Abstract
We have previously reported that the downregulation of MMP-2 by adenovirus-mediated delivery of MMP-2 siRNA (Ad-MMP-2) reduced spheroid invasion and angiogenesis in vitro, and, metastasis and tumor growth in vivo. In this study, we investigated the mechanism of Ad-MMP-2-mediated growth inhibition in vitro and in vivo. Ad-MMP-2 infection led to the induction of apoptosis as determined by TUNEL assay, Annexin-V staining and PARP-1 cleavage in a dose-dependent manner in A549 cells. Ad-MMP-2 decreased the content of the anti-apoptotic members of the Bcl-2 family proteins (Bcl-2 and Bcl-xL) and increased the content of the pro-apoptotic members of the Bcl-2 family (Bax and Bcl-xS) as determined by immunoblotting analysis. Furthermore, Ad-MMP-2-mediated apoptosis was accompanied by increase in truncated Bid, release of cytochrome-c, and the activation of caspases-8, -9 and -3. Immunoblot analysis showed that Ad-MMP-2 infection caused upregulation of Fas/Fas-L and FADD. And Anti-Fas-L antibody reversed Ad-MMP-2-induced apoptosis. TIMP-3, an endogenous inhibitor of MMP-2, which cleaves Fas-L and activates the Fas/Fas-L inducing apoptotic pathway, was increased in Ad-MMP-2-treated cells. Adenovirus-mediated expression of MMP-2 siRNA in human lung xenografts in vivo resulted in increased immunostaining of Fas, Fas-L, cleaved Bid and TIMP-3. This is the first report, to our knowledge, showing that MMP-2 inhibition upregulates TIMP-3 levels, which in turn, promotes apoptosis in lung cancer.
Keywords: CD95 (APO/Fas), Fas-L, caspase-8, Cytochrome-c, Apoptosis, MMP-2, TIMP-3
INTRODUCTION
The matrix metalloproteinases (MMPs) consist of a large family of enzymes that, in combination, can digest components of the extracellular matrix (Birkedal-Hansen et al., 1993). MMP-2 (gelatinase-A) and MMP-9 (gelatinase-B) are the specific MMPs correlated most commonly with breast, ovarian, colorectal, and lung cancer growth (Brown and Giavazzi, 1995; Cawston, 1995). The activity of MMPs is specifically inhibited by tissue inhibitor of metalloproteinases (TIMP), which binds to active MMPs in a 1:1 molar stoichiometry (Brew et al., 2000). Given the ability of TIMPs to inhibit the proteolytic activity of MMPs, it is not surprising to find that TIMPs are capable of blocking tumor metastasis, either by inhibiting tumor invasion of basement membrane or by restraining tumor angiogenesis (Gomez et al., 1997). However, recent studies have suggested that the inhibitory effects of TIMPs on tumor progression are not only due to their ability to inhibit MMP activity, but also because of their ability to directly modulate the cell growth and apoptosis of tumor cells, as well as host endothelial cells. High levels of TIMP-3 promote apoptosis in many cell types in vitro and in vivo (Baker et al., 1998; Bond et al., 2000), this effect is associated with death receptor modulation (Bond et al., 2002).
It is well established that uncontrolled cellular growth, which occurs as a consequence of defects in cell cycle and apoptotic machinery, is responsible for the development of most of the cancers including lung cancer. Therefore those agents that can modulate apoptosis in cancer cells may be able to affect the steady-state cell population and be useful in the management and therapy of cancer. Some studies have reported that MMPs promote tumor growth by releasing growth factors from their inactive membrane-bound form (Haro et al., 2000), thereby suggesting that MMP antagonists would inhibit cell growth. On the other hand, different studies have reported that MMPIs inhibit tumor proliferation by inducing apoptosis via the release of ligands such as TRAIL and TNFα from their membrane-bound inactive forms (Nyormoi et al., 2003). In a past study, we demonstrated that adenoviral-mediated siRNA delivery of MMP-2 inhibited lung cancer growth and metastasis (Chetty et al., 2006). However, the inhibitory mechanism in tumor inhibition in vivo can only be partly explained by the inhibition of the catalytic activity of MMP-2 overexpressed in cancerous tissue. Therefore, we hypothesized that Ad-MMP-2-mediated tumor growth inhibition is mediated by cell growth arrest or apoptosis. In the present study, we demonstrate that Ad-MMP-2 infection induced apoptosis in A549 cells. MMP-2 downregulation induced cytochrome-c release, cleavages of caspases-8, -9 & -3 and PARP-1, and DNA fragmentation by the Fas-mediated signaling pathway in A549 cells and A549 grafted tumors in SCID mice. In addition, we show that TIMP-3 is overexpressed with Ad-MMP-2 infection in A549 cells and tumors. Taken together, these results show that TIMP-3 promotes Fas/Fas-L-mediated apoptosis in lung cancer cells in culture and in vivo.
