Abstract
The high levels of hepatitis B virus (HBV) surface antigen (HBsAg)-bearing subviral particles in the serum of chronically infected individuals are thought to play a role in suppressing the HBV-specific immune response. Current therapeutics are not directed at reducing this viral antigenemia; thus, our group has focused on identifying inhibitors of HBsAg secretion. By using the HBV-expressing cell line HepG2.2.15, high-throughput screening of an 80,288-compound synthetic small-molecule library identified HBF-0259, an aromatically substituted tetrahydro-tetrazolo-(1, 5-a)-pyrimidine. Following resynthesis, HBF-0259 had a 50% effective concentration of approximately 1.5 μM in a secondary, HBV-expressing cell line, with a concentration that exhibited 50% cytotoxicity of >50 μM. The equilibrium concentration of HBF-0259 in aqueous solution at physiological pH was 15 to 16 μM; the selective index was thus >9. As intended by our screening paradigm, HBF-0259 is a selective, potent inhibitor of secretion of both subviral and DNA-containing viral particles, while the secretion of α-1-acid glycoprotein and α-1-antitrypsin was unaffected. The HBV e antigen, which is not a constituent of HBV particles, was also unaffected, suggesting that the secretion of particles bearing HBV structural glycoproteins is targeted directly. Inhibitory activity was also confirmed by transfection of HBsAg, indicating that the action of the compound is independent of those of other viral proteins. HBF-0259 had no effect on HBV DNA synthesis, demonstrating that inhibition is independent of viral genomic replication. Finally, HBF-0259 had little or no effect on the cell-to-cell spread of two unrelated viruses, suggesting that it is a specific inhibitor of secretion of HBsAg. Possible mechanisms of action and the implications for its development are discussed.
Hepatitis B is one of the world's most prevalent diseases. Although most individuals seem to resolve the infection following acute symptoms, approximately 30% of cases become chronic (23). According to current estimates, 350 million to 400 million people worldwide have chronic hepatitis B (24, 50), leading to 500,000 to 1,000,000 deaths per year largely due to the development of hepatocellular carcinoma, cirrhosis, and other complications (2, 33, 37). Despite the availability of an effective vaccine, immunoglobulin therapy, interferon, and antiviral drugs, hepatitis B remains a major global health problem.
The causative agent is hepatitis B virus (HBV), a small DNA virus that is considered to be the prototypical member of the family Hepadnaviridae (16). HBV is an enveloped virus with an unusual mode of replication, centering on the establishment of a covalently closed circular DNA (cccDNA) copy of its genome in the host cell nucleus. This episomal form results from conversion of the partially double-stranded circular DNA (relaxed circular DNA) genome upon initial infection and functions as the template for all HBV mRNAs (17, 29). Unlike the mechanisms of most other DNA viruses, HBV cccDNA replicates through the retrotranscription of a 1.1-genome unit-length RNA copy (pregenomic RNA) which is originally transcribed from the cccDNA template and which is acted upon by a virus-encoded polymerase to yield progeny relaxed circular DNA. HBV DNA synthesis is coupled to the assembly of its capsid, and most copies of the encapsidated genome then efficiently associate with the envelope proteins for virion assembly and secretion (6); a minority of these genomes are shunted to the nucleus, where they are converted to cccDNA, thus amplifying the levels of the episome (51, 52). As the only enzyme encoded by HBV, the polymerase has been well exploited as a target for antiviral drug development, with four nucleoside-analogous polymerase inhibitors already approved by FDA and with others in development (38). Mutations in the primary sequence of the polymerase that confer resistance to lamivudine and adefovir have been identified clinically and underlie a rebound of serum virus titers that 70% of treated patients experience within 3 years of the start of lamivudine therapy (31, 35, 59). Although resistance to telbivudine, adefovir, and entecavir occurs more rarely, it has been recorded (9, 19, 21, 32, 57, 62). Interferon alpha is the other major therapy available for hepatitis B, but it is limited by a poor long-term response (25) and debilitating side effects (25, 61). Hence, there is certainly a medical need for treatments with improved characteristics and for a diversity of approaches in the development of therapies for HBV infection.
Aside from being a critical structural component of the virion, the HBV envelope is a major factor in the disease process. In chronically infected individuals, the serum levels of HBV surface antigen (HBsAg) can be as high as 400 μg/ml, driven by the propensity for infected cells to secrete noninfectious subviral particles at levels far in excess of the levels of infectious (Dane) particles (22, 23). HBsAg comprises the principal antigenic determinant in HBV infection (16, 51) and is composed of the small, middle, and large surface antigens (S, M, and L, respectively). These proteins are produced from a single open reading frame as three separate N-glycosylated polypeptides through utilization of alternative transcriptional start sites (for L and M/S mRNAs) and initiation codons (for L, M, and S) (16, 18). The pathological significance of HBsAg is unknown. A study of duck hepatitis B virus has indicated that the presence of subviral particles in a culture of infected hepatocytes may have a transactivating function on viral genomic replication (5). In addition, a long-held tenet of HBV biology is that this circulating surface antigen functions to suppress the virus-specific immune response. In chronic woodchuck hepatitis virus (WHV) infection, a reduction of antigenemia through clevudine treatment resulted in a positive response to vaccination (43, 44), indicating that circulating antigen may be indeed be suppressing the immune response. Furthermore, the scarcity of virus-specific cytotoxic T lymphocytes, which is a hallmark of chronic WHV and HBV infections (14, 23), may be due to repression of the major histocompatibility complex type I presentation by the intracellular expression of L and M in infected hepatocytes (45, 60). Existing FDA-approved therapies do not significantly affect HBsAg levels in serum (23).
In light of these observations, our group has worked to develop experimental treatments that affect the production of viral antigens from HBV-infected cells. In this work, we present a novel chemical entity that is able to specifically inhibit the secretion of all three HBV antigens expressed in several tissue culture systems. It has no measurable toxicity at effective concentrations and does not affect the general secretion of cellular glycoproteins or the replication of unrelated viruses. We propose that this molecule may represent a starting point for the development of a new anti-HBV therapeutic compound aimed at potentiating the immune response by suppressing antigenemia.
MATERIALS AND METHODS
Cell culture, viruses, antibodies, and plasmids.
For assay development and high-throughput screening, HepG2.2.15 cells (53) were maintained in RPMI medium with additions of penicillin and streptomycin (Invitrogen, Carlsbad, CA), 10% fetal bovine serum (FBS; Atlanta Biologicals, Atlanta, GA), and 0.1 mg/ml of a formulation of three antibiotics (Normocin; InvivoGen, San Diego, CA). The HepDE19 cell line was developed in a manner analogous to that for the HepAD38 cell line (30), with some modifications. Briefly, HepG2 cells were transfected with plasmid pTet-off (Clontech, Mountain View, CA), which expresses tetracycline-responsive transcriptional activator, and plasmid pTREHBVDE, in which HBV pregenomic RNA expression is controlled by a cytomegalovirus early promoter with a tetracycline-responsive element. Transfected HepG2 cells were selected by G418, colonies were expanded in tetracycline-free medium to induce HBV replication, and the viral DNA replication level was determined by Southern blotting. The cells with the highest level of HBV replication were selected and designated HepDE19. In this cell line, the start codon AUG of the precore antigen on the 5′ end of the integrated 1.3-unit-length HBV genome was removed to prevent HBV e antigen (HBeAg) expression from the transgene but could be restored from the 3′ terminal redundancy of pregenomic RNA during viral DNA replication and subsequent cccDNA formation. Therefore, HBeAg is translated only from the precore mRNA transcribed from the episomal cccDNA. HepDE19 cells were maintained in Dulbecco modified Eagle medium (DMEM)-F-12 medium with penicillin and streptomycin (Invitrogen), 10% FBS, 500 μg/ml G418 (Invitrogen), and 1 μg/ml tetracycline (Sigma-Aldrich, St. Louis, MO). HepG2 cells were maintained in medium identical to that used for HepG2.2.15 cells.
