Abstract
Although serotonin (5-HT) can interact with dopamine (DA) systems to negatively modulate the subjective and reinforcing effects of psychostimulants such as cocaine or 3,4-methyldioxymethamphetamine (MDMA, ecstasy), the long-term effects of exposure to psychostimulants on brain 5-HT systems are not well characterized. The present study assessed 5-HT transporter (SERT) availability using positron emission tomography (PET) in rhesus monkeys with the SERT-specific radioligand [11C]3-amino-4-(2-dimethylaminomethylphenylsulfanyl)-benzonitrile (DASB). SERT availability was assessed in regions of interest including the caudate nucleus, putamen, anterior cingulate cortex and cerebellum. [11C]DASB distribution volume ratios (DVRs) were calculated using the cerebellum as the reference region. DVRs were calculated in control monkeys and in cocaine or MDMA self-administering monkeys approximately 24 hours after the last self-administration (SA) session. SERT availability did not differ between monkeys with a history of MDMA SA and control monkeys in any region examined. In contrast, monkeys with a history of cocaine SA showed significantly higher levels of SERT availability in the caudate nucleus and putamen compared to control subjects. These results suggest that chronic SA of cocaine, but not MDMA, leads to alterations in serotonergic function in brain areas relevant to drug abuse. The higher level of SERT availability in cocaine-experienced monkeys may lead to a reduced inhibitory tone of 5-HT on the DA system which may explain, in part, differences in the abuse liability between cocaine and MDMA.
Keywords: cocaine, MDMA, self-administration, PET, serotonin transporters, nonhuman primates
INTRODUCTION
Cocaine and 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) are commonly abused psychostimulants with similar, yet distinct mechanisms of action. Cocaine increases synaptic levels of dopamine (DA), serotonin (5-HT) and norepinephrine (NE) via blockade of the presynaptic transporters (DAT, SERT and NET, respectively; Bennett et al, 1995). MDMA also inhibits the uptake of monoamines at the DAT, SERT and NET (Rothman et al, 2001). However, MDMA also serves as a substrate for these monoamine transporters to stimulate non-exocytotic release of DA, 5-HT and NE (Rudnick and Clark, 1993; Rothman et al, 2001). Another difference between cocaine and MDMA is affinity for DAT, SERT and NET. Cocaine binds with relatively equal affinity at all three transporters (Bennett et al, 1995), whereas MDMA has a 10-fold higher affinity for SERT compared to DAT or NET (Steele et al, 1987; Battaglia et al, 1988). Although cocaine and MDMA both increase DA, which is thought to be the primary mediator of reinforcement (Di Chiara and Imperato, 1988), differences in self-administration (SA) have been reported. For example, MDMA does not maintain rates of responding as high as cocaine in rodents (Ratzenboeck et al, 2001) or nonhuman primates (Beardsley et al, 1986) under fixed-ratio (FR) schedules. Furthermore, using a progressive-ratio (PR) schedule, Lile et al(2005) found that MDMA had lower reinforcing strength than cocaine in nonhuman primates. Thus, behavioral differences in measures of abuse liability exist between cocaine and MDMA.
Increasing brain 5-HT activity can attenuate the behavioral and neurochemical effects of cocaine and MDMA. For example, 5-HT uptake inhibitors have been shown to reduce cocaine and MDMA-induced elevations in extracellular levels of DA in the caudate nucleus (Koch and Galloway, 1997; Czoty et al, 2002). SERT inhibitors and substrates have also been shown to decrease cocaine SA in rats and nonhuman primates (Richardson and Roberts, 1991; Kleven and Woolverton, 1993; Czoty et al, 2002; Glatz et al, 2002) and cue-induced reinstatement (Burmeister et al, 2003) in rodents. Clinically, 5-HT uptake inhibitors have been shown to attenuate the subjective effects of cocaine (Walsh et al, 1994) and MDMA (Tancer and Johanson, 2007). Fenfluramine, a SERT substrate, can also reduce cocaine craving (Buydens-Branchey et al, 1998). These studies support an inhibitory role of the 5-HT system on the neurochemical and behavioral effects of cocaine and MDMA.
A critical question for better understanding drug addiction is how the brain changes in response to long-term drug exposure. Chronic cocaine or MDMA use may lead to differential disruptions of brain serotonergic systems which would suggest different treatment strategies during abstinence. For instance, in studies using single photon emission computed tomography (SPECT), acutely abstinent cocaine-dependent patients had higher SERT binding in striatal brain regions compared to non-drug abusing control subjects (Jacobsen et al, 2000). Also, cocaine overdose victims had higher SERT binding in the caudate and putamen (Mash et al, 2000). In contrast, MDMA users had lower SERT availability within the caudate, anterior cingulate cortex (ACC), thalamus and cortical regions as measured by positron emission tomography (PET; McCann et al, 1998; Buchert et al, 2003; Thomasius et al, 2003; Buchert et al, 2004; McCann et al, 2005; Buchert et al, 2006). A major caveat of these studies is that human drug abusers frequently have used multiple drugs which can complicate identification of the precise contributions of cocaine or MDMA to alterations in SERT availability (Gouzoulis-Mayfrank and Daumann, 2006).