RESULTS
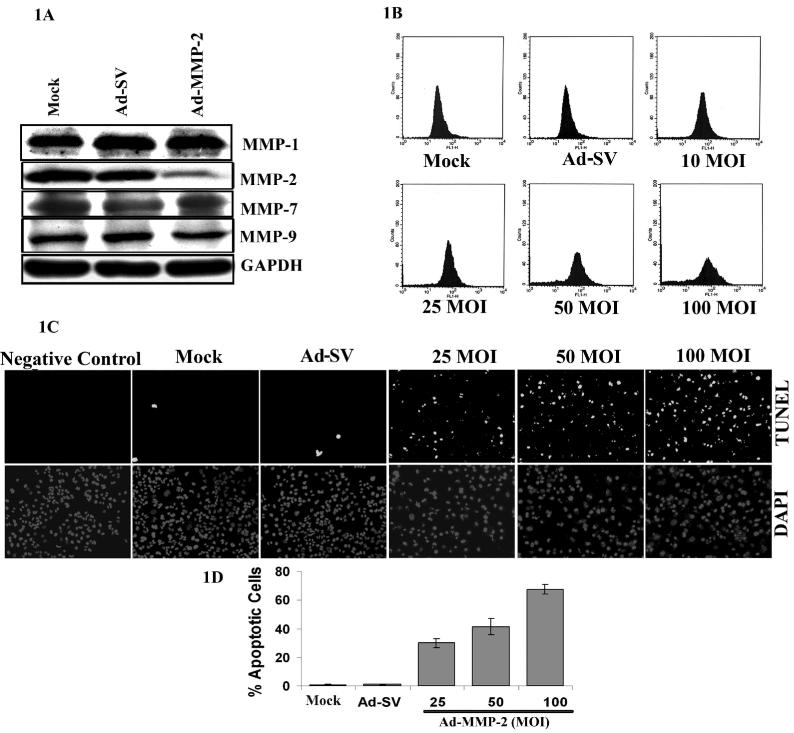
Ad-MMP-2 induces apoptosis in A549 cells
We previously showed that downregulation of MMP-2 with Ad-MMP-2 siRNA reduced invasion, migration and angiogenesis in A549 cells in vitro and inhibited tumor growth and lung metastasis in vivo (Chetty et al., 2006). We have clearly demonstrated the specificity of this virus in our earlier published results and showed that the virus does not activate components of the interferon system. To further characterize the specificity of Ad-MMP-2 infection we determined the expression of other MMP family proteins, MMP-1, MMP-7 and MMP-9. We did not observe any change in the expression of MMP-1, -7 and -9 in Ad-MMP-2 infected cells compared to mock (PBS) and scrambled vector (Ad-SV) controls (Fig. 1A). Of interest, Ad-MMP-2-infected cells displayed morphological signs of apoptosis including cell shrinkage, membrane blebbing, and eventual disintegration into numerous apoptotic bodies within 48−72h of treatment (data not shown). To confirm these initial observations, we analyzed apoptosis by looking for phosphatidylserine externalization by annexin-V binding and DNA fragmentation (TUNEL) assay. To quantify early and late events in the course of apoptosis, A549 cells were stained with annexin-V-FITC, 36h after Ad-MMP-2 infection. As depicted in Fig. 1, infection of A549 cells with Ad-MMP-2-induced annexin-V expression on the cell surface as compared to mock and Ad-SV controls. We observed a dose-dependant increase in annexin-V positive staining with increasing MOI of Ad-MMP-2 infection (Fig. 1B). At 48h post-infection, TUNEL-positive, apoptotic lung cancer cells were meagerly present in mock or Ad-SV-infected cells. Ad-MMP-2 infection resulted in a distinct increase of TUNEL-positive cells in a dose-dependent manner (Fig. 1C). Quantitation of TUNEL-positive cells indicated that at 25 and 50MOI Ad-MMP-2, 30% and 43% of the cells were TUNEL-positive, and 100MOI of Ad-MMP-2, more than 65% of were TUNEL-positive (Fig. 1D).
Figure 1. Ad-MMP-2 infection induces apoptosis in A549 cells.
A549 cells were treated for with mock (PBS Control), 100MOI of Ad-SV or the indicated MOI of Ad-MMP-2. (A) Western blot analysis showing the effect of Ad-MMP-2 infection on MMP family proteins. (B) The externalization of phosphatidylserine was assessed by FACS analyses after 36 h infection by measuring FITC-annexin-V binding. Ad-MMP-2 treatment led to a substantial increase in annexin-V binding in A549 cells. (C) TUNEL staining at 48h after infection. (D) Quantitation of apoptotic cells (P ≤ 0.01). The, bars indicate standard errors (±SE) from the mean of three separate experiments.
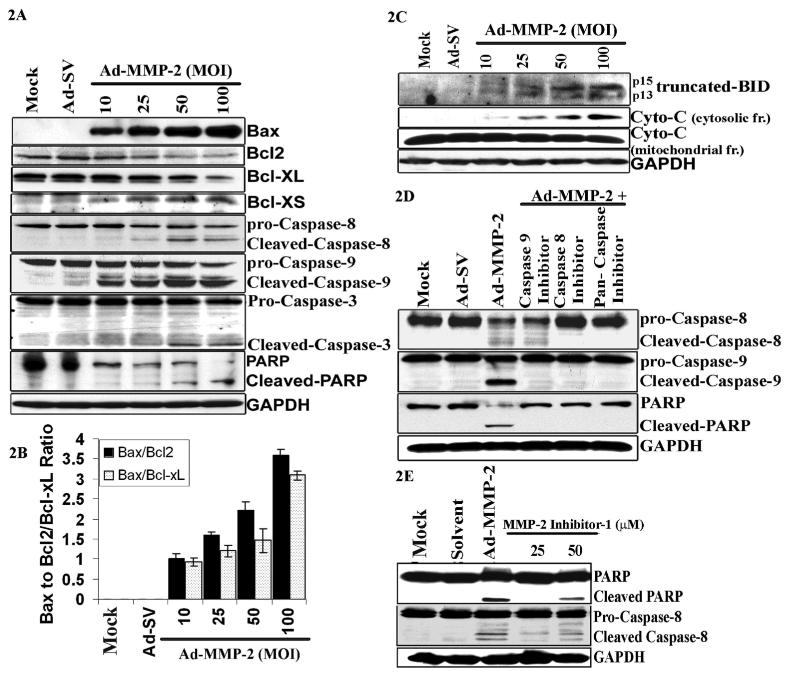
Ad-MMP-2 infection alters Bax/Bcl-2 expression and induces cleavage of caspases-3, -8 and -9 and PARP-1
Since Bax and Bcl-2 play crucial roles in apoptosis, we next determined the effect of Ad-MMP-2 infection on protein levels of Bax and Bcl-2 in A549 cells. The immuno-blot results exhibited a dose-dependent increase in the expression of the pro-apoptotic protein Bax in cells infected with Ad-MMP-2. In contrast, the protein expression of Bcl-2 was significantly decreased with Ad-MMP-2 infection in a dose-dependent fashion. We observed decreased expression of the anti-apoptotic molecules Bcl-xL and increased expression of the pro-apoptotic molecule Bcl-xs in Ad-MMP-2-treated cells (Fig. 2A). The densitometric analysis revealed an increase in the Bax/Bcl2 and Bax/Bcl-xL ratio in a dose-dependent manner (Fig. 2B). Because the alteration in Bax/Bcl-2 is known to initiate caspase signaling, we evaluated the involvement of various caspases in Ad-MMP-2-mediated apoptosis in A549 cells. Figure 2A indicates that Ad-MMP-2 infection induced the cleavage of initiator pro-caspases-8 and -9, and the effector pro-caspase-3 in a dose-dependent manner. We observed no cleavage of these caspases in the mock and Ad-SV controls. We also found that Ad-MMP-2 infection caused cleavage of the PARP a downstream effector of caspases. As shown in Figure 2A, the 85-kDa cleaved fragment of PARP-1 was detected in Ad-MMP-2-treated cells, but not in mock and Ad-SV-infected cells.