Herpes simplex virus type 1 (HSV-1; strain K057) was propagated in Vero cells in DMEM-F-12 medium with penicillin, streptomycin, and 10% FBS. Bovine viral diarrhea virus (BVDV; strain NADL) was propagated in MDBK cells in DMEM-F-12 medium with penicillin, streptomycin, and 10% horse serum (Invitrogen). Viral stock cultures were collected by scraping cell cultures upon observation of a full cytopathic effect (CPE), centrifugation, and freezing-thawing of the cell pellet in appropriate culture medium. The titer was then determined by plaque assay. For both viruses, stocks were diluted in 10-fold steps and plaque assays were carried out in six-well dishes with a 1.5% methylcellulose overlay containing 10% FBS for Vero cells or 10% horse serum for MDBK cells. Plaques were visualized by crystal violet staining and counted.
The antibodies used in the HBsAg enzyme-linked immunosorbent assay (ELISA) for screening were as follows: the primary capture antibody was an anti-HBsAg mouse monoclonal antibody (Fitzgerald Industries, Concord MA), and the detection antibody was a horseradish peroxidase-conjugated anti-surface antigen mouse monoclonal antibody (Abbott Diagnostics, Abbott Park, IL). The antibodies used for Western blotting were an anti-HBV pre-S2 domain rabbit polyclonal antibody (Fitzgerald), an anti-human α-1-acid glycoprotein (AGP) mouse monoclonal antibody (Sigma-Aldrich), an anti-human α-1-antitrypsin mouse monoclonal antibody (Bethyl Laboratories, Montgomery, TX), an antiactin mouse monoclonal antibody (Chemicon International, Temecula, CA), and an anti-hemagglutinin (anti-HA) mouse monoclonal antibody (Covance, Berkeley, CA). Monoclonal anti-HBsAg antibody was used for protein dot blotting (DakoUSA, Carpinteria, CA).
Plasmid pNI2.SHA, which expresses HBV S (subtype ayw) with an HA tag on the C terminus in mammalian cells, was a kind gift from Reinhild Prange, Johannes Gutenberg University, Mainz, Germany. To make plasmid pTRE-MS, which expresses HBV M and S (subtype ayw, isolate V01460) in mammalian cells, the region of nucleotides (nts) 3174 to 1 to 1980 was amplified by PCR with Pfu polymerase (Stratagene, La Jolla, CA) from plasmid pUC119CMV-HBV (13) and cloned into pTRE2 (Clontech). Plasmid pTRE-MSHA, which expresses HBV M and S with an HA tag on the C terminus, was made by replacement of the AvrII-NcoI fragment (nts 650 to 844) in pTRE-MS with the AvrII-NcoI fragment (nts 941 to 2168) from pNI2.SHA.
Compound sources and handling.
The IHVR small-molecule collection consists of 80,288 compounds from the complete libraries of ChemDiv, Inc. (San Diego, CA), Asinex Inc. (Winston-Salem, NC), Chembridge Inc. (San Diego, CA), and Maybridge Inc. (Cornwall, United Kingdom). The compounds were selected from the large libraries of each of these companies through cheminformatic analysis that culled generally reactive compounds, structural motifs known to have nonspecific biological activity, c log P (calculated log10 partition coefficient in octanol versus water) values over 5.0, molecular masses over 500 Da, and other criteria. While the ∼16,000-compound Chemdiv and Asinex portions of the IHVR library are combinatorial, the majority of the library (∼64,000 compounds) is highly diverse. Although we have not determined it with precision, the number of different pharmacophores represented in the collection is in the thousands. In all, the compounds in the IHVR library have an average molecular mass of ∼350 Da and a maximum c log P value of 5.0.
The compounds were purchased as dry powders in 96-well-format “mother” plates, resuspended in ultrapure dimethyl sulfoxide (DMSO) to a final concentration of 10 mM, and diluted in DMSO into working stock (“daughter”) plates at 1 mM. The compounds are stored in covered polypropylene plates at −20°C.
The resynthesis of HBF-0259 and the other compounds was carried out by ChemDiv for analogues of HBF-0259 and the other compounds obtained from ChemDiv, Asinex, Chembridge, and Maybridge. Solubility analysis of HBF-0259 was carried out by pION Inc. (Woburn, MA), by the modified shake flask method.
High-throughput assay and compound screen.
HBsAg ELISA capture wells were made by incubation of 25 μl of monoclonal anti-HBsAg antibody (clone M701077; Fitzgerald) diluted in binding buffer (0.17% Na2CO3, 0.29% NaHCO3, pH 9.6) to 0.9 mg/ml in each well of polystyrene 96-well plates. Incubation was at 4°C overnight, followed by two washes with 150 μl/well of phosphate-buffered saline (PBS)-0.5% Tween 20 (PBST) with shaking. Capture wells were blocked with 150 μl PBST-2.0% bovine serum albumin at 37°C for 1 h, followed by two washes as described above. Following the second wash, the cell culture supernatants from screening plates were transferred and processed as described below.
Stock cultures of HepG2.2.15 cells were grown to confluence, trypsinized, centrifuged, washed once by resuspension in PBS and centrifugation, and seeded in fresh medium in 96-well plates at a density of 5.0 × 104 cells/well to provide a confluent monolayer in medium that was free of previously secreted HBV antigens. Each screening plate consisted of 80 compound test wells, 4 wells of cells with 1.0% DMSO only, 4 wells with DMSO and without cells, and 4 wells of cells with DMSO and 1 mM dithiothreitol (DTT) as a nonspecific reference inhibitor. Immediately following cell seeding, the daughter compound plates were thawed at 37°C and the compounds (final concentrations, 10 μM in 1.0% DMSO) were added to the screening plates by means of automated liquid handling. The screening plates were incubated at 37°C in a 5.0% CO2 atmosphere for 6 days. Following incubation, 150 μl of medium from each well was transferred to blocked capture plates, and the plates were incubated for 4 days at 4°C. The medium was removed, the plates were washed, and 25 μl/well of detection antibody (diluted to 0.625 ng/ml in PBST-2.0% bovine serum albumin) was added. The plates were incubated at 37°C for 1 h and washed twice with 150 μl/well of PBST with shaking, and 50 μl/well of BM Blue peroxidase substrate (Roche, Indianapolis, IN) was added. The plates were allowed to develop for 20 to 30 min (within the linear range of the assay) at room temperature, and the color change was assessed visually. Simultaneously, the cell-containing screening plates were fixed and stained with 50% ethanol-0.5% crystal violet, and the overall toxicity of each compound was assessed by visual comparison of the stained monolayers in compound wells with those in the negative control wells.
To confirm the activities of the hit compounds, the effective concentration that inhibited 50% of the secretion activity (EC50) was determined by incubating the cells with the compounds in duplicate wells at concentrations that ranged from 50 μM to 0.016 μM (in half-log steps), carrying out ELISA as described above, determining the absorbance of the developed assay plates in a SLT Rainbow spectrophotometer (Tecan US, Research Triangle Park, NC) at 650 nm with a reference wavelength of 490 nm, and analyzing the results by best-fit-curve analysis with XLfit software (version 4.0; IDBS; Bridgewater, NJ). The degree of inhibition was calculated for multiple negative control samples in which only DMSO was incubated with the cells. In addition, each plate had multiple wells that contained 1.0 mM DTT as a reference inhibitor. Only curves with R2 values above 0.5 were considered to produce valid EC50 values.
The concentration that exhibited 50% cytotoxicity (CC50) was determined by plating the cells at 1.0 × 104 cells/well (20% confluence) to detect the inhibition of cell growth compared with the level of inhibition that occurred in the absence of compounds. The cell plates were then incubated with compound dilutions and the controls, as described above, and 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT; Sigma-Aldrich) was added to a final concentration of 0.5 mg/ml (47). The plates were again incubated at 37°C for 4 h, after which 10% SDS-0.01 N HCl was added to each well in a volume equal to that of the medium (100 μl), followed by overnight incubation. The absorbance was read in a Rainbow spectrophotometer at 570 nm (reference wavelength, 630 nm) and analyzed with XLfit software (version 4.0) as described above.