The present study was designed to examine SERT availability in the caudate nucleus, putamen and ACC using PET in nonhuman primates with extensive histories of either cocaine (n=4) or (±) MDMA (n=4) SA in comparison with drug-naïve controls responding under a schedule of food reinforcement (n=4). These regions of interest (ROI) were selected based on their association with the reinforcing effects of psychostimulants (Di Chiara and Imperato, 1988; Goldstein and Volkow, 2002). For these studies, the radiotracer [11C]3-amino-4-(2-dimethylaminomethyl-phenylsulfanyl)-benzonitrile (DASB) was used. [11C]DASB has a higher affinity for SERT compared to DAT and NET and appears to be a more selective and improved radioligand for the SERT compared to [11C]McN5652 (Houle et al, 2000; Szabo et al, 2002). We hypothesized that SERT availability would be higher in monkeys self-administering cocaine and lower in monkeys self-administering MDMA compared to controls.
METHODS
Subjects and Apparatus
Twelve adult male rhesus monkeys (Macaca mulatta) served as subjects. Subjects R-1268, R-1326, R-1346 and R-1427 had self-administered cocaine (Table 1; Czoty et al, 2006), but had limited experience self-administering other psychostimulants (Lile et al, 2000, 2003, 2005). Subjects R-1361, R-1496, R-1498 and R-1499 had recently self-administered MDMA (Table 1) and subjects R-1523, R-1524, R-1525 and R-1526 were experimentally and drug-naïve at the start of this study. MDMA and control monkeys were individually housed in stainless steel cages with water available ad libitum and had visual and auditory contact with each other. Monkeys were fitted with a nylon collar (Primate Products, Redwood City, CA) and trained to sit calmly in a standard primate restraint chair using a specially designed stainless steel pole that attached to the collar. Experiments were conducted in ventilated and sound-attenuating chambers (150 × 74 × 76 cm, Med Associates, East Fairfield, VT) designed to accommodate a primate chair (Primate Products). Monkeys were weighed weekly and fed enough food daily (LabDiet Monkey Chow and fresh fruit) to maintain body weights at approximately 95% of free-feeding levels. For the cocaine SA group, monkeys were individually housed in sound-attenuating chambers (91 cm3; Plas Labs, Lansing, MI). The front wall of each cubicle was constructed of Plexiglas to allow the monkey visual access to the laboratory. Each cubicle was equipped with two response levers (BRS/LVE, Beltsville, MD); only the right lever was used in the present studies. Four stimulus lights, alternating white and red, were located in a horizontal row above each lever. Each animal was fitted with a stainless-steel restraint harness and spring arm (Restorations Unlimited, Chicago, IL) that attached to the rear of the cubicle. Monkeys were weighed monthly and fed enough food daily (LabDiet Monkey Chow and fresh fruit) to maintain body weights at approximately 95% of free-feeding levels and had water available ad libitum. All procedures were performed in accordance with established practices as described in the National Institutes of Health Guide for Care and Use of Laboratory Animals. In addition, all procedures were reviewed and approved by the Animal Care and Use Committee of Wake Forest University. Environmental enrichment was provided as outlined in the Animal Care and Use Committee of Wake Forest University Non-Human Primate Environmental Enrichment Plan.
Table 1.
Self-administration histories (mg/kg) prior to [11C]DASB PET studies
|
1Weekly MDMA Intake |
Total MDMA Intake |
2Months of SA | (−) Cocaine | |
|---|---|---|---|---|
| MDMA Group | ||||
| R-1361 | 22.2 | 107.46 | 6 | 118.33 |
| R-1496 | 6.2 | 138.44 | 18 | 69.75 |
| R-1498 | 6.8 | 96.63 | 8 | 80.01 |
| R-1499 | 3.9 | 141.74 | 16 | 0.2 |
| Mean (± SEM) | 121.07 (13.0) | 12 (3.4) | 67.07 (28.4) | |
|
1Weekly Cocaine Intake |
Total Cocaine Intake |
2Months of SA | (±) MDMA | |
| Cocaine Group | ||||
| R-1268 | 1.8 | 1028.79 | 10 | 0.00 |
| R-1326 | 1.83 | 537.27 | 12 | 129.55 |
| R-1346 | 24 | 645.3 | 5 | 40.61 |
| R-1427 | 2.19 | 759.04 | 14 | 0.00 |
| Mean (± SEM) | 742.60 (121.9) | 10.25 (2.2) | 42.54 (35.3) |
Weekly Intake (mg/kg) refers to drug intake over the 5 days before the PET study.
“Months of SA” refers to the number of months each subject had been exclusively self-administering either MDMA or cocaine.
All eight monkeys in the SA groups had been exposed to cocaine at some time in their history (Table 1). However, monkeys in the cocaine SA group had an average lifetime intake ten times higher than monkeys in the MDMA group (approximately 743 vs. 67 mg/kg; Table 1). In addition, monkeys in the MDMA SA group had not self-administered cocaine for at least 9 months prior to the PET imaging studies. Monkeys in the MDMA group had mean MDMA intakes of 121.07 mg/kg; range of intakes was 100-140 mg/kg (Table 1). On average, monkeys in the cocaine and MDMA groups had been self-administering the primary drug of interest for 10 and 12 months, respectively (Table 1).
Catheter Implantation
Under sterile conditions, each monkey was surgically prepared with an indwelling intravenous catheter and vascular access port (Access Technologies, Skokie, IL), placed into a major vein (internal or external jugular, femoral or brachial) as previously described (Czoty et al, 2006).