Figure 2. Ad-MMP-2 infection induces cleavage of caspases and PARP.
A549 cells were infected with mock, 100MOI of Ad-SV or the indicated dose of Ad-MMP-2 for 48h. (A) Cell lysates were used for immuno blot analysis of Bcl-2 family proteins and caspases. (B) Densitometric analysis showing the Bax/Bcl2 and Bax/ Bcl-xL ratios in treatment cells. The, bars indicate standard errors (±SE) from the mean of three separate experiments. (C) Cells were used to evaluate Bid cleavage and cytochrome-c release in the cytosol. (D) The effects of caspase inhibitors on Ad-MMP-2-induced apoptosis. A549 cells were treated with 100μM of various caspase inhibitors or solvent (DMSO) for 1 h and infected with mock, 100MOI of Ad-MMP-2. Caspase cleavage and PARP levels were determined by immuno blotting. Anti-GAPDH antibody was used for visualizing protein loading. immuno blots are representative of three experiments. (E) Cleavage of caspase-8 and PARP-1 in A549 cells treated with MMP-2 inhibitor-1, a MMP-2 specific inhibitor (50μM), indicating that MMP-2 inhibition induces apoptosis.
Ad-MMP-2 induces cleavage of Bid and cytochrome-c release
Truncated Bid can interact with Bax and enhance its translocation into mitochondria, which, in turn, results in cytochrome-c release from mitochondria. Cytochrome-c release is the limiting factor in caspase-9 activation and represents a central coordinating step in the formation of the apoptosome to induce apoptosis via the mitochondrial pathway (Li et al., 1997). Therefore, Ad-MMP-2-infection was tested for its ability to cleave Bid and release cytochrome-c. As shown in Figure 2C, truncated Bid (p13&p15) increased with Ad-MMP-2 infection in a dose-dependent manner. Cytochrome-c was undetectable in the cytosolic fractions of mock and Ad-SV-infected cells. However, cytochrome-c expression was detected even at 10MOI of Ad-MMP-2 and increased in a dose-dependent manner (Fig. 2C).
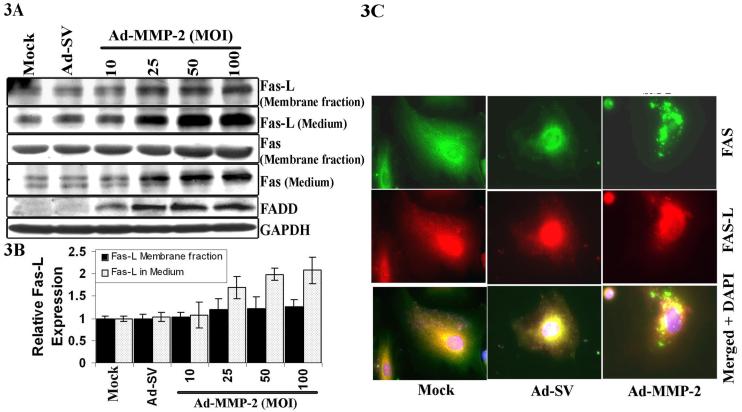
Ad-MMP-2-induces the activation of Fas/Fas-L and recruitment of Fas-Associated Death Domain (FADD) to the Fas Death Domain in A549 cells
Engagement of the Fas antigen with its ligand induces apoptosis. Membrane-bound as well as soluble forms of Fas (sFAS) and Fas-L protein expression levels significantly increased when compared with mock and Ad-SV-treated cells (Fig. 3A). Densitometric analysis indicated a dose-dependent increase in the membrane bound Fas-L as well as sFas-L (Fig. 3B). The binding of Fas-L with Fas initiates receptor oligomerization, which recruits FADD (Chinnaiyan et al., 1995). Levels of FADD protein expression increased with Ad-MMP-2 infection in A549 cells (Fig. 3A). Cellular localization of these proteins by immunofluorescence staining indicated a granular pattern of Fas and Fas-L at the periphery of the cells in Ad-MMP-2 infected cells. (Fig. 3C).