The selective index (SI) for each compound was determined by the equation CC50/EC50.
Compound treatment of cells and immunodetection of HBV antigens.
HepG2.2.15 cells were seeded in triplicate in six-well plates at a density of 1.0 × 106 cells/35-mm well to provide confluent monolayers in medium that was free of previously secreted HBV antigen. The control wells contained 0.5% DMSO, and the test wells contained the indicated concentrations of compounds in a total of 0.5% DMSO on each plate. The plates were incubated for 6 days at 37°C in a 5.0% CO2 atmosphere, after which culture fluids from each sample were collected and centrifuged and the supernatant was retained. Supernatants were mixed 3:1 with 4× sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) loading buffer (200 mM Tris-HCl [pH 6.8], 400 mM DTT, 8% SDS, 0.4% bromophenol blue, 40% glycerol). The cell monolayers from the corresponding samples were lysed in 2× SDS-PAGE loading buffer and collected. Twenty microliters of each sample was boiled for 5 min, separated by 10% SDS-PAGE, and transferred onto a Biotrace polyvinylidene difluoride membrane (Pall Corporation, Pensacola, FL). Western blotting was carried out with WesternBreeze reagents (Invitrogen), according to the manufacturer's specifications. Signals from the adsorbed antibodies were detected by enhanced chemiluminescence (GE Healthcare, United Kingdom), and densitometry of the developed films was carried out with ImageJ software (NIH, Bethesda, MD) (1).
For the detection of HBeAg, 50 μl of the supernatant from each well of the HBsAg ELISA plates set up for EC50 determination was analyzed by using an HBeAg ELISA kit from International Immunodiagnostics (Foster City, CA), but without the final addition of H2SO4. The absorbance was determined at 650 nm with a reference wavelength of 490 nm.
Southern blotting and particle assay.
Intracellular viral core DNA was extracted from compound-treated HepDE19 and HepG2.2.15 cells as described previously (20). Briefly, cells from one 60-mm dish were lysed with 1 ml of lysis buffer (10 mM Tris-HCl [pH 8.0], 1.0 mM EDTA, 1% Nonidet P-40, 2% sucrose) at 37°C for 10 min. Cell debris and nuclei were removed by centrifugation, and the supernatant was mixed with 250 μl of 35% polyethylene glycol 8000 containing 1.5 M NaCl. After 1 h incubation in ice, viral nucleocapsids were pelleted by centrifugation at 12,000 × g for 10 min at 4°C, followed by 1 h digestion at 37°C in 400 μl of digestion buffer (0.5 mg/ml pronase [Calbiochem, San Diego, CA], 0.5% SDS, 150 mM NaCl, 25 mM Tris-HCl [pH 8.0], 10 mM EDTA). The digestion mixture was extracted twice with phenol, and the DNA was precipitated with ethanol and dissolved in TE buffer (10 mM Tris-HCl [pH 8.0], 1 mM EDTA). One-sixth of the DNA sample from each plate was resolved by electrophoresis into a 1.5% agarose gel. The gel was then subjected to denaturation in a solution containing 0.5 M NaOH and 1.5 M NaCl, followed by neutralization in a buffer containing 1 M Tris-HCl (pH 7.4) and 1.5 M NaCl. The DNA was then blotted onto an Hybond-XL membrane (GE Healthcare) in 20× SSC buffer (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). The membranes were probed with an [α-32P]UTP (800 Ci/mmol; Perkin-Elmer)-labeled HBV minus-strand-specific full-length riboprobe. Hybridization was carried out in 5 ml EKONO hybridization buffer (Genotech, St. Louis, MO) with 1 h prehybridization at 65°C and overnight hybridization at 65°C, followed by a 1-h wash with 0.1× SSC and 0.1% SDS at 65°C. The membrane was exposed to a phosphorimager screen, and hybridization signals were quantified with QuantityOne software (Bio-Rad).
For the HBsAg dot blot assay, the culture fluids were harvested at the indicated time points, centrifuged at 1,000 rpm for 10 min, and stored at 4°C. Forty microliters of the supernatant was spotted onto a nitrocellulose membrane (Schleicher & Schuell/Whatman, Florham Park, NJ), air dried, soaked in 2.5% formaldehyde-PBS for 30 min, briefly rinsed with water, and then soaked in 50% methanol for 30 min. After three 5-min washes with water, the membrane was blocked and probed with monoclonal anti-HBsAg antibody (DakoUSA), washed, and incubated with a horseradish peroxidase-conjugated secondary antibody. Binding was visualized with an enhanced chemiluminescence detection system (Amersham Pharmacia Biotech).
For the DNA dot blot assay, 400 μl of supernatant was spotted on nitrocellulose and air dried, and the DNA-containing particles were denatured by soaking the membrane for 30 min in 0.2 M NaOH containing 1.5 M NaCl. The filter was then neutralized with 0.2 M Tris-HCl (pH 7.4) containing 1.5 M NaCl for 30 min, washed in TNE buffer (10 mM Tris-HCl [pH 7.4], 150 mM NaCl, 1 mM EDTA), and dried. Viral DNA was detected by hybridization, as described above.
The HBV particle assay was performed as described previously (34). Briefly, the viral particles were precipitated from 1 ml clarified culture fluid by addition of polyethylene glycol 8000 to a 10% final concentration, followed by incubation at 4°C for 1 h. The precipitates were collected by centrifugation at 925 × g for 20 min and dissolved in 40 μl DMEM-F-12 medium. The viral particles were fractionated by electrophoresis through nondenaturing 1% agarose gels and transferred to a nitrocellulose filter by blotting with TNE buffer. The DNA-containing particles on the filter were then denatured and neutralized, and the viral DNA was detected by hybridization, as described above.
Transfection and compound treatment.
HepG2 cells were seeded into 24-well plates at 1.0 × 105 cells/well (40 to 80% confluence). For each transfection condition, the medium was combined with Fugene 6 transfection reagent (Roche Diagnostics), according to the manufacturer's recommendations, and the mixture was incubated for 5 min at room temperature. Plasmid DNA was added at 1 μg/ml, and the mixture was incubated at room temperature for 30 min. The transfection mixture was then added to the cells dropwise at 100 μl/well, and the cells were incubated overnight at 37°C. On the next day, the transfection mixture was removed and fresh medium with either 4 μM HBF-0259 or 0.5% DMSO was added to each well. The cells were thus treated over 6 days with HBF-0259, with the medium and the compound changed at day 3. Following incubation, the culture supernatant and cell monolayers were harvested and analyzed for either S-HA or M-HA by Western blot analysis.
RESULTS
Identification of HBF-0259 as an inhibitor of HBV surface antigen secretion.
