Abstract
Disorganized attachment is an early predictor of the development of psychopathology in childhood and adolescence. Lyons-Ruth et al. (1999) developed the AMBIANCE coding scheme to assess disrupted communication between mother and infant, and reported the link between maternal behavior and disorganized attachment. The Hungarian group found an association between a polymorphism of the DRD4 gene and disorganized attachment (Lakatos et al., 2000; 2002; Gervai et al., 2005). The present collaborative work investigated the interplay between genetic and caregiving contributions to disorganized attachment. 138 mother-infant dyads, 96 from a Hungarian low-social-risk sample and 42 from a US high-social-risk sample, were assessed for infant disorganized attachment behavior, for DRD4 gene polymorphisms, and for disrupted forms of maternal affective communication with the infant. In accord with literature reports, we found a robust main effect of maternal AMBIANCE scores on infant disorganization. However, this relation held only for the majority of infants who carried the short form of the DRD4 allele. Among carriers of the 7-repeat DRD4 allele, there was no relation between quality of maternal communication and infant disorganization. This interaction effect was independent of degree of social risk and maternal DRD4 genotype.
Keywords: Gene-Environment Interaction, Attachment, DRD4 7-Repeat Allele
Introduction
Advances in molecular genetic techniques and the description of the human genome have made it possible to focus on locating and identifying specific genes underlying genetic variance estimated in earlier twin and adoptive studies. Exploring interactions between genetic and environmental influences that were not easily grasped using quantitative genetic techniques have also become central to recent behavior genetic studies (Rutter, 2006). Such interaction effects may take a variety of forms. Genetic effects may be expressed more strongly under some environmental conditions than others, and environmental effects may be conditional on certain genotypes. Also, exposure to specific environments may be influenced by the individuals' genetic make-up (for a review, see Rutter, Moffitt, & Caspi, 2006). These mechanisms may direct individual development onto different trajectories from early in life and affect the long-term development of mental health and disorder.
Animal and human studies have demonstrated relations between measured aspects of the early nurturing environment and a variety of infant outcomes, including physiological responsiveness to stressors (e.g., Fish et al. 2005; Gunnar, 2005; Liu et al., 1996; Nachmias, Gunnar, Mangelsdorf, Parritz, & Buss, 1996; Spangler, Schieche, Ilg, Maier, & Ackermann, 1994) and cognitive and social development (e.g., NICHD Early Child Care Research Network, 2001; 2005). In both animal models and twin studies, the quality of the early parent-child relationship has been shown to contribute to outcome independently of genetic factors (Caspi et al., 2004; Francis, Champagne, Liu, & Meaney, 1999), with some of these effects being transmitted across generations (Belsky, Jaffee, Sligo, Woodward, & Silva, 2005; Fleming, Kraemer, Gonzalez, Lovic, Rees, & Melo, 2002; Maestripieri, 2005; Meaney, 2001). However, other studies using different samples and sensitive molecular genetic methods suggest that, besides these experience-induced effects of early parenting, behavioral responses to the early caregiving environment show variability, which is moderated by specific genetic factors in rhesus monkeys (Barr et al., 2003; Champoux, Bennett, Shannon, Higley, Lesch, & Suomi, 2002), and also in humans (Caspi et al., 2002; Caspi et al., 2003; Kim-Cohen et al., 2006).
Attachment is the enduring personal bond that develops between infant and caregiver, providing the primary social environment for physical and mental development (Thompson, 1999). Disorganization of infant attachment, i.e. difficulties in developing and maintaining consistent strategies for obtaining comfort from the caregiver at times of fearful arousal, is one of the rare early predictors of later psycho-social maladjustment. It is a major risk factor for aggressive/externalizing behavior in childhood (Lyons-Ruth, Easterbrooks, & Cibelli, 1997; Moss, Bureau, Cyr, Mongeau, & St-Laurent, 2004; Moss, Cyr, & Dubois-Comtois, 2004; van IJzendoorn, Schuengel, & Bakermans-Kranenburg, 1999) and psychopathology in adolescence (Carlson, 1998; Ogawa, Sroufe, Weinfield, Carlson, & Egeland, 1997; Weinfield, Whaley, & Egeland, 2004). About 15–25% of typically developing 1-year-olds and 50–80% of at-risk (e.g., maltreated) infants show disorganized attachment behavior toward the caregiver in situations when comfort and proximity is needed (van IJzendoorn et al., 1999).
Attachment disorganization has been consistently related to a disturbed caregiving environment. In an earlier meta-analysis, the incidence of infant disorganization was related to parental psychiatric impairment but not to serious infant physical disorders, such as cerebral palsy or heart defects (van IJzendoorn, Goldberg, Kroonenberg, & Frenkel, 1992). A more recent meta-analysis confirms that infant disorganization is related to atypical forms of maternal behavior toward the infant as assessed from videotape and mediates a part of the established relation between other maternal risk factors (i.e. maternal unresolved loss or trauma on the Adult Attachment Interview) and infant disorganization (Madigan, Bakermans-Kranenburg, van IJzendoorn, Moran, Pederson, & Benoit, 2006). Assessed in this meta-analysis were both a narrower coding system for frightened or frightening forms of parental behavior (FR; Main & Hesse, 1990) and a broader coding system for parental disrupted affective communication with the infant (AMBIANCE; Lyons-Ruth, Bronfman, & Parsons, 1999), which includes codes for frightened or frightening behavior. Meta-analysis of studies using either coding system confirmed equivalent effect sizes and external validity for the two systems in relation to measures of maternal and infant attachment (Madigan et al., 2006). The AMBIANCE coding system for parental disrupted communication was used in the work reported here. Examples of the various forms of disrupted maternal communication can be seen under Methods.
A central proposition of attachment theory is that individual differences in child-parent attachment security can be explained primarily by shared environmental effects. This hypothesis has been examined in a few, relatively small-size twin studies designed to estimate genetic and environmental variance components of child-parent attachment. In a study including 207 two-year-old twin pairs, Finkel and Matheny (2000) found a significant difference between concordances of organized forms of attachment (secure/avoidant/resistant) of monozygotic (MZ) and dizygotic (DZ) twin pairs, suggesting a genetic effect on attachment behavior. Finkel and Matheny (2000) had no data regarding disorganized attachment. In another study of 110 twin pairs aged 3.5 years (O'Connor & Croft, 2001), only shared and non-shared environmental effects were significant. (The distinction between shared and nonshared environmental effects is based on whether such environmental influences make siblings more or less alike.) Due to the small sample size, O'Connor and Croft (2001) declined to separately test genetic influences on disorganization. In a third study of 138 one-year-old twin pairs (Bokhorst, Bakermans-Kranenburg, Fearon, van IJzendoorn, Fonagy, & Schuengel, 2003), only non-shared environmental factors accounted for the variance in twin concordances of disorganized vs. organized attachment, while both shared and non-shared environmental effects accounted for the variance in secure vs. insecure attachment.