Figure 3. Ad-MMP-2 infection induces accumulation of the membrane Fas receptor, Fas-L and FADD in A549 cells.
A549 cells were treated with mock, 100MOI of Ad-SV and the indicated MOI of Ad-MMP-2. (A) Expression of the Fas receptor, Fas-L and FADD was measured by immuno blotting. (B) Densitometric analysis of Fas-l expression and shedding on membrane and in media in Ad-MMP-2 treated cells. The, bars indicate standard errors (±SE) from the mean of three separate experiments. (C) Immunohistochemistry of Fas and Fas-L.
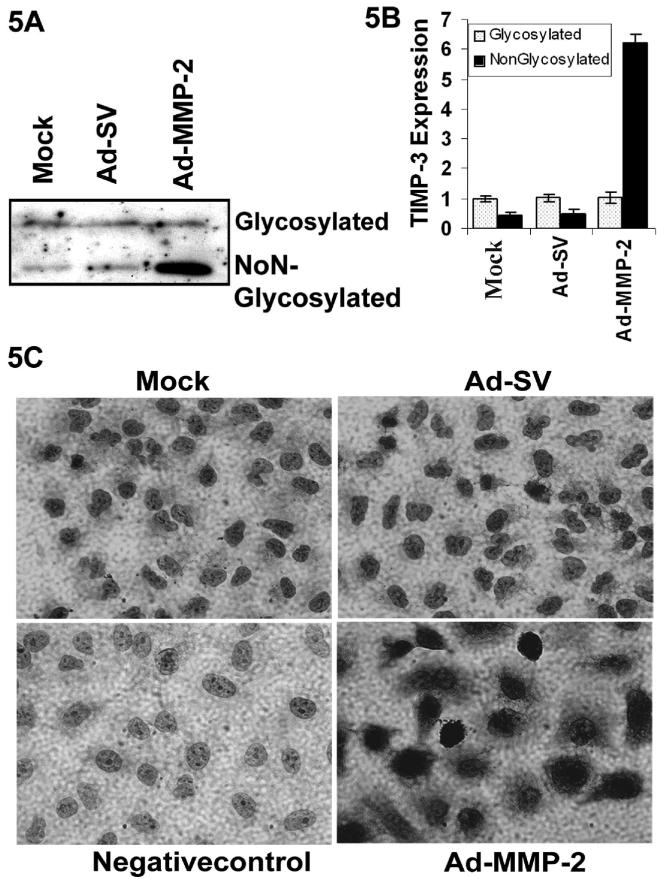
Ad-MMP-2 infection increases expression of TIMP-3
TIMP-3 is one of the endogenous inhibitors for MMP-2 and is a secreted 24-kDa protein that, unlike other TIMP family members, binds to the extracellular matrix and induces apoptosis via the Fas/Fas-L system (Baker et al., 1999). We detected both the non-glycosylated and glycosylated-TIMP-3 forms in immunoblot analysis in Ad-MMP-2-treated cells. The non-glycosylated form of TIMP-3 was increased 6 times in Ad-MMP-2 infected cells (Fig. 5A & B). Cytoplasmic immunoreactivity to TIMP-3 was also significantly increased with Ad-MMP-2 infection (Fig. 5C).
Figure 5. Ad-MMP-2 infection increases expression of TIMP-3.
(A) Immuno blotting analysis for TIMP-3 expression in the ECM of A549 cells infected with mock, 100MOI of Ad-SV or 100MOI of Ad-MMP-2. (B) Densitometric analysis showing the TIMP-3 expression in Ad-MMP-2 treated A549 cells. The, bars indicate standard errors (± SE) from the mean of three separate experiments. (C) Immunohistochemistry showing TIMP-3 expression in A549 cells infected with mock, 100MOI of Ad-SV & Ad-MMP-2.
Tumor regression induced by Ad-MMP-2 treatment is due to apoptosis
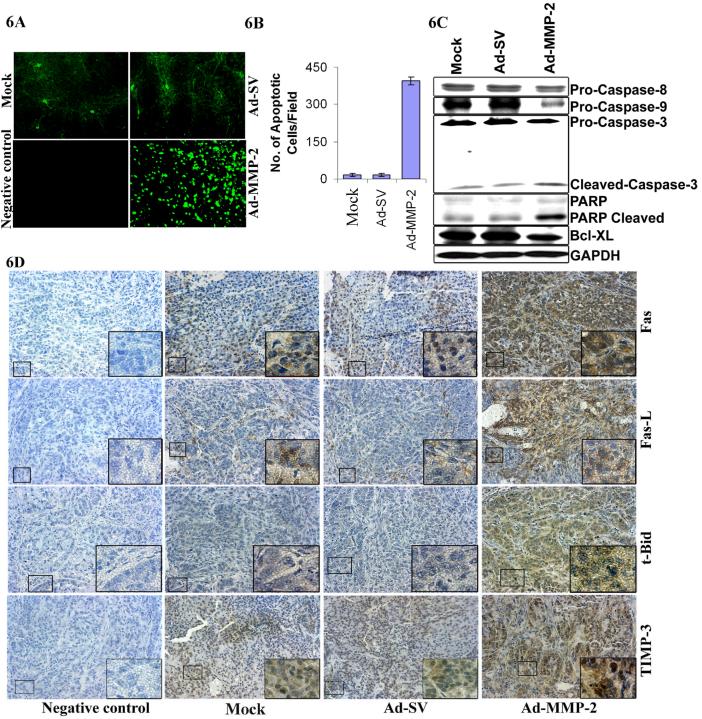
To determine if anti-tumor effect was elicited by apoptosis, we sectioned the tumors for histological and TUNEL analyses. As shown in Figure 6A, very few TUNEL-positive cells were present in the tumors from mice that received mock and Ad-SV treatment. The apoptotic index of the tumor cells quantified by the number of TUNEL-positive cells per field were 25 fold higher in tumors from mice that received Ad-MMP-2 treatment compared to controls. Moreover, the immunoblot analysis of the tumor tissue samples showed that the pro-forms of caspases-8, -9, -3 and the anti-apoptotic molecule Bcl-xL were reduced in tumors from mice that received Ad-MMP-2 treatment. Predictably, the cleaved fraction of PARP (85-kDa) was increased in Ad-MMP-2-treated cells (Fig. 6C). Immunohistochemical analysis of the sections of tumors from mice that received Ad-MMP-2 treatment indicated increased staining for TIMP-3, Bid, Fas-L and Fas compared to mock and Ad-SV controls (Fig. 6D).