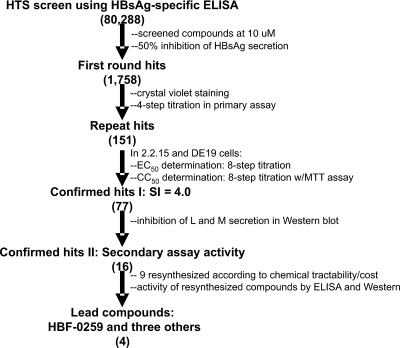
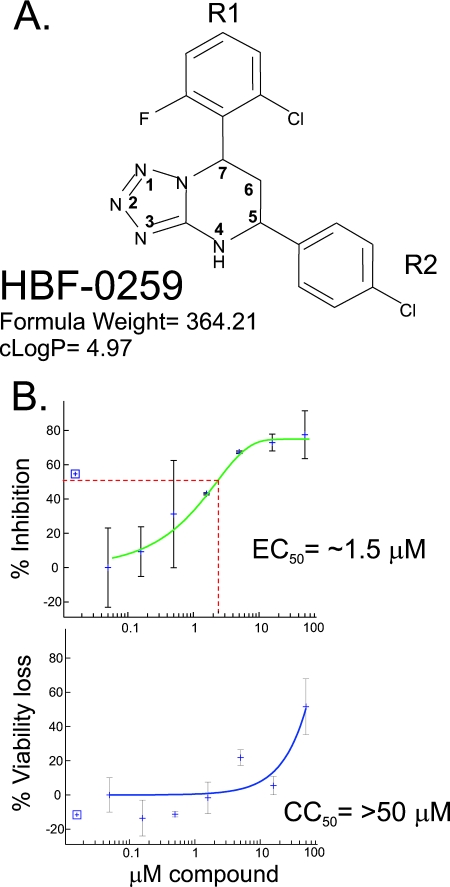
A high-throughput screen of the 80,288 compounds in the IHVR collection was undertaken by using a “sandwich” ELISA designed to quantitatively detect the “a” epitope of HBsAg (58) secreted by HepG2.2.15 cells. As illustrated in Fig. 1, the primary screen yielded 1,758 hits, defined as compounds that inhibited secretion by at least 50%. Of these, 1,607 were identified by crystal violet staining of the cells in the primary screen that were sufficiently cytotoxic at 10 μM to have scored as an inhibitor for that reason alone or that did not repeat the inhibition of HBsAg secretion when they were retested at a variety of concentrations (10, 3.6, 1.0, 0.36 μM) with the primary screening assay. The remaining 151 compounds were analyzed by repeating the primary screening assay with an increased range of compound concentrations (50 to 0.016 μM) to determine the EC50s and by the MTT assay at the same concentrations to determine the CC50s. These assays were carried out with both the HepG2.2.15 cell line and the HepDE19 cell line developed by our group. Of these, 77 compounds were found to have SI values at or above 4.0 and were selected for further investigation. Since the screen scored for the ability to inhibit native “a” epitope secretion, as detected by ELISA, we employed Western blotting as a secondary assay to permit analysis of the effects upon full-length, denatured surface antigen. Thus, the secretion of L and M was analyzed from HepG2.2.15 cells treated with 8, 4, 2, 1, 0.5, and 0.25 μM each compound. Of these, 16 compounds were identified as causing a significant, reproducible inhibition of the secretion of L and M. These compounds were assayed for their purity by high-pressure liquid chromatography (HPLC)-mass spectroscopy (courtesy of Ramila Philip and Jonathan Krakover of the Proteomic Facility, Institute for Hepatitis and Virus Research), and most were found to consist of at least 90% of the predicted substance. On the basis of general predictions for chemical tractability and cost estimates, chemical resynthesis was ordered for nine compounds; retesting of these identified four compounds that were still significantly active in the new preparations. One of these, a tetrahydro-tetrazolo-pyrimidine, specifically, 7-(2 chloro-6-fluoro-phenyl)-5-(4-chorophenyl)-4,5,6,7-tetrahydro-tetrazolo[1,5-a]pyrimidine, referred to as HBF-0259 (Fig. 2A), was chosen for use in follow-up studies, based on potency and medicinal chemistry considerations.
FIG. 1.
High-throughput screening (HTS) paradigm. See the text for details. The values in parentheses represent the numbers of compounds surviving selection at that step, after previous criteria were applied.
FIG. 2.
Basic characteristics of HBF-0259. (A) Structural representation. Most hydrogen atoms are left inferred. The tetrahydro-tetrazolo-pyrimidine ring is conventionally numbered according to double ring positions, with bridge atoms ignored. R1, substituent at carbon 7; R2, substituent at carbon 5. (B) EC50 and CC50 determinations with HepDE19 cells by the HBsAg ELISA and the MTT assay, respectively. All data points are the means for duplicate wells, and error bars represent the standard deviations from the mean. The results shown are those of one typical experiment of five trials. The best-fit curve and calculation were carried out with XLfit software (version 4.0), with outliers removed if necessary to generate R2 values above 0.5. Compound concentrations are expressed on a log10 scale and were 0.0158, 0.05, 0.158, 0.5, 1.58, 5.0, 15.8, and 50.0 μM (from left to right). The percent inhibition of secretion and the percent loss of viability were calculated against the addition of DMSO alone, for which the results were scored as 0% inhibition and 0% loss of viability, respectively.
HBF-0259 is a selective inhibitor of HBsAg secretion.
Using the screening ELISA, we measured the EC50 and CC50 of HBF-0259. In the HepG2.2.15 cell line, the EC50 of HBF-0259 in the primary screening ELISA has been somewhat variable (results not shown), although cytotoxicity has never been observed. This variability (also noted with other compounds) appears to be a property of these cells and is likely related to the highly variable levels of HBV antigen secretion exhibited by the HepG2.2.15 cell line. Alternatively, in the HepDE19 cell line, the EC50 for the inhibition of surface antigen secretion is approximately 1.5 μM. While the CC50 is >50 μM, the equilibrium concentration of HBF-0259 in aqueous solution at physiological pH (6.5 to 7.5) is between 15 and 16 μM, yielding an SI of ≥9 (Fig. 2B).
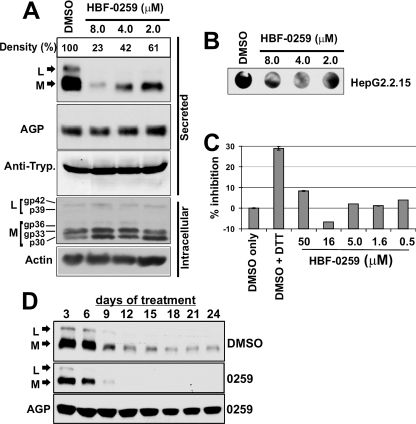
The effects of this compound on the secretion and intracellular processing of HBV L and M were assayed by Western blotting of the supernatants and whole-cell lysates from treated cells by using an antibody specific for the pre-S2 region. In HepG2.2.15 cells treated with HBF-0259 at concentrations of 8.0, 4.0, and 2.0 μM, both L and M are found in significantly reduced abundance in the supernatants of the cells compared to their abundance in a control treated only with the compound solvent (DMSO). Densitometric analysis of this blot indicated that in the sample treated with 4.0 μM, the total inhibition of L and M secretion was 58%, yielding EC50s in this assay of 3.0 to 4.0 μM. In the same samples, the levels of AGP and α-1-antitrypsin appeared to be unaffected by the presence of the compound (Fig. 3A).
FIG. 3.
Characterization of HBF-0259 activity by secondary assays. (A) HepG2.2.15 cells were treated with the indicated concentrations, as described in the text; and cell culture supernatants were analyzed by Western blotting for L, M, AGP, and α-1-antitrypsin (Anti-Tryp.). Whole-cell lysates were analyzed by Western blotting for L, M and actin. p30, gp33, and gp36 represent nonglycosylated, singly glycosylated, and double glycosylated M, respectively; p39 and gp42 represent nonglycosylated and glycosylated L, respectively. The results represent multiple blotting of the same samples, and each assay was repeated (with some variations in the experimental setup) at least three times. (B) Culture supernatants from HepG2.2.15 cells treated with HBF-0259 or DMSO only, as described for panel A, were analyzed for total HBsAg by dot blot enzyme immunoassay under nondenaturing conditions. The results represent those of an experiment repeated twice. (C) Culture supernatants from HepG2.2.15 cells treated with the indicated concentrations of HBF-0259, 1 mM DTT, or DMSO alone were assayed for secreted HBeAg by capture ELISA. The percent inhibition of secretion was calculated by normalizing the reading for the DMSO-only-treated sample to 100% secretion or 0% inhibition. The results represent those of two trials. (D) Effects of extended incubation with HBF-0259 or DMSO alone on L, M, and AGP secretion from HepG2.2.15 cells. Culture supernatants were collected every 3 days from day 3 to day 24. The samples were analyzed by Western blotting, as described for panel B. The results represent the observations from experiments conducted with triplicate samples.