These twin studies aimed at partitioning the population variance due to genetic and environmental effects by comparing MZ and DZ twin correlations or concordances. Sensitivity to detect significant differences between twin correlations (i.e. finding a genetic effect) is dependent on effect size as well as on sample size, with a good deal of power needed to detect such differences between correlations. Also, twin studies often lack the power to test gene-environment correlations and interactions. While two of the above studies converge in finding primarily environmental effects on attachment behavior, the divergent conclusions of the Finkel and Matheny study regarding the detection of genetic effects on attachment could easily result from the relatively modest sample sizes across studies. With only one twin study reporting on disorganized forms of attachment, no firm conclusions on heritability estimates can yet be drawn.
The development of molecular genetic techniques has now made it possible to detect small effects of single genetic polymorphisms. The D4 dopamine receptor gene has been considered as a candidate gene for infant attachment behavior (Lakatos, Toth, Nemoda, Ney, Sasvari-Szekely, & Gervai, 2000), because it is preferentially expressed in the brain regions of the mesocorticolimbic dopamine pathway mediating reward related to social interaction, including mother-infant attachment (Insel, 2003; Muller, Brunelli, Moore, Myers, & Shair, 2005). In the third exon of the DRD4 gene, there is a repeating sequence of 48 base pairs ranging from 2 to 10 repeats (Van Tol et al., 1992). The 7-repeat allele was found to have a lower potency for dopamine-mediated coupling to adenylate cyclase than receptors encoded by the 2- or 4-repeat forms (Asghari, Sanyal, Buchwaldt, Paterson, Jovanovic, & Van Tol, 1995). Although some studies did not confirm this difference (Kazmi, Snyder, Cypess, Graber, & Sakmar, 2000; Watts, Vu, Wiens, Jovanovic, Van Tol, & Neve, 1999), more recent research (Schoots & Van Tol, 2003; Van Craenenbroeck, Clark, Cox, Oak, Liu, & Van Tol, 2005) provided new evidence for the functional significance of the exon III repeat polymorphism. These new results showed that the 7-repeat (D4.7) variant of the gene was significantly less efficient at the levels of transcription, translation and second messenger generation compared to the most frequent short (D4.2, D4.4) forms. The 48 bp repeat polymorphism of the DRD4 gene was associated with normal variations of human neonatal, infant, adult temperament (for a review, see Ebstein, 2006) and attention processes in healthy individuals (Fan, Fossella, Sommer, Wu, & Posner, 2003; Fossella et al., 2002; Schmidt, Fox, Perez-Edgar, Hu, & Hamer, 2001), but has also been related to attention deficit/hyperactivity disorder (ADHD; Faraone et al., 2005).
In a population association study, Lakatos et al. (2000) reported an association between the infant's D4.7 genotype and disorganized attachment. Infants carrying the 7-repeat variant were four times more likely than others (36% vs. 9%) to be classified as disorganized with their mothers in the Strange Situation. Analysis of the -521 C/T regulatory (promoter) polymorphism of the same gene revealed that the association of the 7-repeat allele and disorganized attachment was observed only in the presence of the -521T allele (Lakatos et al., 2002). While a Dutch study failed to replicate this result in a small twin sample (Bakermans-Kranenburg & van IJzendoorn, 2004), further family-based transmission disequilibrium tests (TDT) in the Hungarian sample have found a highly significant non-transmission of the 7-repeat allele to securely attached infants, as well as a trend for preferential transmission to disorganized infants (Gervai et al., 2005).
The present collaborative study was concerned with testing the hypothesis of an interplay between genetic and caregiving contributions to disorganized attachment. In order to increase the incidence and range of key measures of demographic risk, maternal disrupted communication, and infant disorganized attachment behavior, data were aggregated across the low-income, high-risk U.S. sample (N=42), from which the AMBIANCE coding system was developed, and the middle income, low-risk Hungarian sample (N=96), which was the source of the previous reports of an association between DRD4 and infant disorganization. For purposes of the current study, DRD4 genotypes were collected from the US sample, and videotaped parent-infant interaction data previously collected from the Hungarian sample were newly coded using the AMBIANCE system.
We had three aims: a) to evaluate whether the previously observed relations between infant disorganization and maternal disrupted communication, and infant disorganization and the DRD4 allele, would replicate in the larger and socioeconomically more diverse sample created by combining low and high-risk samples; b) to test potential passive and evocative gene-environmental correlations by evaluating whether maternal and infant DRD4 genotypes influence the level of maternal disrupted affective communication; and c) to test for the presence of a gene-environment interaction effect by evaluating whether the quality of maternal-infant communication moderates the relation between the DRD4 gene polymorphism and infant disorganization.
Methods
Participants
138 mothers and infants participated in the study, 96 drawn from a study of a low-risk community sample of 103 middle-class Hungarian families with healthy, full-term, first-born infants (45 female, 58 males, BW>2500g) and 42 from a study of 56 low-income families in the US, recruited to over-represent infants at caregiving risk. The Hungarian sample was recruited during the third trimester of pregnancy from antenatal classes to participate in the longitudinal Budapest Infant-Parent Study (BIPS) to investigate infants' social and emotional development. The sample was ethnically homogeneous, Caucasian, of Hungarian origin. Signed informed consent was obtained from the parents for participating in the whole study, and again for 96 children (41 females, 55 males) separately for the genetic investigation. Families of the remaining 7 children (4 girls, 3 boys) either moved away by the time of the DNA sampling or refused the collection of cheek cells. The research protocol was approved by the Research Ethics Committee of the Institute of Psychology of the Hungarian Academy of Sciences. At the time of the infant's birth, all mothers were married or living with a partner. Mothers' mean age was 27.3 years (SD=3.5, range = 19–38), 53% of the mothers and 59% of the fathers had a college or university degree, and a further 43% and 31%, respectively, had completed secondary school education. US families were recruited during the first 18 months of the infant's life, as part of a study of the impact of social risk factors on infant development. All families met U.S. federal poverty guidelines at study entry and 52% of families had been referred for clinical home-visiting services due to service providers' concerns about the quality of care for the infant. Signed informed consent was obtained from all families for participation in the study. As part of a multi-phase longitudinal study, genetic samples were collected when the infants were young adults, aged 18–22 years. Of 56 infants relocated in young adulthood, 10 refused participation, and 4 specifically refused cheek cell collection, yielding genetic data for 42 families. The research protocol was approved over all phases of the study by the Institutional Review Board of the Cambridge Health Alliance. At the time of entry into the infant study, 19% were single parents; mothers' mean age was 26.2 years (SD = 5.6, range = 16–41); ethnic composition: 35 Caucasian (83%), 7 Black (17%), no Asian; 64% of mothers had completed high school and 36% had some post-secondary education.
Measures
Cumulative demographic risk
The cumulative risk index included (1) low level of maternal education (less than completed high-school), (2) early motherhood (mother younger than 20 at first birth), (3) unplanned pregnancy with the target child, (4) birth of an additional infant before age 18 months of the target child, (5) three or more children under age 6 years in the family, (6) single parenthood, (7) two or more moves within the last year, and (8) low level of parental occupation. Hungarian and US occupation data were coded on a matching 6-level scale ranging from unemployed to major professional (1=unemployed, 2=unskilled worker, 3=skilled worker, 4=technician/semiprofessional, 5=lesser professionals, 6=higher executive/major professional) and a score ≤ 3 was used as a cut-off for dichotomizing socioeconomic risk as reflected by parental occupation. If both parents were employed, the higher level occupation was chosen for risk coding. Hungarian and US samples differed significantly in overall maternal education, occupational level, and single parenthood. Each of the above 8 demographic variables was coded according to absence (0) or presence (1) of risk, and then summed to create the cumulative index.