Figure 6. Ad-MMP-2 treatment induces apoptosis in A549 subcutaneous tumors.
(A) Tumor sections were stained for apoptosis by TUNEL staining. Data shown are representative fields. (B) Quantitation of apoptotic cells indicated an increase in number of TUNEL-positive cells per microscopic field in Ad-MMP-2 treated cells. Bars represent the mean and standard error of the mean of three sections from three animals of the same treatment. (C) Processing of PARP and caspases-8, -9 and -3 was detected in total cell lysates from the subcutaneous tumors of mice, which received mock, Ad-SV and Ad-MMP-2. Representative results obtained from the tumor of one mouse per treatment condition are shown. (D) Immunohistochemical analysis of Fas, Fas-L, TIMP-3 and Bid showing increased expression of these proteins in Ad-MMP-2-treated tumors compared to mock and Ad-SV controls.
DISCUSSION
The present study was designed to define the mechanism(s) underlying the anti-proliferative and pro-apoptotic effects of an adenovirus carrying siRNA against MMP-2 (Ad-MMP-2) in the A549 lung cancer cell line. In this study, we provide evidence that adenoviral-mediated delivery of siRNA against the human MMP-2 gene had a direct apoptosis-inducing effect on A549 lung cancer cells. Ad-MMP-2 infection increased Fas and Fas-L, which, in turn, leads to upregulation of the Bax/BCl-2 ratio and activates caspases resulting in the induction of apoptosis. Our results include annexin-V-positive staining, DNA fragmentation, and cleavage of PARP-1, which are all indicators of apoptosis. We also observed a decrease in Bcl-2 protein expression, an upstream effector molecule in the apoptotic pathway in A549 cells following Ad-MMP-2 infection. Of note, Bax protein expression was up regulated in these cells at 48h post-treatment; the ratio of Bax to Bcl-2 observed in the Ad-MMP-2 infected cells favored apoptosis.
In addition to degrading ECM components, MMPs are shown to confer apoptosis resistance by modulating Fas-mediated death signaling (Mitsiades et al., 2001). Decreased sensitivity to Fas-L-mediated apoptosis is a common trait shared by many different types of cancer cells, which provides cancer cells with critical survival advantages that ultimately lead to malignant progression (Khong and Restifo, 2002). Patient survival analysis revealed that the patients with negative Fas stage-III NSCLC tumors have a worse prognosis (Uramoto et al., 1999). This fact may indicate that Fas-mediated apoptosis inhibits tumor progression especially in advanced lung cancer patients. Our studies demonstrate that Fas and Fas-L expression increased significantly with Ad-MMP-2 treatment. Other studies have shown that cleavage of Fas by MMP-2 results in decreased sensitivity of HT-29 colon carcinoma cells to Fas-mediated apoptosis (Strand et al., 2004). Next, to determine whether Ad-MMP-2 affects death signaling downstream of the receptor-ligand interaction, we examined caspase activation. Two major pathways of caspase cascade activation have been characterized. One is initiated by ligation of death receptors and the activation of caspase-8. Caspase-8 is activated by the Fas/Fas-L signal, resulting in the caspase cascade (Medema et al., 1997). Two pathways are regulated by caspase-8 (Scaffidi et al., 1998). In one, pro-caspase-3 is directly cleaved by the active form of caspase-8 and transmits apoptotic signals by degrading target molecules. In the other pathway, caspase-8 degrades Bid, a member of the Bcl-2 family. The carboxyl fragment of Bid acts as an input signal to the mitochondria and is transported from the cytosol to the mitochondria where it induces the translocation of cytochrome-c (Luo et al., 1998). Next, cytochrome-c associated with Apaf-1, and pro-caspase-9 activates caspases-9 and -3 (Hengartner, 2000). Therefore, activated caspase-8 can switch on both the death receptor pathway and the mitochondrial pathway via Bid degradation. The results of the present study clearly show increased activity of caspases-3, -8 and -9 as well as Bid degradation and cytochrome-c translocation from the mitochondria membrane into the cytosol of A549 cells after Ad-MMP-2 infection. These findings indicate that both the mitochondrial and death receptor pathways are involved in apoptosis mediated by MMP-2 inhibition. To further determine the order of caspase activation, we treated the cells with caspase inhibitors prior to Ad-MMP-2 infection. Our results indicated that caspase-8 activity was required for caspase-9-cleavage. However, the caspase-9 inhibitor did not have any effect on caspase-8 cleavage. These results suggest that the Ad-MMP-2-induced apoptosis functions via the extrinsic II pathway.