Self-administration conditions
Three groups of monkeys (n=4/group) were studied: cocaine SA, MDMA SA and food-reinforced control monkeys. For monkeys in the cocaine SA group, cocaine doses (saline, 0.03 – 0.3 mg/kg/inj) were made available, in random order, under a PR schedule of reinforcement as described previously (Czoty et al, 2006). Briefly, under the PR schedule, white lights were illuminated above the right lever and 50 responses resulted in a 10-s injection, extinguishing of white lights and illumination of red lights for 10-s. A 10-min timeout (TO) period, during which no lights were illuminated and responding had no scheduled consequences, followed each injection. The response requirement for subsequent injections was determined by the exponential equation used by Richardson and Roberts (1996): ratio = 5 × exponent (SR# × 0.2) − 5. Sessions ended when 2-h elapsed without an injection. For monkeys in the MDMA SA group, responding was maintained under a concurrent FR 30 schedule of MDMA and food pellet presentation. Briefly, 30 consecutive responses on one lever (counterbalanced across monkeys) activated the infusion pump, resulting in a MDMA injection; 30 consecutive responses on the other lever resulted in presentation of a banana-flavored food pellet. Each completion of the FR requirement was followed by a 30-s TO. If a response was made on the alternate lever before an FR 30 was completed, the response requirement was reset. The session ended after 30 total reinforcers had been earned or 60-min had elapsed. MDMA doses (saline, 0.03–0.3 mg/kg/inj) were examined in random order for at least five consecutive sessions and until responding was deemed stable (% injection-lever responding ± 20% of the mean of three consecutive sessions with no trend). For monkeys in the control group, responding was maintained under an FR 30 schedule of food pellet presentation; a 30-s TO followed each food presentation and sessions ended after a maximum of 30 reinforcers or after 1-h.
MR and PET imaging
Magnetic resonance imaging (MRI) scans were acquired for each monkey. Approximately 20-min before the MRI, subjects were anesthetized with ketamine (10 mg/kg, i.m.) and transported to the MRI facility. Anesthesia was maintained during the scanning procedure with ketamine supplements when necessary. T1- weighted images of the entire brain were acquired with a 1.5-Tesla GE Signa NR scanner (GE Medical Systems). Images were used to anatomically define ROI, including the caudate nucleus, putamen, ACC and cerebellum, for later registration with PET images.
On the day of a PET study, no behavioral experiments were conducted. Prior to the start of the PET study, monkeys were anesthetized with 8 mg/kg ketamine and intubated. Anesthesia was maintained throughout the scan by inhaled isoflurane (1.5%). A Catheter (22-gauge angiocath; Becton Dickinson Vascular Access, Sandy, Utah, USA) was placed in an external vein by percutaneous stick for administration of [11C]DASB at the start of the scan and delivery of lactated ringer's solution (i.v.) to the monkey throughout the study. A paralytic (0.07 mg/kg vecuronium bromide) was administered i.v. and respiration was maintained by a ventilator. Supplemental doses of vecuronium bromide (0.1 mg/hr) were administered throughout the study. [11C]DASB was injected at the start of the scan, followed by 3 ml heparinized saline. The average dose injected and specific activity for each group was: Control (8.3 mCi; 1090±386 mCi/μmole), MDMA (8.5 mCi; 676 ± 140 mCi/μmole), Cocaine (7.5 mCi; 3189 ± 943 mCi/μmole).
Each subject was imaged for 90 minutes from the time of radioligand injection on a GE Advance NXi PET Scanner (General Electric Systems, Milwaukee, Wisc.). This device provides 35 contiguous transverse slices with a 4.25 mm center-to-center spacing over a 15.2 cm axial field of view (see DeGrado, 1994). The first five frames of each study's PET image data were then added together. This summed image represents tracer uptake in the early part of the study and approximates a blood flow image. This image was then registered to the animal's MRI using the AIR algorithm (Woods et al, 1993) after extracting the brain from the MRI using the method of Smith (2002). This method provides excellent registration of cortical and subcortical regions. Spherical ROIs for the caudate nucleus, putamen, ACC and cerebellum were then drawn on each subject's MRI and transferred to their co-registered PET scans. Time-activity curves were generated and distribution volumes ratios (DVR) were calculated using the cerebellum, a region relatively devoid of SERT, as the reference region and the graphical method of Logan et al (1996). This method has previously been shown to provide valid, reproducible measures of SERT binding potentials with low variance between subjects (Meyer et al, 2004). For all regions, DVRs from the right and left sides were not statistically different and were averaged.
Statistical Analysis
The primary dependent variable examined was the DVR for each ROI (caudate nucleus, putamen and ACC). DVRs across all three experimental groups were analyzed using a one-way ANOVA with a post-hoc Tukey's test for each ROI. In all cases, differences were considered significant at the 95% level of confidence (p < 0.05).