Attachment classification
In both studies, the infant's attachment to the mother was assessed in the standard Strange Situation assessment (Ainsworth, Blehar, Waters, & Wall, 1978). Assessments were conducted at 12 months of age in the Hungarian study and at 18 months in the US study. Infant attachment classifications have been found to be stable over this age period (Waters, 1978). Classification was carried out blind to infant genotype (Lakatos et al., 2000; Lyons-Ruth et al., 1990). Videotapes were evaluated for attachment security as described by Ainsworth et al. (1978). In the Hungarian study, infant disorganized behavior was rated on a 9-point ordinal scale (D-scale) and infants with a D-score ≥ 5 were classified as disorganized (Main & Solomon, 1990). The original 5-point version of the D-scale, later replaced by a 9-point version, was used in the US study, where the cut-off value for D classification was ≥ 4. The later 9-point version of the scale uses the same criteria for classification as the earlier 5-point version, but adds a broader range of description for variations within the D classification (values 5–9). To use D-scale values in regression analyses, the Hungarian D-scale scores were adjusted to the US scale by matching the cut-off values (5 was recoded as 4) and compressing values 3–4 and 5–9 by using half-points.
Maternal behavior
Disrupted maternal affective communication was assessed in the Strange Situation using the AMBIANCE coding scheme (Lyons-Ruth et al., 1999). Five types of maternal atypical behavior were recorded: Disorientation (e.g. appears confused, hesitant, or frightened with infant; incongruous affect), Negative-intrusive behavior (e.g. mocks or teases infant), Role confusion (e.g. draws attention to self when infant is in need), Withdrawal (e.g. fails to initiate interaction, does not greet infant after separation) and Affective communication errors (including contradictory affective communications, e.g. talks in inviting voice but physically blocks infant's access, and failures to respond to clear infant cues). Based on both the frequency and quality of the behavior observed, a 7-point rating was assigned by the coders on the Level of Disrupted Communication Scale. ‘Disrupted’ classification was defined by a score of 5–7 on the qualitative level scale. Reliabilities and procedures for coding of the US tapes have been published previously (Lyons-Ruth et al., 1999). A recent meta-analysis of the relation of the AMBIANCE scale to infant attachment disorganization has shown an effect size of .35 (N = 384) (Madigan et al., 2006). In addition, meta-analytic test-retest data for the AMBIANCE coding of disrupted maternal communication yielded a stability coefficient of .56 (N = 203), for periods ranging from 8 months to 5 years. For the current study, two Hungarian researchers were trained to reliability. Following the detailed coding protocol, 101 Hungarian mothers were rated on the 7-point Level of Disrupted Communication Scale and classified as Disrupted or Not-disrupted. Reliability coefficients: Disrupted Scale score, ri = .74; classification kappa = .60; classification agreement 80%. Coders were naïve to coding procedures for attachment security or disorganization, and they were blind to both genetic data and infant attachment classifications. Regarding potential common method variance from coding both infant disorganization and maternal behavior from the same assessment, meta-analytic data confirm that AMBIANCE codes have similar magnitudes of association with infant disorganization when coded in a separate laboratory observation (Madigan et al., 2006).
DRD4 genotype
For both the US and Hungarian samples, DNA was isolated from buccal epithelial cells as described elsewhere (Boor et al., 2002), except that Purgene DNA Purification kits (Gentra) were used for DNA isolation in the US sample. For the US sample, DNA samples were collected during the 19-year follow-up study and Schleicher & Schuell IsoCode ID kits were used for 6 samples that were collected by mail. Purified DNA samples from the US were transported and analyzed for the DRD4 exon III repeat polymorphism in Hungary. Genotyping for the DRD4 exon III repeat polymorphism was carried out as described previously (Barta et al., 2001; Ronai, Guttman, Nemoda, Staub, Kalasz, & Sasvari-Szekely, 2000) and blind to behavioral data for both studies.
Control for genetic stratification
In the ethnically homogeneous Hungarian sample, DRD4 genotypes in both the infant and the parental groups were in Hardy-Weinberg equilibrium, reflecting a random combination and stable frequencies of the DRD4 repeat alleles in this population (Gervai et al., 2005; Lakatos et al., 2000). For the ethnically heterogeneous US sample, 40 random marker polymorphisms distributed evenly along the human genome (1–3 markers per chromosome) were analyzed. Population stratification was investigated using the L-POP population substructure detection software (Purcell & Sham, 2004). This program employs the latent class analysis model, which is based on the assumption that unlinked markers are in Hardy-Weinberg equilibrium and in linkage equilibrium in a homogenous population (Pritchard, Stephens, Rosenberg, & Donnelly, 2000). Based on this approach, no significant population stratification was observed in the US study sample.
Analytic Plan
First, the low-social-risk Hungarian data were tested for replication of the association between maternal disrupted communication and infant disorganized attachment to the mother. Further analyses were carried out on the combined Hungarian-US data set. Combining the high-risk US longitudinal and the low-risk Hungarian samples with complete data sets (N=42 and 96, respectively) increased power and also resolved the problem of restriction of range present in both samples by increasing the variability of demographic risk, maternal atypical behavior and infant attachment behavior. Control variables were first assessed for their relation to maternal disrupted communication and infant disorganization, and if significantly associated, were included as covariates in further multivariate modeling. Continuous scales for infant disorganization and maternal disrupted communication were used in regression models to preserve maximum power. Parallel secondary analyses assessed whether the pattern of results also held when attachment disorganization was analyzed as a categorical variable, using the standard well-validated scale point for classification as disorganized.
Imputing missing data
All 138 infants had genotype data, but there were missing data on other variables of interest in 8 cases. Attachment data were available for all but 3 US infants. AMBIANCE data were missing for 4 mothers in the US sample. Two Hungarian tapes had sound problems, so these could not be coded for maternal atypical behavior. To impute complete data for the samples, we used the Multiple Imputation (MI) procedure of the SAS/STAT, designed to follow the multiple imputation guidelines by Rubin (1987) and Schafer (1997). The procedure creates multiply imputed data sets for incomplete multivariate data. It uses methods that incorporate appropriate variability across the m imputations. In the present study, five data sets were generated. Regression analyses were then computed for each data set with unstandardized coefficients (B) and their standard errors pooled and analyzed through the SAS/STAT's MIANALYZE procedure.
Results
Relations Between Maternal Disrupted Communication and Disorganized Attachment in the Low-Social-Risk Hungarian Sample
The first set of analyses revealed that the rated level of disrupted maternal communication in the Hungarian sample was significantly associated with the level of infant disorganization, Spearman's rho = .21, p <.05, replicating previous results in the US sample (Lyons-Ruth et al., 1999). As shown in Table 1, the relation found for the continuous ratings was also significant using dichotomized classification cut-points both for disrupted communication and for infant disorganization.
Table 1.