MMPs and their endogenous inhibitors (TIMPs) are also involved in modulating cell surface receptors for signaling molecules. A549 cells showed a dramatic increase in non-glycosylated form of TIMP-3 with Ad-MMP-2 which was shown to induce apoptosis (Drynda et al., 2005). The cell-death domain of TIMP-3 has been localized to the amino terminus and its apoptotic effect appears to correlate with the inhibition of metalloproteinase activity (Bond et al., 2000). Several studies indicate that the loss of TIMP-3 in the tumor cell is an important event during tumorigenesis. Lack of TIMP-3 leads to an early unscheduled activation of MMP-2 and greater matrix degradation (Fata et al., 2001). Overexpression of TIMP-3 has been shown to restrict tumor cell invasiveness in vitro (Baker et al., 1999) and to inhibit tumor growth in vivo (Spurbeck et al., 2002). TIMP-3 silencing through promoter methylation is often found in gastric cancer (Kang et al., 2000). Recombinant TIMP-3 induces apoptosis in melanoma cell lines in vivo by a mechanism that directly involves stabilization of death receptors, including Fas-L (Ahonen et al., 2002). Our studies show that Ad-MMP-2 infection induces TIMP-3 expression resulting in apoptosis by a mechanism that involves increased expression of Fas and Fas-L. Quantitative analysis of Fas-L expression on the cell surface and sFas-L indicated that Ad-MMP-2 infection resulted in a dose-dependent increase in membrane-bound Fas-L and sFas-L, both of which can activate Fas (Tanaka et al., 1995).
In summary, we have provided evidence here that RNAi-mediated MMP-2 inhibition reduces tumor growth occurs as a result of apoptotic induction. Our data also indicate that inhibition of MMP-2 leads to accumulation of TIMP-3, which, in turn, results in the upregulation of Fas and Fas-L followed by the induction of the pro-apoptotic genes Bax and Bcl-xs. In addition, caspase-8 activation precedes caspase-9 activation, which leads to subsequent apoptotic events, including cytochrome-c release and caspase-3 activation in A549 cells. Furthermore, our data demonstrates that a similar pathway functions in the animal model. One possible mechanism of TIMP-3 upregulation in our study could be due to increased phosphorylation of ERK and JNK (data not shown) with Ad-MMP-2 as TIMP-3 induction was shown to be mediated by tyrosine and MAP kinase-signaling pathways (Liand and Zafarullah, 1998). Although the exact mechanisms that mediate the effect of TIMP-3 require further investigations, these data have important implications for understanding the relation between the MMP-2 down-regulation induced apoptosis via TIMP-3. The majority of clinical trials using synthetic MMP inhibitors have proven disappointing, principally owing to lack of efficacy and untoward side effects (Baker et al., 2002). However, survival benefits in some trials in pancreatic (Evans et al., 2001;) and gastric cancer (Ramhall et al., 2002) are indicative of the clinical value of MMP inhibition, and the basic principle of therapeutic MMP inhibition remains intact. In this context our study demonstrates that siRNA-mediated targeting of MMP-2 could have significant therapeutic value over small molecule inhibitors due to two reasons. We show that Ad-MMP-2 infection specifically inhibits MMP-2 expression reducing the local proteolysis, and induced TIMP-3 expression whose biological characteristics have also been exploited in cancer gene therapy.
MATERIALS & METHODS
Antibodies and Reagents
Primary antibodies against MMP-1, MMP-2, MMP-7, MMP-9, PARP1, GAPDH, HRP/FITC/Texas Red conjugated secondary antibodies were obtained from Biomeda (Foster City, CA), anti-caspase-8, -3, -9 were from Cell Signaling (Boston, MA), Cleaved Bid, Bax, Bcl-xL, Fas-L, FADD, Mouse IgG were from Santa Cruz Biotechnology (Santa Cruz, CA), Fas, cytochrome-c and MMP-2 inhibitor-1 were from Calbiochem (San Diego, CA). BCA reagent (Pierce, Rockford, IL), ECL reagent (Amersham Pharmacia, Piscataway, NJ), DAPI nuclear staining mounting solution (Vector Laboratories, Burlingame, CA), DAB peroxidase substrate (Sigma, St Louis, MO), TUNEL Detection kit (Roche Molecular Biochemicals Indianapolis, IN) and Annexin-V-FITC Apoptosis Detection Kit (BioVision Mountain View, CA) were used for this study.
Cell lines and culture conditions
A549 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA). Cells were maintained as a monolayer in RPMI 1640 medium (ATCC Manassas, VA) supplemented with 10% FBS, 50units/mL penicillin, and 50μg/mL streptomycin (Life Technologies, Inc., Frederick, MD), at 37°C in a humidified 5% CO2 atmosphere.
Adenoviral siRNA constructs and infection
The adenoviral siRNA for MMP-2 (Ad-MMP-2) and scrambled vector (Ad-SV) were constructed and amplified as described by us previously (Chetty et al., 2006). Viral titers were quantified as pfu/ml following infection of 293 cells. Titers were obtained for the viruses used in this work are Ad-SV, 7.6 × 1011 pfu/ml and Ad-MMP-2, 5.0 × 1011. The amount of infective adenoviral vector per cell (pfu/cell) in culture media was expressed as multiplicity of infection (MOI). Virus constructs were diluted in serum-free culture media to the desired concentration and added to cells and incubated at 37°C for 1h. The necessary amount of complete medium was then added and cells were incubated for the desired time periods.
Immunoblot analysis
A549 lung cancer cells were infected with mock, 100MOI of Ad-SV or the indicated MOI of Ad-MMP-2 for 48h. Whole cell lysates were by lysing cells in RIPA buffer as described by us previously (Chetty et al., 2006). For tissue samples, ground frozen tissue (∼ 0.2g) to a powder with liquid nitrogen and homogenized in 1ml of RIPA buffer, kept on ice for 15min, centrifuged at 13500rpm for 20min at 4°C and supernatants were collected. Equal concentration of protein were resolved by SDS-PAGE and transferred to a polyvinylidene fluoride membrane according to the manufacturer's instructions. After blocking with 5% nonfat dry milk and 0.1% Tween-20 in PBS, membranes were incubated with 1:1000 dilution of primary antibodies and horseradish peroxidase conjugate secondary antibodies were used to detect chemiluminescent signals using the ECL system. GAPDH levels served as loading control.
