:1148–1154. doi: 10.1093/humrep/deh201. [DOI] [PubMed] [Google Scholar]
- Murakami M, Otoi T, Karja NW, Wongsrikeao P, Agung B, Suzuki T. Blastocysts derived from in vitro-fertilized cat oocytes after vitrification and dilution with sucrose. Cryobiology. 2004;48:341–348. doi: 10.1016/j.cryobiol.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Newton H, Pegg DE, Barrass R, Gosden RG. Osmotically inactive volume, hydraulic conductivity, and permeability to dimethyl sulphoxide of human mature oocytes. J Reprod Fert. 1999;117:27–33. doi: 10.1530/jrf.0.1170027. [DOI] [PubMed] [Google Scholar]
- Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: A meta-analysis. Fert Steril. 2006;86:70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- Paynter SJ. A rational approach to oocyte cryopreservation. Reprod Biomed Online. 2005;10:578–586. doi: 10.1016/s1472-6483(10)61664-1. [DOI] [PubMed] [Google Scholar]
- Pukazhenthi B, Comizzoli P, Travis AJ, Wildt DE. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod Fert Dev. 2006;18:77–90. doi: 10.1071/rd05117. [DOI] [PubMed] [Google Scholar]
- Rho GJ, Kim S, Yoo JG, Balasubramanian S, Lee HJ, Choe SY. Microtubulin configuration and mitochondrial distribution after ultra-rapid cooling of bovine oocytes. Mol Reprod Dev. 2002;63:464–470. doi: 10.1002/mrd.10196. [DOI] [PubMed] [Google Scholar]
- Ruppert-Lingham CJ, Paynter SJ, Godfrey J, Fuller BJ, Shaw RW. Developmental potential of murine germinal vesicle stage cumulus–oocyte complexes following exposure to dimethylsulph-oxide or cryopreservation: Loss of membrane integrity of cumulus cells after thawing. Hum Reprod. 2003;18:392–398. doi: 10.1093/humrep/deg071. [DOI] [PubMed] [Google Scholar]
- Ruppert-Lingham CJ, Paynter SJ, Godfrey J, Fuller BJ, Shaw RW. Membrane integrity and development of immature murine cumulus–oocyte complexes following slow cooling to −60 degrees C: The effect of immediate rewarming, plunging into LN2 and two-controlled-rate-stage cooling. Cryobiology. 2006;52:219–227. doi: 10.1016/j.cryobiol.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Songsasen N, Ratterree MS, VandeVoort CA, Pegg DE, Leibo SP. Permeability characteristics and osmotic sensitivity of rhesus monkey (Macaca mulatta) oocytes. Hum Reprod. 2002;17:1875–1884. doi: 10.1093/humrep/17.7.1875. [DOI] [PubMed] [Google Scholar]
- Spindler RE, Wildt DE. Circannual variations in intraovarian oocyte but not epididymal sperm quality in the domestic cat. Biol Reprod. 1999;61:188–194. doi: 10.1095/biolreprod61.1.188. [DOI] [PubMed] [Google Scholar]
- Stachecki JJ, Cohen J, Willadsen S. Detrimental effects of sodium during mouse oocyte cryopreservation. Biol Reprod. 1998;59:395–400. doi: 10.1095/biolreprod59.2.395. [DOI] [PubMed] [Google Scholar]
- Stojkovic M, Machado SA, Stojkovic P, Zakhartchenko V, Hutzler P, Goncalves PB, Wolf E. Mitochondrial distribution and adenosine triphosphate content of bovine oocytes before and after in vitro maturation: Correlation with morphological criteria and developmental capacity after in vitro fertilization and culture. Biol Reprod. 2001;64:904–909. doi: 10.1095/biolreprod64.3.904. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Davis P, Mathwig V, Alexander S. Domains of high-polarized and low-polarized mitochondria may occur in mouse and human oocytes and early embryos. Hum Reprod. 2002;17:393–406. doi: 10.1093/humrep/17.2.393. [DOI] [PubMed] [Google Scholar]
- Van der Elst J, Nerinckx S, Van Steirteghem AC. In vitro maturation of mouse germinal vesicle-stage oocytes following cooling, exposure to cryoprotectants and ultrarapid freezing: Limited effect on the morphology of the second meiotic spindle. Hum Reprod. 1992;7:1440–1446. doi: 10.1093/oxfordjournals.humrep.a137591. [DOI] [PubMed] [Google Scholar]
- Velilla E, Rodriguez-Gonzalez E, Vidal F, Izquierdo D, Paramio MT. Mitochondrial organization in prepubertal goat oocytes during in vitro maturation and fertilization. Mol Reprod Dev. 2006;73:617–626. doi: 10.1002/mrd.20426. [DOI] [PubMed] [Google Scholar]
- Vozzi C, Formenton A, Chanson A, Senn A, Sahli R, Shaw P, Nicod P, Germond M, Haefliger JA. Involvement of connexin 43 in meiotic maturation of bovine oocytes. Reproduction. 2001;122:619–628. [PubMed] [Google Scholar]
- Wang WH, Abeydeera LR, Prather RS, Day BN. Polymerization of nonfilamentous actin into microfilaments is an important process for porcine oocyte maturation and early embryo development. Biol Reprod. 2000;62:1177–1783. doi: 10.1095/biolreprod62.5.1177. [DOI] [PubMed] [Google Scholar]
- Webb RJ, Marshall F, Swann K, Carroll J. Follicle-stimulating hormone induces a gap junction-dependent dynamic change in (cAMP) and Protein Kinase A in mammalian oocytes. Dev Biol. 2002;246:441–454. doi: 10.1006/dbio.2002.0630. [DOI] [PubMed] [Google Scholar]
- Wildt DE. Genome resource banking for wildlife research, management, and conservation. ILAR J. 2000;41:228–234. doi: 10.1093/ilar.41.4.228. [DOI] [PubMed] [Google Scholar]
- Wood TC, Wildt DE. Effect of the quality of the cumulus–oocyte complex in the domestic cat on the ability of oocytes to mature, fertilize and develop into blastocysts in vitro. J Reprod Fert. 1997;110:355–360. doi: 10.1530/jrf.0.1100355. [DOI] [PubMed] [Google Scholar]
- Wu C, Rui R, Dai J, Zhang C, Ju S, Xie B, Lu X, Zheng X. Effects of cryopreservation on the developmental competence, ultrastructure and cytoskeletal structure of porcine oocytes. Mol Reprod Dev. 2006;73:1454–1462. doi: 10.1002/mrd.20579. [DOI] [PubMed] [Google Scholar]