The intracellular levels of L and M and their respective glycoforms did not appear to be reduced and actually exhibited a slight accumulation in the presence of the compound, especially the accumulation of the nonglycosylated p30 form of M (Fig. 3A). The effects on total S, M, and L secretion were also assessed by dot blot enzyme immunoassay with an anti-S (“a” epitope) antibody and indicated a notable reduction of the total signal in the culture supernatants from HBF-0259-treated HepG2.2.15 cells (Fig. 3B), thus corroborating the results observed by the primary screening ELISA and L- and M-specific Western blotting. Interestingly, HBF-0259 did not affect HBeAg secretion in HepG2.2.15 cells (Fig. 3C) compared to the secretion observed by treatment with DTT. Because HBeAg secretion is distinct from that of HBV subviral particles (46, 48) and the extracellular levels of two nonviral proteins are also unaffected, HBF-0259 appears to specifically target secretion of S-, M-, and L-containing particles.
To investigate the effects of long-term HBF-0259 treatment on the secretion of subviral particles, HepG2.2.15 cells were plated under high-density conditions and incubated with 4.0 μM compound in 0.5% DMSO or 0.5% DMSO alone for 24 days. Samples of medium were collected every 3 days, and medium and compound or DMSO were refreshed at those intervals. The samples were analyzed for L, M, and AGP by Western blotting. In the absence of compound, there was a marked decrease in the levels of L and M after 6 days of incubation, and the levels stabilized after 18 days. In the presence of compound, the levels of L and M secretion were attenuated at as early as 3 days and became undetectable after 9 days, while the secretion of AGP remained relatively unaffected (Fig. 3D). These observations indicate that HBF-0259 may be potentiating a tendency on the part of the cells to attenuate the secretion of HBV subviral particles over an extended period and that the HBV secretion pathway does not become habituated to the presence of the inhibitor.
HBF-0259 reduces HBV particle secretion without affecting viral replication.
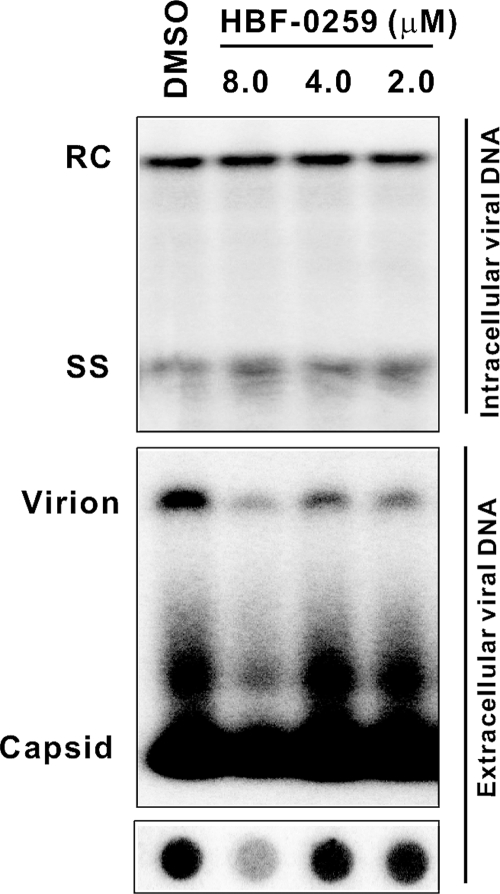
To determine the effects of this compound on intracellular viral replication, HepDE19 cells were subjected to tetracycline withdrawal to permit the synthesis of HBV pregenomic RNA and mRNAs, capsid assembly, and DNA synthesis by reverse transcription. After 7 days, HBF-0259 was added at 2.0, 4.0, and 8.0 μM or DMSO alone was added for an additional 6 days in the absence of tetracycline, with changes of medium and fresh compound made after 3 days. The cell monolayers and culture supernatants were then harvested. Intracellular viral replicative intermediates (relaxed circular and single-stranded DNA) were analyzed by Southern blot assay as described above, and the results were compared with previous results to confirm the sensitivities of these bands to the HBV polymerase inhibitor lamivudine (63). The culture supernatants were subjected to separation by nondenaturing agarose gel electrophoresis and were probed for virion DNA by DNA hybridization. In addition, the supernatants were also analyzed by dot blotting and DNA hybridization.
We observed no changes in the levels of intracellular DNA in the presence of HBF-0259 (Fig. 4, top panel), indicating that the compound has no effect on the steps of viral genomic replication which are reproduced in this HepG2-derived stably transfected system, namely, the posttranscriptional events of HBV replication. A similar analysis of viral genomic replication in HepG2.2.15 cells yielded the same result (data not shown). However, we cannot exclude the possibility that the compound may affect the steps of replication that are not reproduced here, specifically, virus binding to the receptor, entry, and uncoating. However, the inhibition of pretranscriptional steps cannot affect envelope secretion in either the HepG2.2.15 or the HepDE19 cell system and so would be unrelated to the observations reported in this paper.
FIG. 4.
Effects of HBF-0259 treatment on intracellular HBV DNA synthesis and the secretion of HBV DNA-containing particles. HepDE19 cells were withdrawn from tetracycline for 7 days, after which treatment with HBF-0259 or DMSO was initiated and continued for 6 days. Cell monolayers and culture supernatants were harvested and processed for intracellular core-associated DNA (top panel), secreted particles (particle assay; middle panel), and total extracellular viral DNA (bottom panel), as described in the text. The results represent those of two experimental trials. RC, relaxed circular DNA; SS, single-stranded DNA.
In contrast, treatment with HBF-0259 had a significant inhibitory effect on the levels of virion-associated DNA secreted from these cells, as demonstrated by particle assay and DNA dot blotting (Fig. 4, middle and bottom panels, respectively). This observation is in agreement with the finding of the inhibition of secretion of the envelope proteins that comprise subviral particles. A slight lessening in the amount of “naked” HBV capsid was also observed. Although nucleocapsid exit from the cell is commonly seen, the mechanism whereby these nucleocapsids exit the cell is unknown, but this effect most likely corroborates the observed inhibition of intact virus levels. Therefore, the stage of the intracellular HBV life cycle targeted by HBF-0259 appears to be the secretion of enveloped particles at a step that occurs downstream of capsid assembly and reverse transcription.
HBF-0259 inhibits secretion of transiently expressed HBV envelope proteins.
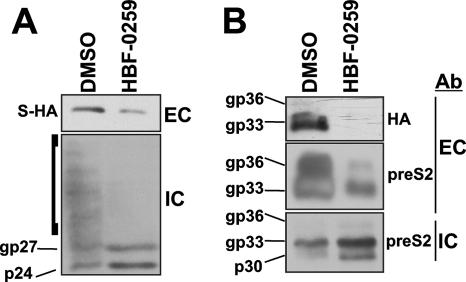
To corroborate our observations with the stably expressing HepG2.2.15 and HepDE19 cell systems, we employed transient transfection of the parental HepG2 cell line with the plasmid constructs pNI2.SHA and pTRE-MSHA, which encode S and M with C-terminal HA epitope tags, respectively. Due to the inherent cytotoxicity of exogenously expressed L (15), that protein was not used in these experiments. Compound treatment was initiated immediately following transfection and continued for a total of 48 h, when both the medium and the cell monolayers were harvested and analyzed by Western blotting by using both anti-HA (for S and M) and anti-pre-S2 (for M) antibodies. As shown in Fig. 5A, incubation with 4.0 μM HBF-0259 resulted in a significant decrease in the levels of secreted S-HA; the intracellular levels of S-HA were not reduced, and the cells exhibited an accumulation of the nonglycosylated form of S-HA (p24) (49). In addition, a “smearing” of the intracellular S-HA signal, seen in the DMSO-treated sample, was notably absent from the HBF-0259-treated sample (Fig. 5A, bracketed area); it is possible that this observation represents the oligomerization of S, as studied by Mangold and colleagues (39-41) and Huovila et al. (26), and which has been observed even under mildly reducing gel conditions (i.e., in the presence of DTT) (39). The oligomerization of S occurs during transport through the endoplasmic reticulum (26), suggesting that HBF-0259 may prevent the transport of HBsAg through the secretory pathway.