Animal experiments
Animal experiments were performed as described by previously (Chetty et al., 2006). Subcutaneous tumors were either frozen immediately at −80°C or fixed in 10% phosphate-buffered formaldehyde.
Statistical Analysis
Results were analyzed using a two-tailed Student's t test to assess statistical significance. Values of P<0.05 were considered statistically significant.
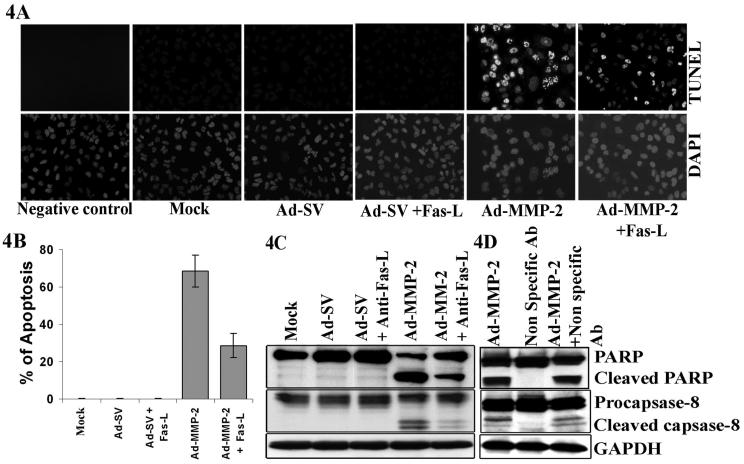
Figure 4. Antagonistic Fas-L antibodies block Ad-MMP-2-induced apoptosis.
A549 cells were incubated for 1h with 10μg/mL of antagonistic Fas-L antibody or non specific antibody before infection with 100MOI of Ad-SV and 100MOI of Ad-MMP-2. Forty-eight hours later, apoptosis was determined by (A) TUNEL staining. (B) Quantitation of TUNEL positive cells. The, bars indicate standard errors (±SE) from the mean of three separate experiments. (C) Immuno blot analysis for cleavage of caspase-8 and PARP, (D) Apoptosis was not antagonized with Non-specific antibody. Immuno blots are representative of three experiments.
ACKNOWLEDGEMENT
We thank Noorjehan Ali for technical assistance, Shellee Abraham for manuscript preparation and Diana Meister and Sushma Jasti for manuscript review.
This research was supported by National Cancer Institute Grant CA75557, CA92393, CA95058, CA116708 and N.I.N.D.S. NS47699, NS57529 and Caterpillar, Inc., OSF Saint Francis, Inc. Peoria, IL (to J.S.R.). and American Cancer Society Grant #06−03 (S.S.L.)
Supplementary Material
Reference List
- Ahonen M, la-Aho R, Baker AH, George SJ, Grenman R, Saarialho-Kere U, et al. Antitumor activity and bystander effect of adenovirally delivered tissue inhibitor of metalloproteinases-3. Mol Ther. 2002;5:705–715. doi: 10.1006/mthe.2002.0606. [DOI] [PubMed] [Google Scholar]
- Anand-Apte B, Bao L, Smith R, Iwata K, Olsen BR, Zetter B, et al. A review of tissue inhibitor of metalloproteinases-3 (TIMP-3) and experimental analysis of its effect on primary tumor growth. Biochem Cell Biol. 1996;74:853–862. doi: 10.1139/o96-090. [DOI] [PubMed] [Google Scholar]
- Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer. 1999;79:1347–1355. doi: 10.1038/sj.bjc.6690217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker AH, Zaltsman AB, George SJ, Newby AC. Divergent effects of tissue inhibitor of metalloproteinase-1, -2 or -3 overexpression on rat vascular smooth muscle cell invasion, proliferation, and death in vitro. TIMP-3 promotes apoptosis. J Clin Invest. 1998;101:1478–1487. doi: 10.1172/JCI1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. doi: 10.1177/10454411930040020401. [DOI] [PubMed] [Google Scholar]
- Bond M, Murphy G, Bennett MR, Amour A, Knauper V, Newby AC, et al. Localization of the death domain of tissue inhibitor of metalloproteinase-3 to the N terminus. Metalloproteinase inhibition is associated with proapoptotic activity. J Biol Chem. 2000;275:41358–41363. doi: 10.1074/jbc.M007929200. [DOI] [PubMed] [Google Scholar]
- Bond M, Murphy G, Bennett MR, Newby AC, Baker AH. Tissue inhibitor of metalloproteinase-3 induces a Fas-associated death domain-dependent type II apoptotic pathway. J Biol Chem. 2002;277:13787–13795. doi: 10.1074/jbc.M111507200. [DOI] [PubMed] [Google Scholar]
- Brand K, Baker AH, Perez-Canto A, Possling A, Sacharjat M, Geheeb M, et al. Treatment of colorectal liver metastases by adenoviral transfer of tissue inhibitor of metalloproteinases-2 into the liver tissue. Cancer Res. 2000;60:5723–5730. [PubMed] [Google Scholar]
- Brew K, Dinakarpandian D, Nagase H. Tissue inhibitors of metalloproteinases: evolution, structure and function. Biochim Biophys Acta. 2000;1477:267–283. doi: 10.1016/s0167-4838(99)00279-4. [DOI] [PubMed] [Google Scholar]
- Brown PD, Giavazzi R. Matrix metalloproteinase inhibition: a review of anti-tumour activity. Ann Oncol. 1995;6:967–974. doi: 10.1093/oxfordjournals.annonc.a059091. [DOI] [PubMed] [Google Scholar]
- Cawston TE. Proteinases and inhibitors. Br Med Bull. 1995;51:385–401. doi: 10.1093/oxfordjournals.bmb.a072968. [DOI] [PubMed] [Google Scholar]
- Chetty C, Bhoopathi P, Joseph P, Chittivelu S, Rao JS, Lakka SS. Adenovirus-mediated siRNA against MMP-2 suppresses tumor growth and lung metastasis in mice. Mol Cancer Ther. 2006;5:2289–2299. doi: 10.1158/1535-7163.MCT-06-0169. [DOI] [PubMed] [Google Scholar]