Infant Attachment Classification and Maternal Disrupted Communication in the Hungarian Sample
| Infant attachment classification | Strength of association | ||
|---|---|---|---|
| Organized | Disorganized | ||
| n = 80 | n = 21 | ||
| Level of disrupted communication | η = .20 | ||
| 3.38 ± 1.50 | 4.14 ± 1.80 | p < .05 | |
| Maternal disrupted classification | |||
| Not disrupted | 75% (60) | 52% (11) | Φ= .20 |
| Disrupted | 25% (20) | 48% (10) | p < .05 |
Study Sample Comparisons
Frequencies of gender categories were nearly identical, with 57.3% and 57.1% males in the Hungarian and US samples, respectively. Table 2 summarizes data for key measures for the two samples. There was no significant difference in the frequency of the DRD4 7-repeat genotype by sample, χ2 (1, N=138) = 1.97, p = .23. As expected, the samples differed by demographic risk status, t (136) = −9.78, p < .001, by extent of infant disorganization t (136) = −4.41, p < .001, and by extent of maternal disrupted communication, t (136) = −3.19, p < .002.
Table 2.
Frequencies and Means of Key Variables in the Hungarian and US Samples
| Variable | Hungarian | US |
|---|---|---|
| N = 96 | N = 42 | |
| Frequency of DRD4 7-repeat genotype | 38.5% | 26.2% |
| Cumulative demographic risk | .27 | 2.60 |
| (.53) | (1.50) | |
| Level of infant disorganization | 2.13 | 3.43 |
| (1.46) | (1.25) | |
| Level of maternal disrupted communication | 3.51 | 4.45 |
| (1.56) | (1.65) |
Note. SD in parentheses.
Univariate Analyses for Control Variables
Gender
Gender was first assessed for its relation to infant disorganization, DRD4 7-repeat allele, and maternal disrupted communication. Consistent with meta-analytic findings (van IJzendoorn et al., 1999), there was no significant effect of gender on infant disorganization, t (136) = .18, p > .85. Also consistent with meta-analytic findings (Madigan et al., 2006), child gender was unrelated to level of maternal disrupted communication, t (136) = 1.27, p > .20. There was also no relation between presence of the 7-repeat DRD4 allele and infant gender χ2(1, N=138) = .13, p > .70. Relations with gender were also non-significant within the national groups, so gender was not considered further in the analyses.
Cumulative Demographic Risk
As expected from prior literature, there were significant effects of cumulative demographic risk both on levels of infant disorganization, Spearman rho = .37, p < .001, and on maternal disrupted communication, rho = .25, p < .005. There was no association between cumulative demographic risk and presence of the 7-repeat allele in the infant, t (136) = 1.25, p > .20. Since variability of the cumulative risk index was considerably restricted within both national groups (see Table 2), correlations within the samples were not significant (infant disorganization, rho = .07 and −.08, p > .40; maternal disrupted communication, rho = .02 and −0.01, p > .80, for the Hungarian and the US samples, respectively). Cumulative demographic risk, therefore, was controlled for in later analyses of genetic and caregiving effects.
Assessment of Gene-Environment Correlation
There was no significant relation between the presence of the DRD4 7-repeat allele in the infant and the level of maternal disrupted communication, t (136) = −.26, p > .70. Therefore, infant genotype did not appear to elicit disturbed forms of maternal interaction. Relation between the presence of the DRD4 7-repeat allele in the mother and the level of maternal disrupted communication was not significant either, t (132) = 1.27, p > .20, so maternal DRD4 genotype could not account for the correlation between maternal disrupted communication and infant disorganization.
Assessment of Genetic Effects and Gene-Environment Interaction on Infant Disorganization
Level of infant disorganization was regressed on presence of the DRD4 7-repeat allele and level of maternal disrupted communication, with level of cumulative demographic risk entered first into the equation as a control variable. Main effects, as well as the interaction term between infant DRD4 genotype and maternal disrupted communication, were tested.
With cumulative demographic risk controlled, the main effect of maternal disrupted communication was significant, t (134) = 3.18, p < .002, B = .37. The main effect of infant genotype was not significant, t (134) = 1.02, p > .30. The interaction term was entered on the third step and was significant, t (133) = −2.18, p = .03, B = .35.
The genotype by level of maternal disrupted communication interaction term was further explored as recommended (Aiken & West, 1991, p. 130) by computing regression analyses for the relation between maternal disruption and infant disorganization separately for infants who did not carry the DRD4 7-repeat allele (n = 90) and for those who did (n = 48). Among infants who did not carry the allele, with demographic risk controlled, there was a significant relation between level of disorganization and maternal disrupted communication, t (87) = 4.35, p < .0001, B = .37. Among infants who did carry the 7-repeat allele, with demographic risk controlled, there was no relation between maternal disruption and infant disorganization, t (45) = .13, p > .80. Results (not shown) were similar without controlling for demographic risk.
We also computed regression analyses for the relation between DRD4 7-repeat allele and infant disorganization separately within maternal groups categorized as non-disrupted and disrupted at the standard classification point of 5 or above on the 7-point scale (Lyons-Ruth et al., 1999). For infants whose mothers were non-disrupted (n = 88), with demographic risk controlled, there was a significant relation between the level of disorganization and the DRD4 genotype, t (85) = 2.31, p < .025, B= .69. Among infants whose mothers displayed high levels of disrupted behavior (n = 50), with demographic risk controlled, the relation between the level of disorganization and DRD4 genotype was not significant, t (45) = −1.20, p > .20.
Because cumulative demographic risk was also a correlate of infant disorganization, a demographic risk by genotype interaction term was entered into the primary regression analysis to test whether a similar gene-environment interaction effect on dysregulation would be associated with cumulative demographic risk. This term did not reach significance, t (132) = −1.61, p = .11, indicating some specificity to the role of early care in the interaction with infant genotype.
Since the categorical classification of infant disorganization is widely used and variability at the lower ranges of the disorganization scale may have less prognostic significance than variation across the classification point, the analysis was repeated regressing the dichotomous disorganized classification on the same set of variables as above. After controlling for demographic risk as before, the genotype by maternal disruption interaction term was again significant, t (133) = 2.10, p < .04, B = .60. This interaction is displayed graphically in Figure 1. A final follow up analysis confirmed that the relation between disorganized attachment and maternal disrupted behavior was significant only in the 7-absent groups both for the Hungarian and US samples (t (56) = 2.03, p < .05, B = .19 and t (28) = 2.84, p < .01, B = .39, respectively).
Figure 1.