RESULTS
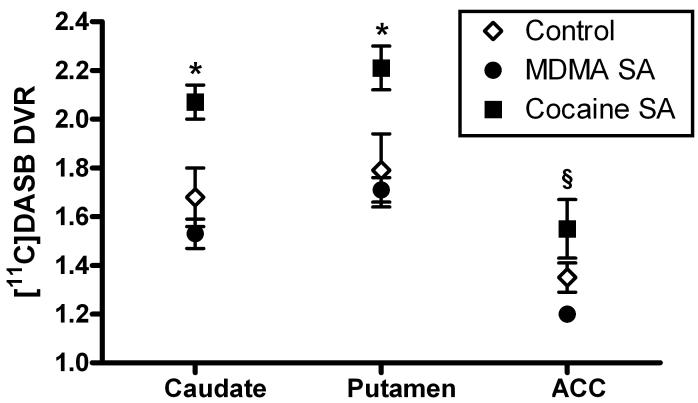
The mean weekly intakes of MDMA ranged from approximately 4.0 to 22 mg/kg and for cocaine ranged from approximately 2.0 to 24 mg/kg (Table 1). Individual and group mean DVRs are shown in Table 2. ANOVA revealed a main effect of SA history in the caudate nucleus (F2,9=10.99, p<0.01), putamen (F2,9=6.34, p<0.05) and ACC (F2,9=4.84, p<0.05). Post-hoc analysis indicated significantly higher SERT availability in monkeys from the cocaine SA group compared to both MDMA SA and control monkeys in the caudate nucleus and putamen (p<0.05; Fig. 1). There were no statistically significant differences between monkeys in the MDMA SA group and control subjects in these brain regions. In the ACC, SERT availability was significantly higher in the cocaine SA group compared to the MDMA SA group (p<0.05). In the ACC, mean DVRs were not different from the control group in either cocaine or MDMA SA monkeys
Table 2.
Individual and Group DVRs for each Region of Interest
| Caudate nucleus |
Putamen | Anterior cingulate cortex |
|
|---|---|---|---|
| Control Group | |||
| R-1523 | 1.8 | 2.0 | 1.46 |
| R-1524 | 1.39 | 1.36 | 1.19 |
| R-1525 | 1.92 | 1.97 | 1.43 |
| R-1526 | 1.61 | 1.82 | 1.31 |
| Mean (± SEM) | 1.68 (0.12) | 1.79 (0.15) | 1.35 (0.06) |
| MDMA Group | |||
| R-1361 | 1.43 | 1.63 | 1.18 |
| R-1496 | 1.69 | 1.83 | 1.28 |
| R-1498 | 1.48 | 1.62 | 1.18 |
| R-1499 | 1.5 | 1.77 | 1.17 |
| Mean (± SEM) | 1.53 (0.06) | 1.71 (0.05) | 1.20 (0.03) |
| % Difference | −9.2 (3.4) | −4.3 (2.9) | −10.9 (1.9) |
| Cocaine Group | |||
| R-1268 | 2.05 | 2.43 | 1.44 |
| R-1326 | 2.26 | 2.46 | 1.89 |
| R-1346 | 2.02 | 2.21 | 1.51 |
| R-1427 | 1.94 | 2.02 | 1.35 |
| Mean (± SEM) | 2.07 (0.07) | 2.21 (0.09) | 1.55 (0.12) |
| % Difference | 23.1 (4.1) | 27.4 (5.8) | 14.6 (8.8) |
Figure 1.
SERT availability in the caudate nucleus, putamen and anterior cingulate cortex (ACC) in monkeys with extensive histories of cocaine or MDMA self-administration (SA) or food reinforcement (Control). Data are shown as mean (±SEM) distribution volume ratios (DVR) for each group (n=4). * indicates significantly different from control and MDMA SA groups (p<0.05). § indicates significantly different from MDMA SA group only (p<0.05).
DISCUSSION
The goal of the present study was to compare SERT availability in monkeys with histories of cocaine or MDMA exposure to drug-naïve control subjects using PET imaging. Compared to controls, monkeys with long-term cocaine SA histories had significantly higher levels of SERT availability in the caudate nucleus and putamen. In contrast, levels of SERT availability in monkeys with long-term MDMA SA histories were not significantly different from control subjects. These data provide evidence for differences in the neuropharmacological consequences of long-term cocaine and MDMA exposure, which may explain differences in the reinforcing strength of cocaine compared to MDMA (Lile et al, 2005).
In the present study, a history of cocaine SA was associated with higher levels of SERT, extending preclinical rodent studies (Cunningham et al, 1992) to nonhuman primates and to PET imaging. Acutely abstinent cocaine-dependent patients (Jacobsen et al, 2000) or cocaine overdose victims (Mash et al, 2000) also had higher SERT binding in the anterior and posterior striatal regions compared to controls. Interestingly, these higher levels of SERT parallel the effects of cocaine on DAT densities reported in monkeys with long-term cocaine SA histories (Letchworth et al, 2001) and humans (Little et al, 1993). The present results are consistent with the hypothesis that long-term cocaine exposure may lead to a reduced 5-HT tone due to increased SERT function. However, randomized clinical trials have failed to show any effectiveness of 5-HT uptake inhibitors alone in treating cocaine dependence (Grabowski et al, 1995; Schmitz et al, 2001). Since both DAT and SERT are higher after long-term cocaine SA histories in nonhuman primates (Letchworth et al, 2001; present study) and humans (Little et al, 1993; Mash et al, 2000), pharmacotherapies that target both these monoamine transporters might be more efficacious for treating cocaine dependence (cf. Rothman and Baumann, 2003 and Howell et al, 2007).