- Drynda A, Quax PH, Neumann M, van der Laan WH, Pap G, Drynda S, et al. Gene transfer of tissue inhibitor of metalloproteinases-3 reverses the inhibitory effects of TNF-alpha on Fas-induced apoptosis in rheumatoid arthritis synovial fibroblasts. J Immunol. 2005;174:6524–6531. doi: 10.4049/jimmunol.174.10.6524. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur J Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- Guedez L, Courtemanch L, Stetler-Stevenson M. Tissue inhibitor of metalloproteinase (TIMP)-1 induces differentiation and an antiapoptotic phenotype in germinal center B cells. Blood. 1998;92:1342–1349. [PubMed] [Google Scholar]
- Haro H, Crawford HC, Fingleton B, Shinomiya K, Spengler DM, Matrisian LM. Matrix metalloproteinase-7-dependent release of tumor necrosis factor-alpha in a model of herniated disc resorption. J Clin Invest. 2000;105:143–150. doi: 10.1172/JCI7091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Kang SH, Choi HH, Kim SG, Jong HS, Kim NK, Kim SJ, et al. Transcriptional inactivation of the tissue inhibitor of metalloproteinase-3 gene by dna hypermethylation of the 5'-CpG island in human gastric cancer cell lines. Int J Cancer. 2000;86:632–635. doi: 10.1002/(sici)1097-0215(20000601)86:5<632::aid-ijc5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Khong HT, Restifo NP. Natural selection of tumor variants in the generation of "tumor escape" phenotypes. Nat Immunol. 2002;3:999–1005. doi: 10.1038/ni1102-999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leighl NB, Paz-Ares L, Douillard JY, Peschel C, Arnold A, Depierre A, et al. Randomized phase III study of matrix metalloproteinase inhibitor BMS-275291 in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: National Cancer Institute of Canada-Clinical Trials Group Study BR.18. J Clin Oncol. 2005;23:2831–2839. doi: 10.1200/JCO.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem. 2003;278:8516–8525. doi: 10.1074/jbc.M210432200. [DOI] [PubMed] [Google Scholar]
- Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES, et al. Cytochrome-c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- Luo X, Budihardjo I, Zou H, Slaughter C, Wang X. Bid, a Bcl2 interacting protein, mediates cytochrome-c release from mitochondria in response to activation of cell surface death receptors. Cell. 1998;94:481–490. doi: 10.1016/s0092-8674(00)81589-5. [DOI] [PubMed] [Google Scholar]
- Medema JP, Toes RE, Scaffidi C, Zheng TS, Flavell RA, Melief CJ, et al. Cleavage of FLICE (caspase-8) by granzyme B during cytotoxic T lymphocyte-induced apoptosis. Eur J Immunol. 1997;27:3492–3498. doi: 10.1002/eji.1830271250. [DOI] [PubMed] [Google Scholar]
- Mitsiades N, Poulaki V, Mitsiades CS, Anderson KC. Induction of tumour cell apoptosis by matrix metalloproteinase inhibitors: new tricks from a (not so) old drug. Expert Opin Investig Drugs. 2001;10:1075–1084. doi: 10.1517/13543784.10.6.1075. [DOI] [PubMed] [Google Scholar]
- Nyormoi O, Mills L, Bar-Eli M. An MMP-2/MMP-9 inhibitor, 5a, enhances apoptosis induced by ligands of the TNF receptor superfamily in cancer cells. Cell Death Differ. 2003;10:558–569. doi: 10.1038/sj.cdd.4401209. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurbeck WW, Ng CY, Strom TS, Vanin EF, Davidoff AM. Enforced expression of tissue inhibitor of matrix metalloproteinase-3 affects functional capillary morphogenesis and inhibits tumor growth in a murine tumor model. Blood. 2002;100:3361–3368. doi: 10.1182/blood.V100.9.3361. [DOI] [PubMed] [Google Scholar]
- Strand S, Vollmer P, van den AL, Gottfried D, Alla V, Heid H, et al. Cleavage of CD95 by matrix metalloproteinase-7 induces apoptosis resistance in tumour cells. Oncogene. 2004;23:3732–3736. doi: 10.1038/sj.onc.1207387. [DOI] [PubMed] [Google Scholar]
- Uramoto H, Osaki T, Inoue M, Taga S, Takenoyama M, Hanagiri T, et al. Fas expression in non-small cell lung cancer: its prognostic effect in completely resected stage III patients. Eur J Cancer. 1999;35:1462–1465. doi: 10.1016/s0959-8049(99)00157-4. [DOI] [PubMed] [Google Scholar]
- Valente P, Fassina G, Melchiori A, Masiello L, Cilli M, Vacca A, et al. TIMP-2 over-expression reduces invasion and angiogenesis and protects B16F10 melanoma cells from apoptosis. Int J Cancer. 1998;75:246–253. doi: 10.1002/(sici)1097-0215(19980119)75:2<246::aid-ijc13>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.