FIG. 5.
HBF-0259 inhibits the secretion of exogenously expressed S and M. The results represent those from three experimental trials. (A) HepG2 cells were transfected with plasmid pNI2.SHA, which encodes S-HA, and treated with 4 μM HBF-0259 or DMSO, as indicated, for 48 h. The culture supernatants and cell monolayers were harvested and analyzed by Western blotting for S-HA. p24 and gp27 represent nonglycosylated and glycosylated S-HA, respectively, as predicted by their migration in SDS-polyacrylamide gels. (B) HepG2 cells were transfected with plasmid pTRE-MSHA, which encodes M-HA; treated as described for panel A; and analyzed by Western blotting for M-HA or pre-S2 by using the appropriate antibody (Ab). p30, gp33, and gp36 represent nonglycosylated, singly glycosylated, and double glycosylated M-HA. EC, extracellular; IC, intracellular.
A reduction in M-HA secretion was observed by detection with both anti-HA and anti-pre-S2 antibodies, although the level of inhibition between these two detection conditions varied (Fig. 5B). Similar to S, analysis of intracellular M indicated that HBF-0259 causes the accumulation of the nonglycosylated (p30) and also the single glycosylated (p33) forms of M-HA, suggesting that HBF-0259 may be affecting the full glycosylation of M-HA. Overall, the data presented in Fig. 5 indicate that the inhibitory effects of HBF-0259 on HBV subviral particles are independent of nonenvelope viral proteins and viral replication and that the compound may be modifying the transport of HBsAg through the secretory pathway at a preglycosylation or an early glycosylation step.
Selective antiviral spectrum of HBF-0259.
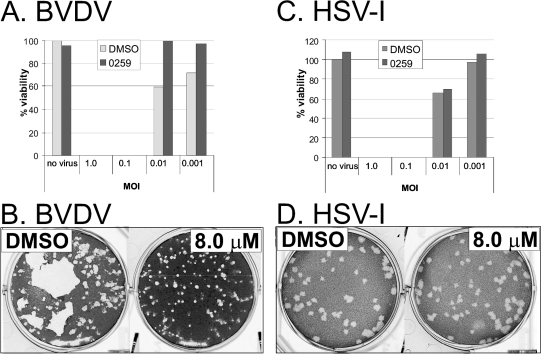
Because the exact mechanism of action of HBF-0259 is not yet known, it is possible that this compound may have a general activity that may be extended to other viruses. To ascertain the potential for HBF-0259 to be used as a general antiviral compound, we tested its effects on the cell-to-cell spread of wild-type HSV-1 (a member of the family Herpesviridae) strain K057 and BVDV (a member of the family Flaviviridae) strain NADL. HSV-1 was used to infect the Vero cell line, and BVDV was used to infect the MDBK cell line. For both viruses, cells were mock infected or infected at various multiplicities of infection (MOIs) of 1.0, 0.1, 0.01, and 0.001 in the presence of 5 μM HBF-0259 or DMSO alone. Under each MOI condition, the cell monolayers were inspected by microscopy for evidence of CPE and were harvested on the day on which full CPE was observed for the DMSO-only control. The samples that were mock infected or in which a full CPE was not observed were harvested at 4 days postinfection for the BVDV plates and at 9 days postinfection for the HSV-1 plates. At harvest, cell viability was determined by the MTT assay as a percentage of viability of the mock-infected, DMSO-only-treated sample. As depicted in Fig. 6 A and C, HBF-0259 did not protect cells infected at an MOI of 1.0 or 0.1 from full CPE for either virus. However, in cells infected with BVDV at MOIs of 0.01 and 0.001, the presence of HBF-0259 did correlate with a slight decrease in the level of the BVDV-induced CPE compared to that achieved with DMSO alone (Fig. 6A). This effect was not observed in the HSV-1-infected samples (Fig. 6C).
FIG. 6.
Selective antiviral spectrum of HBF-0259. (A) MDBK cells were infected with BVDV at the indicated MOIs on 96-well microtiter plates in the presence of HBF-0259 or DMSO and were processed as described in the text. The values for percent viability are the averages for quadruplicate samples. Percent viability was calculated by setting the average for uninfected, DMSO-treated controls at 100%. The results represent those from two experimental trials. (B) MDBK cells were seeded onto six-well plates and infected with an approximate total of 100 PFU/ml of BVDV, under a semisolid overlay, in the presence of HBF-0259 or DMSO. Plaques were visualized by crystal violet staining. The results represent those from five trials. (C) Vero cells were infected with HSV-1 at the indicated MOIs on microtiter plates and processed as described for A. (D) Vero cells were infected with an approximate total of 100 PFU of HSV-1 and treated and processed as described for panel B. The results in panels C and D represent those from two experimental trials.
For both virus systems, the effect of HBF-0259 on plaque size and therefore on the efficiency of cell-to-cell spread was determined by plating approximately 100 PFU/35-mm well on MDBK and Vero cell monolayers grown to approximately 75% confluence. The cells were overlaid with appropriate medium containing methylcellulose and 8.0, 4.0, or 2.0 μM HBF-0259 or DMSO alone. Plaque development was monitored visually and by microscopy and was allowed to proceed to the point where the plaque size was readily observable. The overlay was then removed and the cells were fixed and stained with crystal violet. The presence of HBF-0259 at all three concentrations seemed to result in only a very slight size reduction of the BVDV plaques compared to the numbers of plaques obtained on plates treated with DMSO alone (Fig. 6B). The size of the HSV-1 plaques was unaffected by the compound (Fig. 6D); because wild-type HSV-1 spreads primarily by extracellular virus rather than by syncytium formation (8), these results indicate that HBF-0259 has no ability to restrict the efficiency of the cell-to-cell spread of HSV-1. However, the compound may be able to weakly inhibit the efficiency of BVDV cell-to-cell spread, as evidenced by the protection of a monolayer from a CPE at a very low MOI (albeit not at an MOI at which 10% or more of the cells would be infected).
DISCUSSION
HBF-0259 is a specific inhibitor of HBV particle secretion.
Our group carried out a high-throughput screen of a carefully assembled small-molecule library in an effort to identify novel compounds that would inhibit the egress of viral antigens from the HBV-replicating HepG2.2.15 cell line. The screening campaign was specifically directed to identify inhibitors of this particular step of the HBV life cycle and to minimize the identification of compounds affecting other steps. For example, because the envelope proteins are primarily expressed from the integrated HBV transgene in these cells rather than established cccDNA templates (36, 53), our screening assay would not have scored inhibitors of viral genomic replication or inhibitors of HBV DNA nuclear transport and cccDNA establishment. Similarly, compounds affecting core protein dimerization and general capsid assembly would have not been identified, since the secretion of subviral particles (which comprise the vast majority of extracellular HBsAg compared to the amount of Dane particles) is independent of capsid assembly. Through secondary assays, we eliminated compounds with significant cytotoxicities and confirmed the activities of many of the hits scored in the primary screen. Finally, after further investigation we focused on the compounds that we judged were acting in an HBV-specific manner. Of these, HBF-0259 is the first to be reported. In tissue culture, it has an EC50 in the low micromolar range and a CC50 of over 50 μM, constituting a “window” large enough to suggest that this compound's inhibitory activity does not stem from nonspecific cytotoxic effects on cellular metabolism. Its activity is persistent over lengthy incubations; it appears to act on all three HBV surface antigens and on subviral as well as infectious particles. In direct contrast to the recently reported activity of ellagic acid (54), it does not inhibit the secretion of HBeAg, singling out its effects on HBsAg. Initial observations of its effects on the intracellular nonglycosylated and glycosylated forms of HBsAg suggest that it blocks secretion at an early step in the pathway. It has no effect on HBV DNA synthesis and, hence, cannot be affecting capsid assembly or retrotranscription. It has little or no effect on the ability of two unrelated viruses to spread from cell to cell. Finally, limited structure-activity relationship studies with 10 analogues suggest that the activity of HBF-0259 extends to other members of this class of molecules (results not shown), indicating that an exploration of the chemistry of this compound may lead to optimization of its activity. Overall, HBF-0259 is a novel inhibitor of a crucial step in the HBV life cycle and in chronic disease progression.