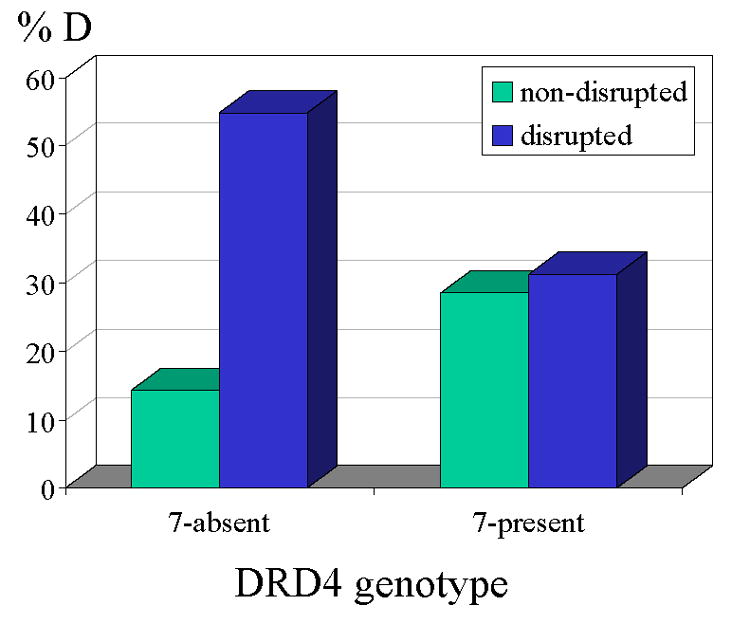
Maternal disrupted communication and disorganized infant attachment by DRD4 genotype
Discussion
In relation to the first aim of the current work, maternal disrupted communication was related to infant disorganization, both in the newly coded Hungarian data set and in the socioeconomically diverse combined Hungarian-US sample. However, with a more diverse sample, the main effect of the infant DRD4 7-repeat genotype, previously found to relate to infant disorganized attachment in the Hungarian sample alone, was not significant. Infant attachment disorganization was related to cumulative demographic risk, as well as to maternal disrupted communication, but, after controlling for demographic risk, the link between maternal disrupted communication and infant disorganization still remained significant.
In relation to the second aim, neither maternal nor infant DRD4 7-repeat genotype accounted for maternal disrupted affective communication with the infant.
Most importantly, in relation to the third aim, the strong relation between maternal disrupted communication and infant disorganization was moderated by the infant's DRD4 7-repeat genotype. The nature of this moderation was that attachment disorganization was only strongly related to the quality of mother-infant communication in the larger group of infants with the 7-absent genotype. Only in this group did we find the predicted relationship between maternal caregiving behavior and infant disorganization. Among infants who carried the 7-repeat allele, infant disorganization was unrelated to the level of maternal disrupted communication, suggesting that infants with the 7-repeat allele were less sensitive to regulation by the caregiving relationship.
It is important to note that the data presented here moderate the previous findings relating both caregiving and DRD4 gene polymorphism to attachment disorganization. The current findings do not argue for main effects on disorganized attachment. On the contrary, genetic variation appears to modulate infant sensitivity to care. Infant attachment behavior among those with the more frequent genotype (7-absent) was strongly related to quality of care. Sensitivity to quality of care promoted secure attachment behavior under conditions of sensitive care and vulnerability to disorganization only when parent-infant communication was disrupted. Conversely, the 7-repeat allele appeared to mute the infant's responsiveness to care, providing some protection in the context of very disrupted interactions but providing less regulation in the context of higher quality care.
These results are consistent with, but also extend, previous findings from both samples. First, the relation between attachment disorganization and maternal disrupted communication previously described for the US sample as well as other samples (Lyons-Ruth et al., 1999; Madigan et al., 2006) was replicated in the Hungarian sample. Secondly, the addition of the maternal caregiving data to the Hungarian sample newly revealed the significant moderation by maternal behavior of our previously reported genetic effect on attachment (Gervai et al, 2005; Lakatos et al., 2000; 2002). Thirdly, the relation between maternal behavior and infant disorganization was conditional on the 7-absent genotype not only in the combined sample but also in both samples analyzed separately.
What these findings imply about attachment is that secure attachment is aided not only by the parent's sensitivity to the infant's cues, but also by the infant's sensitivity to the parent's sensitivity. For parental sensitivity to provide effective regulation of infant stressful arousal, the infant must register the sensitive and contingent nature of parental responses to the infant's cues. If the infant is inattentive to these contingencies, the regulatory effect will be lessened. We would hypothesize that infants who carry the DRD4 7-repeat allele are relatively less attentive or less sensitive to the more subtle social rewards involved in affective communications, while infants with the more common short forms of the DRD4 allele are relatively more sensitive. These results are consistent with a number of other findings describing heterogeneity of behavioral responses to early rearing conditions due to specific genotypes. In the Dunedin study, Caspi and colleagues (2002; 2003) found that the relation of childhood maltreatment with later psychological maladjustment was moderated by genetic factors. The functional polymorphism of the regulatory region of the monoamine oxidase A (MAOA) gene has been shown to moderate the relation between early maltreatment and later antisocial behavior (Caspi et al., 2002), and a regulatory polymorphism of the serotonin transporter (5-HTT) gene has been shown to moderate the effect of early maltreatment on adult depression (Caspi et al., 2003). Both findings have since been replicated (Foley et al., 2004; Kaufman et al., 2004; Kim-Cohen et al., 2006). The homologous MAOA and 5-HTT gene polymorphisms in rhesus monkeys also moderated the effects of adverse rearing conditions on infants' aggressive behavior (Newman et al., 2005), on CNS function (Bennett et al., 2002), on early behavioral development (Champoux et al., 2002), and on hormonal responses to separation stress (Barr et al., 2004). Although links of temperament and personality with DRD4 gene polymorphisms have been reported previously (Ebstein, 2006), there is very sparse data demonstrating DRD4 gene-environment interaction effects. In a small low-social-risk twin sample (Bakermans-Kranenburg & van IJzendoorn, 2006), low maternal sensitivity in infancy predicted higher levels of mother-reported externalizing behavior problems at 2–3 years of age, but only if infants carried the 7-repeat allele of the DRD4 gene. Since this study used an earlier measure of maternal sensitivity that has not shown a robust relation to disorganization of attachment in infancy (van IJzendoorn et al., 1999) and a very different outcome measure, results can not be meaningfully compared to results of the current study.
Animal studies have shown that mesocorticolimbic dopamine is involved in both reward-related learning and in mammalian maternal caregiving (Insel, 2003), but the role of dopamine in the infant's complementary attachment behavior is much less clear. The mesolimbic dopamine system has been implicated in the processing of natural and artificial rewards by mediating the hedonic aspects of rewarding stimuli and acting as a learning signal for behavioral reinforcement (Schultz, 1998). Dopamine may be integral to the formation of mother-infant attachment through specific pathways that enhance the hedonic value and salience of stimuli associated with the mother, perhaps especially emotional communicative signals. We may hypothesize that the development of the mother-infant bond may be supported and guided by the motivational/rewarding aspects of maternal social behavior and infant emotional learning. The role of the D4 dopamine receptor in emotional learning (both in encoding and expression) in the medial prefrontal cortex is supported by the results of a recent animal study (Laviolette, Lipski, & Grace, 2005). It has also been shown that administration of a D2 receptor antagonist in prairie voles blocks male-female bonding by removing the hedonic value of stimuli associated with the sexual partner (Young & Wang, 2004), congruent with the hypothesis that dopamine plays a role in attachment processes. In this neural model of pair bond formation, dopamine reward is activated in social recognition circuits involving the prefrontal cortex, the nucleus accumbens and the medial amygdala, so that social cues associated with a specific partner acquire enhanced hedonic value. It may be hypothesized that functional variations in the DRD4 gene expressed preferentially in brain regions of the reward circuit (prefrontal/cingulate cortex, nucleus accumbens and ventral tegmental area) modulate sensitivity to maternal stimuli resulting in differential sensitivity to caregiving behavior. In view of the present results on moderation of infant sensitivity to maternal care by the DRD4 gene variation, further work is needed to explore interrelations among infant attachment and genetic variations affecting the neurotransmitter systems involved in attachment processes and distress regulation.