In contrast to cocaine, long-term MDMA SA did not affect SERT availability. Human imaging studies involving active ecstasy abusers and controls have reported conflicting results regarding SERT availability in brain regions similar to those examined in the present study. Two radioligands, [11C]McN5652 and [11C]DASB, have been used to examine SERT availability in MDMA users. Studies using [11C]McN5652 have reported either decreases (McCann et al, 1998; Buchert et al, 2003; Thomasius et al, 2003; Buchert et al, 2004) or a lack of effect (Buchert et al, 2006; Thomasius et al, 2006) in SERT availability in the caudate, putamen or ACC. McCann et al (2005) compared [11C]McN5652 and [11C]DASB in the same MDMA users and found a high correlation and no significant differences between the radiotracers. This study reported significantly lower levels of SERT availability in the ACC, but not the caudate or putamen, compared to control subjects. Thus, the results of the present study are consistent with data from human subjects demonstrating no effect of MDMA on SERT availability in the caudate nucleus or putamen (McCann et al, 2005). Importantly, the present study demonstrates no effect of MDMA under conditions in which a history of cocaine exposure resulted in higher levels of SERT availability compared to controls.
Because SERT availability is influenced by both numbers of transporters and levels of competition from endogenous 5-HT, the functional tone of the serotonergic system is difficult to determine using PET data alone. There is a paucity of studies examining the effects of endogenous 5-HT on DASB binding. In vitro evidence suggests that at relatively high concentrations, 5-HT can inhibit DASB binding (Hummerich et al, 2004). In contrast, in vivo evidence for 5-HT competing with DASB at the SERT is conflicting (Ginovart et al, 2003; Milak et al, 2005; Praschak-Rieder et al, 2005; Talbot et al, 2005). Thus, the influence of endogenous levels of 5-HT on differences in SERT availability between cocaine SA monkeys and monkeys from the MDMA or control groups cannot be ruled out. Future studies using in vivo microdialysis and PET imaging examining 5-HT levels in nonhuman primates self-administering cocaine and MDMA could help explain how chronic drug exposure alters 5-HT levels and influences [11C]DASB measures.
Previous research in nonhuman primates treated with MDMA (5 mg/kg, twice daily for four days) showed acute (9-40 days) reductions in SERT availability, as measured by PET, in the caudate nucleus, putamen and cortical regions, with decreases in cortical regions still apparent 13 months after MDMA (Scheffel et al, 1998; Szabo et al, 2002). Rhesus monkeys treated with a similar dosing regimen as in Scheffell et al (1998) also showed acute (4-31 days) decreases in SERT density as measured by SPECT (Reneman et al, 2002). Nonetheless, monkeys from the present study had only modest, non-significantly lower SERT availability (approximately 7%) compared to controls. Reasons for the discrepancy between the earlier reports and the present study may include differences in MDMA dose and regimen. In the studies involving nonhuman primates showing lower SERT availability, MDMA was administered by the experimenter (5 mg/kg, sc, twice daily for four consecutive days) rather than self-administered (average total session intake ranged from 0.3 mg/kg to 2.3 mg/kg dependent upon the dose available) as in the present study. Contingent vs. non-contingent drug administration can profoundly influence the behavioral and neurochemical effects of drugs (Dworkin et al, 1995; Hemby et al, 1997). In fact, Fantegrossi (2007) has argued that the non-contingent, experimenter-administered dosing regimens employed in the preclinical studies described above do not model the human abuse condition and that the model of intravenous self-administration is more appropriate for determining the neurochemical consequences of MDMA exposure. Moreover, using “effect scaling” to determine the dose administered to rats, MDMA does not significantly decrease SERT protein expression (Wang et al, 2004, 2005). Therefore, it is possible that the method and dosage of MDMA administration may have contributed to discrepancies between present and previous observations. The use of MDMA doses that are more analogous to humans based on self-administration studies (present study; Fantegrossi et al, 2004) or using effect scaling (Wang et al, 2004; 2005) might be more appropriate in ascertaining the neurochemical consequences of MDMA.
Another potential reason for statistically insignificant differences between monkeys in the MDMA SA group and control subjects observed in our study may relate to the amount of drug exposure or that the present study was not a within-subjects design. One advantage of using nonhuman primates is the ability to perform multiple scans within subject over prolonged periods of time which was not used in the present study due to experimental constraints. It is possible, although unlikely (see Fantegrossi et al, 2004), that greater intakes of MDMA than were self-administered in the present study are necessary for neuroadaptations to occur (see Table 1).
It should also be mentioned that although all subjects had been self-administering either cocaine or MDMA for at least six months prior to assessing SERT availability, previous exposure to other drugs could be a confound. However, previous preclinical and clinical studies would argue against this point since differences were reported in cocaine exposed rodents (Cunningham et al, 1992) and humans (Jacobsen et al, 2000; Mash et al, 2000). Also, one potential reason why we did not detect significantly lower levels of SERT availability in select ROI in the monkeys self-administering MDMA could be related to their prior exposure to cocaine, which may have increased SERT availability prior to the predicted MDMA-induced reductions. This might also explain why Fantegrossi et al (2004) did not observe lower 5-HT neurochemical markers since those monkeys were “co-abusing” MDMA and cocaine. Therefore, future longitudinal, PET imaging studies are warranted to address the issue of how cocaine-induced changes in SERT are affected by subsequent MDMA exposure.