HBF-0259 is a novel chemical class to exhibit anti-HBV activity.
With the exception of interferon, all of the FDA-approved antiviral drugs for hepatitis B treatment are nucleoside- and nucleotide-based polymerase inhibitors (38). Furthermore, experimental anti-HBV compounds, such as Bay 41-4109 and the analogous heteroaryldihydropyrimidines (11, 56), 5,5′-bis[8-(phenylamino)-1-naphthalenesulfonate] (64), and the phenylpropenamide derivatives AT-61 and AT-130 (10, 28), are not functionally or structurally related to HBF-0259. Ellagic acid, an interesting compound derived from extracts of the plant Phyllanthus urinaria, appears to specifically inhibit the secretion of HBeAg in vivo (27) and in vitro (54), without affecting the secretion of surface antigen or HBV genomic replication. Our group has reported on the inhibitory effects on the secretion of HBV and other viral glycoproteins of the iminosugar derivatives of deoxynojirimicim and related glycolipids, which work primarily by competitive inhibition of α-glucosidase (4) and also by stimulation of the intrinsic interferon response (3, 42). To this collection of approved and experimental antivirals we add the tetrahydropyrimidine class of compounds, represented by HBF-0259, which have not previously been reported to have antiviral activity.
The significance of this new chemical class of HBV inhibitors can be divided into the implications for therapy and the possible utility of this class of inhibitors in uncovering novel aspects of HBV particle assembly and secretion. In addressing the former, we speculate that antigen secretion inhibitors may prove useful in ameliorating antigenemia in patients with chronic HBV. This clinical end point may then make it possible to employ immunotherapy (i.e., vaccination) to reduce or perhaps even eliminate the population of infected hepatocytes in the liver. The reduction of antigenemia in the WHV infection model has yielded an increase in anti-HBV cellular immune function and antibody titers (43, 44). Secretion inhibitors and immunotherapy, in combination with interferon and/or polymerase inhibitors, may make it possible to develop an anti-HBV cocktail that would effectively reduce both intracellular viral replication and the levels of circulating antigen in the form of both infectious and subviral particles.
One possible confounding factor for this therapeutic approach is that the intracellular accumulation of L has been implicated in HBV-associated fulminant cholestatic hepatitis (FCH), an acute and often fatal form of liver failure. Chisari et al. have reported that the “ground glass” morphology of FCH-affected hepatocytes is reproduced in the livers of transgenic mice that overexpress L protein (7). Moreover, cell culture studies have determined that L expression promotes apoptosis and the appearance of the distinct FCH cytopathology in hepatoma-derived cell lines (15). It is unlikely that L-induced cytoxicity plays a significant role in the work described here, because the concentrations of HBF-0259 and the other compounds under study that cause L accumulation do not cause a concomitant increase in cell death. It is possible that the level of accumulation of L during drug treatment simply does not rise to the levels required to induce cytotoxicity in cell culture, but this may, indeed, turn out to be a factor in future animal studies.
Studies of mechanism of action.
HBF-0259 may also prove to be a useful tool in furthering understanding of HBV assembly and secretion. Unlike the α-glucosidase inhibitors, HBF-0259 does not appear to affect postglycosylation processing of the HBV envelope proteins. In addition, we observed a distinct intracellular accumulation of nonglycosylated or early glycosylated forms of the envelope proteins, whereas the inhibition of secretion effected by the iminosugars causes the accelerated proteosomal processing of M (55). This distinction suggests that HBF-0259 functions through a mechanism different from that for deoxynojirimicim and its derivatives and is of investigational interest for that reason alone.
A principal distinguishing characteristic of this compound is the remarkable degree of specificity of its effects on HBV antigen secretion. It has negligible cytotoxicity at active concentrations; it does not inhibit the secretion of at least two abundant cellular glycoproteins; it does not effect the phenotypes of two unrelated enveloped virus families that also encode glycoproteins; and, surprisingly, it does not inhibit the secretion of HBeAg, an HBV protein that is not a structural virion or subviral particle component (46, 48). Although the exact mechanism of action of HBF-0259 remains to be elucidated, these observations suggest that its effects occur through a direct action on either the HBV viral and subviral particles themselves, the antigens that they contain, or the cellular processes or factors that are uniquely required for the secretion of these particles. Our group is currently focused on dissecting these possibilities to define the mechanism of action.
Optimization of HBF-0259.
Chemical tractability was a criterion that we addressed when deciding which active compounds identified in the screen would be further investigated. The substituted tetrahydro-tetrazolo-pyrimidine structure of HBF-0259 is amenable to most modifications that would be made to investigate structure-activity relationships and structural optimization. In our initial attempts to do so, a variety of analogues with different substitutions in the tetrahydropyrimidine ring were tested in the primary screening assay; while no clear structure-activity relationship pattern has emerged yet, we are encouraged by the observation that some compounds retained activity, indicating that the HBF-0259 structure is indeed the basis for the inhibition of HBV antigen secretion. Also, the toxicity of the series did not increase appreciably, suggesting that there is a high threshold for modifications before any nonspecific adverse effects are seen.
A salient feature of HBF-0259 is the presence of two chiral centers, at carbon 5 and carbon 7 of the tetrahydropyrimidine ring. Although our samples of HBF-0259 consist of mixtures of enantiomers that are at least 99% pure (as determined by HPLC-mass spectroscopy; results not shown), we as yet have no information on the comparative levels of each stereoisomer in that mixture. Because it is likely that only one of the stereoisomers is biologically active, it is possible that the EC50 of this compound may actually be four times lower than our determinations for either HBF-0259 or any of the analogues that we have tested, if it is assumed that approximately equal portions of each isomer are present. However, the original synthesis of the HBF-0259 family was described as favoring the formation of the cis isomers (12), and therefore, it is likely that our sample consists mostly of molecules in the two possible cis conformations. To address these points, we are moving in two separate directions, in conjunction with commercial vendors: (i) we will attempt separation of the mixture by chiral chromatography, with subsequent testing of the isolated stereoisomers in our primary ELISA, and (ii) analogues of IHVR compounds modified by insertion of double bonds into the tetrahydropyrimidine ring to reduce or eliminate the chirality will be synthesized and tested. Through these approaches, we hope to determine both the identity of the active stereoisomer and the importance of the chirality to the biological activity.
Acknowledgments
We thank Xiaodong Xu of Pharmabridge Inc. for critical reading of the manuscript, Courtney Mills and Bertha Conyers for assistance with follow-up studies, Reinhild Prange for plasmid pNI2.SHA, Ramila Philip and Jonathan Krakover of Immunotope Inc. for HPLC-mass spectrometry analysis, and Ying-Hsiu Su for HSV-1.
A. Cuconati is the Bruce Witte Fellow of the Hepatitis B Foundation. This work was supported by an appropriation from the Commonwealth of Pennsylvania and the Hepatitis B Foundation.
Footnotes
Published ahead of print on 17 September 2007.