Acknowledgments
This work was supported by grants to K. Lyons-Ruth (NIMH RO1062030; Fogarty International Research Center Award RO306014), J. Gervai (Hungarian Science Fund - OTKA T 038407) and Krisztina Lakatos (OTKA D 45940).
References
- Abrams KY, Rifkin A, Hesse E. Examining the role of parental frightened/frightening subtypes in predicting disorganized attachment within a brief observational procedure. Development and Psychopathology. 2006;18:345–361. doi: 10.1017/S0954579406060184. [DOI] [PubMed] [Google Scholar]
- Aiken SL, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks: Sage; 1991. [Google Scholar]
- Ainsworth MD, Blehar MC, Waters E, Wall S. Patterns of attachment: A psychological study of the Strange Situation. Hillsdale: Erlbaum; 1978. [Google Scholar]
- Asghari V, Sanyal S, Buchwaldt S, Paterson A, Jovanovic V, Van Tol HH. Modulation of intracellular cyclic AMP levels by different human dopamine D4 receptor variants. Journal of Neurochemistry. 1995;65:1157–1165. doi: 10.1046/j.1471-4159.1995.65031157.x. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. No association of dopamine D4 receptor (DRD4) and -521 C/T promoter polymorphisms with infant attachment disorganization. Attachment and Human Development. 2004;6:211–218. doi: 10.1080/14616730412331281584. [DOI] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Gene-environment interaction of the dopamine D4 receptor (DRD4) and observed maternal insensitivity predicting externalizing behavior in preschoolers. Developmental Psychobiology. 2006;48:406–409. doi: 10.1002/dev.20152. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Becker ML, Parker CC, Champoux M, Lesch KP, et al. The utility of the non-human primate; model for studying gene by environment interactions in behavioral research. Genes, Brain and Behavior. 2003;2:336–340. doi: 10.1046/j.1601-1848.2003.00051.x. [DOI] [PubMed] [Google Scholar]
- Barr CS, Newman TK, Shannon C, Parker C, Dvoskin RL, Becker, et al. Rearing condition and rh5-HTTLPR interact to influence limbic-hypothalamic-pituitary-adrenal axis response to stress in infant macaques. Biological Psychiatry. 2004;55:733–738. doi: 10.1016/j.biopsych.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Barta C, Ronai Z, Nemoda Z, Szekely A, Kovacs E, Sasvari-Szekely M, et al. Analysis of dopamine D4 receptor gene polymorphism using microchip electrophoresis. Journal of Chromatography A. 2001;27(924):285–290. doi: 10.1016/s0021-9673(01)00915-3. [DOI] [PubMed] [Google Scholar]
- Belsky J, Jaffee SR, Sligo J, Woodward L, Silva PA. Intergenerational transmission of warm-sensitive-stimulating parenting: a prospective study of mothers and fathers of 3-year-olds. Child Development. 2005;76:384–396. doi: 10.1111/j.1467-8624.2005.00852.x. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Lesch KP, Heils A, Long JC, Lorenz JG, Shoaf SE, et al. Early experience and serotonin transporter gene variation interact to influence primate CNS function. Molecular Psychiatry. 2002;7:118–122. doi: 10.1038/sj.mp.4000949. [DOI] [PubMed] [Google Scholar]
- Bokhorst CL, Bakermans-Kranenburg MJ, Fearon RMP, van IJzendoorn MH, Fonagy P, Schuengel C. The importance of shared environment in mother-infant attachment security: a behavioral genetic study. Child Development. 2003;74:1769–1782. doi: 10.1046/j.1467-8624.2003.00637.x. [DOI] [PubMed] [Google Scholar]
- Boor K, Ronai Z, Nemoda Z, Gaszner P, Sasvari-Szekely M, Guttman A, et al. Noninvasive genotyping of dopamine receptor D4 (DRD4) using nanograms of DNA from substance-dependent patients. Current Medical Chemistry. 2002;9:793–797. doi: 10.2174/0929867024606821. [DOI] [PubMed] [Google Scholar]
- Carlson EA. A prospective longitudinal study of attachment disorganization/disorientation. Child Development. 1998;69:1107–1128. [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig I, et al. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Morgan J, Rutter M, Taylor A, Arseneault L, et al. Maternal expressed emotion predicts children's antisocial behavior problems: using monozygotic-twin differences to identify environmental effects on behavioral development. Developmental Psychology. 2004;40:149–161. doi: 10.1037/0012-1649.40.2.149. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Champoux M, Bennett A, Shannon C, Higley JD, Lesch KP, Suomi SJ. Serotonin transporter gene polymorphism, differential early rearing, and behavior in rhesus monkey neonates. Molecular Psychiatry. 2002;7:1058–1063. doi: 10.1038/sj.mp.4001157. [DOI] [PubMed] [Google Scholar]
- Ebstein RP. The molecular genetic architecture of human personality: beyond self-report questionnaires. Molecular Psychiatry. 2006;11:427–445. doi: 10.1038/sj.mp.4001814. [DOI] [PubMed] [Google Scholar]
- Fan J, Fossella J, Sommer T, Wu J, Posner MI. Mapping the genetic variation of executive attention onto brain activity. Proceedings of the National Academy of Sciences US. 2003;100:7406–7411. doi: 10.1073/pnas.0732088100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraone SV, Perlis RH, Doyle AE, Smoller JW, Goralnick JJ, Holmgren MA, Sklar P. Molecular genetics of attention-deficit/hyperactivity disorder. Biological Psychiatry. 2005;57:1313–23. doi: 10.1016/j.biopsych.2004.11.024. [DOI] [PubMed] [Google Scholar]
- Finkel D, Matheny AP., Jr Genetic and environmental influences on a measure of infant attachment security. Twin Research. 2000;3:242–250. doi: 10.1375/136905200320565210. [DOI] [PubMed] [Google Scholar]
- Fish EW, Shahrokh D, Bagot R, Caldji C, Bredy T, Szyf M, et al. Epigenetic programming of stress responses through variations in maternal care. Annals of the New York Academy of Sciences. 2004;1036:167–180. doi: 10.1196/annals.1330.011. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Kraemer GW, Gonzalez A, Lovic V, Rees S, Melo A. Mothering begets mothering: the transmission of behavior and its neurobiology across generations. Pharmacology Biochemistry and Behavior. 2002;73:61–75. doi: 10.1016/s0091-3057(02)00793-1. [DOI] [PubMed] [Google Scholar]
- Foley DL, Eaves LJ, Wormly B, Silberg JL, Maes HH, Kuhn J, et al. Childhood adversity, monoamine oxidase A genotype, and risk for conduct disorder. Archives of General Psychiatry. 2004;61:1–7. doi: 10.1001/archpsyc.61.7.738. [DOI] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, Posner MI. Assessing the molecular genetics of attention networks. BMC Neuroscience. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis DD, Champagne FA, Liu D, Meaney MJ. Maternal care, gene expression, and the development of individual differences in stress reactivity. Annals of the New York Academy of Sciences. 1999;896:66–84. doi: 10.1111/j.1749-6632.1999.tb08106.x. [DOI] [PubMed] [Google Scholar]