ACKNOWLEDGEMENTS
This research was supported by National Institute on Drug Abuse grants DA-06634 (MAN), DA-14637 (MAN) and DA-020281 (MLB). We acknowledge the technical assistance of Jennifer Martelle, Susan Nader, Kimberly Black and Jessica Stukes and appreciate the insightful comments of Beth Reboussin, Ph.D, Jon Sprague, Ph.D., and Linda Porrino, Ph.D. on an earlier version of this manuscript.
Footnotes
DISCLOSURE/CONFLICT OF INTEREST
We declare that there is no conflict of interest for any of the authors.
REFERENCES
- Battaglia G, Brooks BP, Kulsakdinun C, De Souza EB. Pharmacologic profile of MDMA (3,4-methylenedioxymethamphetamine) at various brain recognition sites. Eur J Pharmacol. 1988;149:159–163. doi: 10.1016/0014-2999(88)90056-8. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. Self-administration of methylenedioxymethamphetamine (MDMA) by rhesus monkeys. Drug Alcohol Depend. 1986;18:149–57. doi: 10.1016/0376-8716(86)90047-5. [DOI] [PubMed] [Google Scholar]
- Bennett BA, Wichems CH, Hollingsworth CK, Davies HM, Thornley C, Sexton T, et al. Novel 2-substituted cocaine analogs: uptake and ligand binding studies at dopamine, serotonin and norepinephrine transport sites in the rat brain. J Pharmacol Exp Ther. 1995;272:1176–86. [PubMed] [Google Scholar]
- Buchert R, Thomasius R, Nebeling B, Petersen K, Obrocki J, Jenicke L, et al. Long-term effects of “ecstasy” use on serotonin transporters of the brain investigated by PET. J Nucl Med. 2003;44:375–84. [PubMed] [Google Scholar]
- Buchert R, Thomasius R, Wilke F, Petersen K, Nebeling B, Obrocki J, et al. A Voxel-based PET investigation of the long-term effects of “Ecstasy” consumption on brain serotonin transporters. Am J Psychiatry. 2004;161:1181–9. doi: 10.1176/appi.ajp.161.7.1181. [DOI] [PubMed] [Google Scholar]
- Buchert R, Thomasius R, Petersen K, Wilke F, Obrocki J, Nebeling, et al. Reversibility of ecstasy-induced reduction in serotonin transporter availability in polydrug ecstasy users. Eur J Nucl Med Mol Imaging. 2006;33:188–99. doi: 10.1007/s00259-005-1850-8. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren Em, Neisewander JL. Effects of fluoxetine and d-fenfluramine on cocaine-seeking behavior in rats. Psychopharmacology. 2003;168:146–54. doi: 10.1007/s00213-002-1307-8. [DOI] [PubMed] [Google Scholar]
- Buydens-Branchey L, Branchey M, Hudson J, Rothman M, Fergeson P, McKernin C. Effect of fenfluramine challenge on cocaine craving in addicted male users. Am J Addict. 1998;7:142–55. [PubMed] [Google Scholar]
- Cunningham KA, Paris JM, Goeders NE. Chronic cocaine enhances serotonin autoregulation and serotonin uptake binding. Synapse. 1992;11:112–123. doi: 10.1002/syn.890110204. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther. 2002;300:831–7. doi: 10.1124/jpet.300.3.831. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Martelle JL, Nader MA. Influence of abstinence and conditions of cocaine access on the reinforcing strength of cocaine in nonhuman primates. Drug Alcohol Depend. 2006;85:213–20. doi: 10.1016/j.drugalcdep.2006.04.009. [DOI] [PubMed] [Google Scholar]
- DeGrado TR, Turkington TG, Williams JJ, Stearns CW, Hoffman JM, Coleman RE. Performance characteristics of a whole-body PET scanner. J Nucl Med. 1994;35:1398–406. [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin SI, Mirkis S, Smith JE. Response-dependent versus response-independent presentation of cocaine: differences in the lethal effects of the drug. Psychopharmacology. 1995;117:262–6. doi: 10.1007/BF02246100. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE, Woolverton WL, Kilbourn M, Sherman P, Yuan J, Hatzidmitriou G, et al. Behavioral and neurochemical consequences of long-term self-administration of MDMA and its enantiomers in rhesus monkeys. Neuropsychopharmacology. 2004;29:1270–81. doi: 10.1038/sj.npp.1300442. [DOI] [PubMed] [Google Scholar]
- Fantegrossi WE. Reinforcing effects of methylenedioxy amphetamine congeners in rhesus monkeys: are intravenous self-administration experiments relevant to MDMA neurotoxicity? Psychopharmacology. 2007;189:471–82. doi: 10.1007/s00213-006-0320-8. [DOI] [PubMed] [Google Scholar]
- Glatz AC, Ehrlich M, Bae RS, Clarke MJ, Quinlan PA, Brown EC, et al. Inhibition of cocaine self-administration by fluoxetine or d-fenfluramine combined with phentermine. Pharmacol Biochem Behav. 2002;71:197–204. doi: 10.1016/s0091-3057(01)00657-8. [DOI] [PubMed] [Google Scholar]