- Gervai J, Nemoda Z, Lakatos K, Ronai Z, Toth I, Ney K, et al. Transmission Disequilibrium Tests confirm the link between DRD4 gene polymorphism and infant attachment. American Journal of Medical Genetics, Part B (Neuropsychiatric Genetics) 2005;132B:126–130. doi: 10.1002/ajmg.b.30102. [DOI] [PubMed] [Google Scholar]
- Goldberg S, Benoit D, Blokland K, Madigan S. Atypical maternal behavior, maternal representations, and infant disorganized attachment. Development and Psychopathology. 2003;15:239–257. doi: 10.1017/s0954579403000130. [DOI] [PubMed] [Google Scholar]
- Grienenberger JF, Kelly K, Slade A. Maternal reflective functioning, mother-infant affective communication, and infant attachment: exploring the link between mental states and observed caregiving behavior in the intergenerational transmission of attachment. Attachment and Human Development. 2005;7:299–311. doi: 10.1080/14616730500245963. [DOI] [PubMed] [Google Scholar]
- Gunnar M. Attachment and stress in early development. In: Carter CS, Ahnert L, Grossmann KE, et al., editors. Attachment and bonding: a new synthesis. Cambridge: The MIT Press; 2005. pp. 245–255. [Google Scholar]
- Insel TR. Is social attachment an addictive disorder? Physiology and Behavior. 2003;79:351–357. doi: 10.1016/s0031-9384(03)00148-3. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, Douglas-Palumberi H, Houshyar S, Lipschitz D, Krystal J, et al. Social supports and serotonin transporter gene moderate depression in maltreated children. Proceedings of the National Academy of Sciences of the U S A. 2004;101:17316–17321. doi: 10.1073/pnas.0404376101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmi MA, Snyder LA, Cypess AM, Graber SG, Sakmar TP. Selective reconstitution of human D4 dopamine receptor variants with Gi alpha subtypes. Biochemistry. 2000;39:3734–3744. doi: 10.1021/bi992354c. [DOI] [PubMed] [Google Scholar]
- Kim-Cohen J, Caspi A, Taylor A, Williams B, Newcombe R, Craig IW, et al. MAOA, maltreatment, and gene-environment interaction predicting children's mental health: new evidence and a meta-analysis. Molecular Psychiatry. 2006;11:903–913. doi: 10.1038/sj.mp.4001851. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Nemoda Z, Toth I, Ronai Z, Ney K, Sasvari-Szekely M, et al. Further evidence for the role of the dopamine D4 receptor gene (DRD4) in attachment disorganization: interaction of the III exon 48 bp repeat and the -521 C/T promoter polymorphisms. Molecular Psychiatry. 2002;7:27–31. doi: 10.1038/sj.mp.4000986. [DOI] [PubMed] [Google Scholar]
- Lakatos K, Toth I, Nemoda Z, Ney K, Sasvari-Szekely M, Gervai J. Dopamine D4 receptor (DRD4) gene polymorphism is associated with attachment disorganization. Molecular Psychiatry. 2000;5:633–637. doi: 10.1038/sj.mp.4000773. [DOI] [PubMed] [Google Scholar]
- Laviolette SR, Lipski WJ, Grace AA. A subpopulation of neurons in the medial prefrontal cortex encodes emotional learning with burst and frequency codes through a dopamine D4 receptor-dependent basolateral amygdala input. Journal of Neuroscience. 2005;25:6066–6075. doi: 10.1523/JNEUROSCI.1168-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science. 1997;277:1659–1662. doi: 10.1126/science.277.5332.1659. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Bronfman E, Parsons E. Maternal frightened, frightening, or atypical behavior and disorganized infant attachment patterns. In: Vondra JI, Barnett D, editors. Atypical attachment in infancy and early childhood among children at developmental risk, Monographs of the Society for Research in Child Development. Vol. 64. 1999. pp. 67–96. [DOI] [PubMed] [Google Scholar]
- Lyons-Ruth K, Connell D, Grunebaum H, Botein S. Infants at social risk: Maternal depression and family support services as mediators of infant development and security of attachment. Child Development. 1990;61:85–98. doi: 10.1111/j.1467-8624.1990.tb02762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons-Ruth K, Easterbrooks MA, Cibelli CD. Infant attachment strategies, infant mental lag, and maternal depressive symptoms: predictors of internalizing and externalizing problems at age 7. Developmental Psychology. 1997;33:681–692. doi: 10.1037//0012-1649.33.4.681. [DOI] [PubMed] [Google Scholar]
- Madigan S, Bakermans-Kranenburg M, van IJzendoorn M, Moran G, Pederson D, Benoit D. Unresolved states of mind, anomalous parental behavior, and disorganized attachment: a review and meta-analysis of a transmission gap. Attachment and Human Development. 2006;8:89–111. doi: 10.1080/14616730600774458. [DOI] [PubMed] [Google Scholar]
- Madigan S, Moran G, Pederson DR. Unresolved states of mind, disorganized attachment relationships, and disrupted interactions of adolescent mothers and their infants. Developmental Psychology. 2006;42:293–304. doi: 10.1037/0012-1649.42.2.293. [DOI] [PubMed] [Google Scholar]
- Maestripieri D. Early experience affects the intergenerational transmission of infant abuse in rhesus monkeys. Proceedings of the National Academy of Sciences of the U S A. 2005;102:9726–9729. doi: 10.1073/pnas.0504122102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Main M, Hesse E. Parent's unresolved traumatic experiences are related to infant disorganized/disoriented attachment status: Is frightened and/or frightening parental behavior the linking mechanism? In: Greenberg M, Cicchetti D, Cummings EM, editors. Attachment in the preschool years: Theory, research, and intervention. Chicago: University of Chicago Press; 1990. pp. 161–182. [Google Scholar]
- Main M, Solomon J. Procedures for identifying infants as disorganized/disoriented during the Ainsworth Strange Situation. In: Greenberg MT, Cicchetti D, Cummings EM, editors. Attachment in the preschool years: Theory, research, and intervention. Chicago: University of Chicago Press; 1990. pp. 121–160. [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annual Reviews of Neuroscience. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Moss E, Bureau JF, Cyr C, Mongeau C, St-Laurent D. Correlates of attachment at age 3: construct validity of the preschool attachment classification system. Developmental Psychology. 2004;40:323–334. doi: 10.1037/0012-1649.40.3.323. [DOI] [PubMed] [Google Scholar]