- Ginovart N, Wilson AA, Meyer JH, Hussey D, Houle S. [11C]-DASB, a Tool for In Vivo Measurement of SSRI-Induced Occupancy of the Serotonin Transporter: PET Characterization and Evaluation in Cats. Syanpse. 2003;47:123–33. doi: 10.1002/syn.10155. [DOI] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. Am J Psychiatry. 2002;159:1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouzoulis-Mayfrank E, Daumann J. The confounding problem of polydrug use in recreational ecstasy/MDMA users: a brief overview. J Psychopharmacol. 2006;20:188–93. doi: 10.1177/0269881106059939. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Elk R, Schmitz J, Davis C, Creson D, et al. Fluoxetine is ineffective for the treatment of cocaine dependence or current opiate and cocaine dependence: two placebo-controlled double-blind trials. J Clin Psychopharmacol. 1995;15:163–74. doi: 10.1097/00004714-199506000-00004. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology. 1997;133:7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Houle S, Ginovart N, Hussey D, Meyer JH, Wilson AA. Imaging the serotonin transporter with positron emission tomography: initial human studies with [11C] DAPP and [11C] DASB. Eur J Nucl Med. 2000;27:1719–22. doi: 10.1007/s002590000365. [DOI] [PubMed] [Google Scholar]
- Howell LL, Carroll FI, Votaw JR, Goodman MM, Kimmel HL. Effects of combined dopamine and serotonin transporter inhibitors on cocaine self-administration in rhesus monkeys. J Pharmacol Exp Ther. 2007;320:757–65. doi: 10.1124/jpet.106.108324. [DOI] [PubMed] [Google Scholar]
- Hummerich R, Geischl G, Ehrlichmann W, Machulla HJ, Heinz A, Schloss P. DASB – in vitro binding characteristics on human recombinant monoamine transporters with regard to its potential as positron emission tomography (PET) tracer. J Neurochem. 2004;90:1218–26. doi: 10.1111/j.1471-4159.2004.02585.x. [DOI] [PubMed] [Google Scholar]
- Jacobsen LK, Staley JK, Malison RT, Zoghbi SS, Seibyl JP, Kosten TR, et al. Elevated central serotonin transporter binding availability in acutely abstinent cocaine-dependent patients. Am J Psychiatry. 2000;157:1134–40. doi: 10.1176/appi.ajp.157.7.1134. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Woolverton WL. Effects of three monoamine reuptake inhibitors on behavior maintained by cocaine or food presentation in rhesus monkeys. Drug Alcohol Depend. 1993;31:149–58. doi: 10.1016/0376-8716(93)90067-z. [DOI] [PubMed] [Google Scholar]
- Koch S, Galloway MP. MDMA induced dopamine release in vivo: role of endogenous serotonin. J Neural Transm. 1997;104:135–46. doi: 10.1007/BF01273176. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lile JA, Morgan D, Freedland CS, Sinnott RS, Davies HM, Nader MA. Self-administration of two long-acting monoamine transport blockers in rhesus monkeys. Psychopharmacology. 2000;152:414–421. doi: 10.1007/s002130000554. [DOI] [PubMed] [Google Scholar]
- Lile JA, Morgan D, Birmingham AM, Wang Z, Woolverton WL, Davies HM, et al. The reinforcing efficacy of psychostimulants in rhesus monkeys: the role of pharmacokinetics and pharmacodynamics. J Pharmacol Exp Ther. 2003;307:356–66. doi: 10.1124/jpet.103.049825. [DOI] [PubMed] [Google Scholar]
- Lile JA, Ross JT, Nader MA. A comparison of the reinforcing efficacy of 3,4-methylenedioxymethamphetamine (MDMA, “ecstasy”) with cocaine in rhesus monkeys. Drug Alcohol Depend. 2005;78:135–140. doi: 10.1016/j.drugalcdep.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Little KY, Kirkman JA, Carroll FI, Clark TB, Duncan GE. Cocaine use increases [3H]WIN 35428 binding sites in human striatum. Brain Res. 1993;628:17–25. doi: 10.1016/0006-8993(93)90932-d. [DOI] [PubMed] [Google Scholar]
- Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- Mash DC, Staley JK, Izenwasser S, Basile M, Ruttenber AJ. Serotonin transporters upregulate with chronic cocaine use. J Chem Neuroanat. 2000;20:271–280. doi: 10.1016/s0891-0618(00)00102-2. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Houle S, Sagrati S, Carella A, Hussey DF, Ginovart N, et al. Brain serotonin transporter binding potential measured with carbon 11-labeled DASB positron emission tomography: Effects of major depressive episodes and severity of dysfunctional attitudes. Arch Gen Psychiatry. 2004;61:1271–1279. doi: 10.1001/archpsyc.61.12.1271. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA. Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet. 1998;352:1433–7. doi: 10.1016/s0140-6736(98)04329-3. [DOI] [PubMed] [Google Scholar]
- McCann UD, Szabo Z, Seckin E, Rosenblatt P, Mathews WB, Ravert HT, et al. Quantitative PET Studies of the Serotonin Transporter in MDMA Users and Controls Using [(11)C]McN5652 and [(11)C]DASB. Neuropsychopharmacology. 2005;30:1741–50. doi: 10.1038/sj.npp.1300736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milak MS, Ogden RT, Vinocur DN, Van Heertum RL, Cooper TB, Mann JJ, et al. Effects of Tryptophan Depletion on the Binding of [11C]-DASB to the Serotonin Transporter in Baboons: Response to acute Serotonin Deficiency. Biol Psychiatry. 2005;57:102–6. doi: 10.1016/j.biopsych.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Praschak-Rieder N, Wilson AA, Hussey D, Carella A, Wei C, Ginovart N, et al. Effects of Tryptophan Depletion on the Serotonin Transporter in Healthy Humans. Biol Psychiatry. 2005;58:825–30. doi: 10.1016/j.biopsych.2005.04.038. [DOI] [PubMed] [Google Scholar]