- Moss E, Cyr C, Dubois-Comtois K. Attachment at early school age and developmental risk: examining family contexts and behavior problems of controlling-caregiving, controlling-punitive, and behaviorally disorganized children. Developmental Psychology. 2004;40:519–532. doi: 10.1037/0012-1649.40.4.519. [DOI] [PubMed] [Google Scholar]
- Muller JM, Brunelli SA, Moore H, Myers MM, Shair HN. Maternally modulated infant separation responses are regulated by D2-family dopamine receptors. Behavioral Neuroscience. 2005;119:1384–1388. doi: 10.1037/0735-7044.119.5.1384. [DOI] [PubMed] [Google Scholar]
- Nachmias M, Gunnar M, Mangelsdorf S, Parritz RH, Buss K. Behavioral inhibition and stress reactivity: the moderating role of attachment security. Child Development. 1996;67:508–522. [PubMed] [Google Scholar]
- Newman TK, Syagailo YV, Barr CS, Wendland JR, Champoux M, Graessle M, et al. Monoamine oxidase A gene promoter variation and rearing experience influences aggressive behavior in rhesus monkeys. Biological Psychiatry. 2005;57:167–172. doi: 10.1016/j.biopsych.2004.10.012. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. Child care and children's peer interaction at 24 and 36 months: the NICHD study of early child care. Child Development. 2001;72:1478–1500. doi: 10.1111/1467-8624.00361. [DOI] [PubMed] [Google Scholar]
- NICHD Early Child Care Research Network. Duration and developmental timing of poverty and children's cognitive and social development from birth through third grade. Child Development. 2005;76:795–810. doi: 10.1111/j.1467-8624.2005.00878.x. [DOI] [PubMed] [Google Scholar]
- O'Connor TG, Croft CM. A twin study of attachment in preschool children. Child Development. 2001;72:1501–1511. doi: 10.1111/1467-8624.00362. [DOI] [PubMed] [Google Scholar]
- Ogawa JR, Sroufe LA, Weinfield NS, Carlson EA, Egeland B. Development and the fragmented self: longitudinal study of dissociative symptomatology in a nonclinical sample. Development and Psychopathology. 1997;9:855–879. doi: 10.1017/s0954579497001478. [DOI] [PubMed] [Google Scholar]
- Pritchard JK, Stephens M, Rosenberg NA, Donnelly P. Association mapping in structured populations. American Journal of Human Genetics. 2000;67:170–181. doi: 10.1086/302959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S, Sham P. Properties of structured association approaches to detecting population stratification. Human Heredity. 2004;58:93–107. doi: 10.1159/000083030. [DOI] [PubMed] [Google Scholar]
- Ronai Z, Guttman A, Nemoda Z, Staub M, Kalasz H, Sasvari-Szekely M. Rapid and sensitive genotyping of dopamine D4 receptor tandem repeats by automated ultra-thin-layer gel electrophoresis. Electrophoresis. 2000;21:2058–2061. doi: 10.1002/1522-2683(20000601)21:10<2058::AID-ELPS2058>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Multiple imputation for nonresponse in surveys. New York: John Wiley & Sons, Inc; 1987. [Google Scholar]
- Rutter M. Genes and behavior: Nature-nurture interplay explained. Oxford: Blackwell; 2006. [Google Scholar]
- Rutter M, Moffitt TE, Caspi A. Journal of Child Psychology and Psychiatry. Vol. 47. 2006. Gene-environment interplay and psychopathology: multiple varieties but real effects; pp. 226–261. [DOI] [PubMed] [Google Scholar]
- Schafer JL. Analysis of incomplete multivariate data. London: Chapman & Hall; 1997. [Google Scholar]
- Schmidt LA, Fox NA, Perez-Edgar K, Hu S, Hamer DH. Association of DRD4 with attention problems in normal childhood development. Psychiatric Genetics. 2001;11:25–29. doi: 10.1097/00041444-200103000-00005. [DOI] [PubMed] [Google Scholar]
- Schoots O, Van Tol HH. The human dopamine D4 receptor repeat sequences modulate expression. Pharmacogenomics Journal. 2003;3:343–348. doi: 10.1038/sj.tpj.6500208. [DOI] [PubMed] [Google Scholar]
- Schuengel C, Bakermans-Kranenburg MJ, van IJzendoorn MH. Frightening maternal behavior linking unresolved loss and disorganized infant attachment. Journal of Consulting and Clinical Psychology. 1999;67:54–63. doi: 10.1037//0022-006x.67.1.54. [DOI] [PubMed] [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. Journal of Neurophysiology. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Spangler G, Schieche U, Ilg U, Maier U, Ackermann C. Maternal sensitivity as an external organizer for biobehavioral regulation in infancy. Developmental Psychobiology. 1994;27:425–437. doi: 10.1002/dev.420270702. [DOI] [PubMed] [Google Scholar]
- Thompson RA. Early attachment and later development. In: Cassidy J, Shaver PR, editors. Handbook of attachment. New York: Guilford; 1999. pp. 265–286. [Google Scholar]
- True MM, Pisani L, Oumar F. Infant-mother attachment among the Dogon of Mali. Child Development. 2001;72:1451–1466. doi: 10.1111/1467-8624.00359. [DOI] [PubMed] [Google Scholar]
- Van Craenenbroeck K, Clark SD, Cox MJ, Oak JN, Liu F, Van Tol HH. Folding efficiency is rate-limiting in dopamine D4 receptor biogenesis. Journal of Biological Chemistry. 2005;280:19350–19357. doi: 10.1074/jbc.M414043200. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Goldberg S, Kroonenberg PM, Frenkel OJ. The relative effects of maternal and child problems on the quality of attachment: A meta-analysis of attachment in clinical samples. Child Development. 1992;63:840–858. doi: 10.1111/j.1467-8624.1992.tb01665.x. [DOI] [PubMed] [Google Scholar]
- van IJzendoorn MH, Schuengel C, Bakermans-Kranenburg MJ. Disorganized attachment in early childhood: meta-analysis of precursors, concomitants, and sequelae. Development and Psychopathology. 1999;11:225–249. doi: 10.1017/s0954579499002035. [DOI] [PubMed] [Google Scholar]
- Van Tol HH, Wu CM, Guan HC, Ohara K, Bunzow JR, Civelli O, et al. Multiple dopamine D4 receptor variants in the human population. Nature. 1992;358:149–152. doi: 10.1038/358149a0. [DOI] [PubMed] [Google Scholar]
- Waters E. The reliability and stability of individual differences in infant-mother attachment. Child Development. 1978;49:483–494. [PubMed] [Google Scholar]
- Watts VJ, Vu MN, Wiens BL, Jovanovic V, Van Tol HH, Neve KA. Short- and long-term heterologous sensitization of adenylate cyclase by D4 dopamine receptors. Psychopharmacology. 1999;141:83–92. doi: 10.1007/s002130050810. [DOI] [PubMed] [Google Scholar]
- Weinfield NS, Whaley GJ, Egeland B. Continuity, discontinuity, and coherence in attachment from infancy to late adolescence: sequelae of organization and disorganization. Attachment and Human Development. 2004;6:73–97. doi: 10.1080/14616730310001659566. [DOI] [PubMed] [Google Scholar]
- Young LJ, Wang Z. The neurobiology of pair bonding. Nature Neuroscience. 2004;7:1048–1054. doi: 10.1038/nn1327. [DOI] [PubMed] [Google Scholar]