- Ratzenboeck E, Saria A, Kriechbaum N, Zernig G. Reinforcing effects of MDMA (“ecstasy”) in drug-naive and cocaine-trained rats. Pharmacology. 2001;62:138–44. doi: 10.1159/000056086. [DOI] [PubMed] [Google Scholar]
- Reneman L, Booij J, Habraken JB, De Bruin K, Hatzidimitriou G, Den Heeten GJ, et al. Validity of [123]beta-CIT SPECT in detecting MDMA-induced serotonergic neurotoxicity. Synapse. 2002;46:199–205. doi: 10.1002/syn.10130. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DCS. Fluoxetine pretreatment reduces breaking points on a progressive ratio schedule reinforced by intravenous cocaine self-administration in the rat. Life Sci. 1991;49:833–840. doi: 10.1016/0024-3205(91)90248-a. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, et al. Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse. 2001;39:32–41. doi: 10.1002/1098-2396(20010101)39:1<32::AID-SYN5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH. Monoamine transporters and psychostimulant drugs. Eur J Pharmacol. 2003;479:23–40. doi: 10.1016/j.ejphar.2003.08.054. [DOI] [PubMed] [Google Scholar]
- Rudnick G, Clark J. From synapse to vesicle: the reuptake and storage of biogenic amine neurotransmitters. Biochim Biophys Acta. 1993;1144:249–63. doi: 10.1016/0005-2728(93)90109-s. [DOI] [PubMed] [Google Scholar]
- Scheffel U, Szabo Z, Mathews WB, Finley PA, Dannals RF, Ravert HT, et al. In vivo detection of short- and long-term MDMA neurotoxicity--a positron emission tomography study in the living baboon brain. Synapse. 1998;29:183–192. doi: 10.1002/(SICI)1098-2396(199806)29:2<183::AID-SYN9>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Averill P, Stotts AL, Moeller FG, Rhoades HM, Grabowski J. Fluoxetine treatment of cocaine-dependent patients with major depressive disorder. Drug Alcohol Depend. 2001;63:207–14. doi: 10.1016/s0376-8716(00)00208-8. [DOI] [PubMed] [Google Scholar]
- Smith S. Fast robust automated brain extraction. Human Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele TD, Nichols DE, Yim GK. Stereochemical effects of 3,4-methylenedioxymethamphetamine (MDMA) and related amphetamine derivatives on inhibition of uptake of [3H]monoamines into synaptosomes from different regions of rat brain. Biochem Pharmacol. 1987;36:2297–2303. doi: 10.1016/0006-2952(87)90594-6. [DOI] [PubMed] [Google Scholar]
- Szabo Z, McCann UD, Wilson AA, Scheffel U, Owonikoko T, Mathews WB, et al. Comparison of (+)-11C-McN5652 and 11C-DASB as serotonin transporter radioligands under various experimental conditions. J Nucl Med. 2002;43:678–92. [PMC free article] [PubMed] [Google Scholar]
- Talbot PS, Frankle WG, Hwang DR, Huang Y, Suckow FR, Slifstein M, et al. Effects of reduced endogenous 5-HT on the in vivo binding of the serotonin transporter radioligand 11C-DASB in healthy humans. Synapse. 2005;55:164–75. doi: 10.1002/syn.20105. [DOI] [PubMed] [Google Scholar]
- Tancer M, Johanson CE. The effects of fluoxetine on the subjective and physiological effects of 3,4-methylenedioxymethamphetamine (MDMA) in humans. Psychopharmacology. 2007;189:565–73. doi: 10.1007/s00213-006-0576-z. [DOI] [PubMed] [Google Scholar]
- Thomasius R, Petersen K, Buchert R, Andresen B, Zapletalova P, Wartberg L, et al. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users. Psychopharmacology. 2003;167:85–96. doi: 10.1007/s00213-002-1383-9. [DOI] [PubMed] [Google Scholar]
- Thomasius R, Zapletalova P, Petersen K, Buchert R, Andresen B, Wartberg L, et al. Mood, cognition and serotonin transporter availability in current and former ecstasy (MDMA) users: the longitudinal perspective. J Psychopharmacol. 2006;20:211–25. doi: 10.1177/0269881106059486. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Preston KL, Sullivan JT, Fromme R, Bigelow GE. Fluoxetine alters the effects of intravenous cocaine in humans. J Clin Psychopharmacol. 1994;14:396–407. [PubMed] [Google Scholar]
- Wang X, Baumann MH, Xu H, Rothman RB. 3,4-Methylenedioxymethamphetamine Administration to Rats Decreases Brain Tissue Serotonin but not Serotonin Transporter Protein and Glial Fibrillary Acidic Protein. Synapse. 2004;53:240–8. doi: 10.1002/syn.20058. [DOI] [PubMed] [Google Scholar]
- Wang X, Bauman MH, Xu H, Morales M, Rothman RB. (±)-3,4-Methylenedioxymethamphetamine administration to rats does not decrease levels of the serotonin transporter protein or alter its distribution between endosomes and the plasma membrane. J Pharmacol Exp Ther. 2005;314:1002–12. doi: 10.1124/jpet.105.088476. [DOI] [PubMed] [Google Scholar]
- Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]