REFERENCES
- 1.Abramoff, M. D., P. J. Magelhaes, and S. J. Ram. 2004. Image processing with ImageJ. Biophotonics Int. 11:36-42. [Google Scholar]
- 2.Beasley, R. P. 1988. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer 61:1942-1956. [DOI] [PubMed] [Google Scholar]
- 3.Block, T. M., and R. Jordan. 2001. Iminosugars as possible broad spectrum anti hepatitis virus agents: the glucovirs and alkovirs. Antivir. Chem. Chemother. 12:317-325. [DOI] [PubMed] [Google Scholar]
- 4.Block, T. M., X. Lu, A. S. Mehta, B. S. Blumberg, B. Tennant, M. Ebling, B. Korba, D. M. Lansky, G. S. Jacob, and R. A. Dwek. 1998. Treatment of chronic hepadnavirus infection in a woodchuck animal model with an inhibitor of protein folding and trafficking. Nat. Med. 4:610-614. [DOI] [PubMed] [Google Scholar]
- 5.Bruns, M., S. Miska, S. Chassot, and H. Will. 1998. Enhancement of hepatitis B virus infection by noninfectious subviral particles. J. Virol. 72:1462-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bruss, V. 2004. Envelopment of the hepatitis B virus nucleocapsid. Virus Res. 106:199-209. [DOI] [PubMed] [Google Scholar]
- 7.Chisari, F. V., P. Filippi, J. Buras, A. McLachlan, H. Popper, C. A. Pinkert, R. D. Palmiter, and R. L. Brinster. 1987. Structural and pathological effects of synthesis of hepatitis B virus large envelope polypeptide in transgenic mice. Proc. Natl. Acad. Sci. USA 84:6909-6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cocchi, F., L. Menotti, P. Dubreuil, M. Lopez, and G. Campadelli-Fiume. 2000. Cell-to-cell spread of wild-type herpes simplex virus type 1, but not of syncytial strains, is mediated by the immunoglobulin-like receptors that mediate virion entry, nectin1 (PRR1/HveC/HIgR) and nectin2 (PRR2/HveB). J. Virol. 74:3909-3917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colonno, R. J., R. Rose, C. J. Baldick, S. Levine, K. Pokornowski, C. F. Yu, A. Walsh, J. Fang, M. Hsu, C. Mazzucco, B. Eggers, S. Zhang, M. Plym, K. Klesczewski, and D. J. Tenney. 2006. Entecavir resistance is rare in nucleoside naive patients with hepatitis B. Hepatology 44:1656-1665. [DOI] [PubMed] [Google Scholar]
- 10.Delaney, W. E., IV, R. Edwards, D. Colledge, T. Shaw, P. Furman, G. Painter, and S. Locarnini. 2002. Phenylpropenamide derivatives AT-61 and AT-130 inhibit replication of wild-type and lamivudine-resistant strains of hepatitis B virus in vitro. Antimicrob. Agents Chemother. 46:3057-3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deres, K., C. H. Schroder, A. Paessens, S. Goldmann, H. J. Hacker, O. Weber, T. Kramer, U. Niewohner, U. Pleiss, J. Stoltefuss, E. Graef, D. Koletzki, R. N. Masantschek, A. Reimann, R. Jaeger, R. Gross, B. Beckermann, K. H. Schlemmer, D. Haebich, and H. Rubsamen-Waigmann. 2003. Inhibition of hepatitis B virus replication by drug-induced depletion of nucleocapsids. Science 299:893-896. [DOI] [PubMed] [Google Scholar]
- 12.Desenko, S. M., E. S. Gladkov, S. A. Komykhov, O. V. Shishkin, and V. D. Orlov. 2001. Partially hydrogenated aromatic substituted tetrazolo[1,5-a]pyrimidines. Chem. Heterocyclic Compounds 37:747-754. [Google Scholar]
- 13.Fallows, D. A., and S. P. Goff. 1995. Mutations in the epsilon sequences of human hepatitis B virus affect both RNA encapsidation and reverse transcription. J. Virol. 69:3067-3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrari, C., A. Penna, A. Bertoletti, A. Valli, A. D. Antoni, T. Giuberti, A. Cavalli, M. A. Petit, and F. Fiaccadori. 1990. Cellular immune response to hepatitis B virus-encoded antigens in acute and chronic hepatitis B virus infection. J. Immunol. 145:3442-3449. [PubMed] [Google Scholar]
- 15.Foo, N. C., B. Y. Ahn, X. Ma, W. Hyun, and T. S. Yen. 2002. Cellular vacuolization and apoptosis induced by hepatitis B virus large surface protein. Hepatology 36:1400-1407. [DOI] [PubMed] [Google Scholar]
- 16.Ganem, G., and R. J. Schneider. 2001. Hepadnaviridae: the viruses and their replication, p. 2923-2969. In D. Knipe and P. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 17.Gerlich, W. H. 1992. Structure and molecular virology, p. 83-112. In A. Maclachlan (ed.), Molecular biology of HBV. CRC Press, Boca Raton, FL.
- 18.Gerlich, W. H., K. H. Heermann, and X. Lu. 1992. Functions of hepatitis B surface proteins. Arch. Virol. Suppl. 4:129-132. [DOI] [PubMed] [Google Scholar]
- 19.Gish, R. G. 2005. Clinical trial results of new therapies for HBV: implications for treatment guidelines. Semin. Liver Dis. 25(Suppl. 1):29-39. [DOI] [PubMed] [Google Scholar]
- 20.Guo, H., W. S. Mason, C. E. Aldrich, J. R. Saputelli, D. S. Miller, A. R. Jilbert, and J. E. Newbold. 2005. Identification and characterization of avihepadnaviruses isolated from exotic anseriformes maintained in captivity. J. Virol. 79:2729-2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hadziyannis, S. J., N. C. Tassopoulos, E. J. Heathcote, T. T. Chang, G. Kitis, M. Rizzetto, P. Marcellin, S. G. Lim, Z. Goodman, J. Ma, S. Arterburn, S. Xiong, G. Currie, and C. L. Brosgart. 2005. Long-term therapy with adefovir dipivoxil for HBeAg-negative chronic hepatitis B. N. Engl. J. Med. 352:2673-2681. [DOI] [PubMed] [Google Scholar]
- 22.Heermann, K. H., U. Goldmann, W. Schwartz, T. Seyffarth, H. Baumgarten, and W. H. Gerlich. 1984. Large surface proteins of hepatitis B virus containing the pre-S sequence. J. Virol. 52:396-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hollinger, F. B., and T. J. Liang. 2001. Hepatitis B virus, p. 2971-3036. In D. Knipe and P. Howley (ed.), Fields virology, vol. 2. Lippincott Williams & Wilkins, Philadelphia, PA. [Google Scholar]
- 24.Hoofnagle, J. H. 1998. Therapy of viral hepatitis. Digestion 59:563-578. [DOI] [PubMed] [Google Scholar]
- 25.Hoofnagle, J. H., and A. M. D. Bisceglie. 1997. The treatment of chronic viral hepatitis. N. Engl. J. Med. 336:347-356. [DOI] [PubMed] [Google Scholar]
- 26.Huovila, A. P., A. M. Eder, and S. D. Fuller. 1992. Hepatitis B surface antigen assembles in a post-ER, pre-Golgi compartment. J. Cell Biol. 118:1305-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang, E. H., T. Y. Kown, G. T. Oh, W. F. Park, S. I. Park, S. K. Park, and Y. I. Lee. 2006. The flavonoid ellagic acid from a medicinal herb inhibits host immune tolerance induced by the hepatitis B virus-e antigen. Antivir. Res. 72:100-106. [DOI] [PubMed] [Google Scholar]
- 28.King, R. W., S. K. Ladner, T. J. Miller, K. Zaifert, R. B. Perni, S. C. Conway, and M. J. Otto. 1998. Inhibition of human hepatitis B virus replication by AT-61, a phenylpropenamide derivative, alone and in combination with (−)beta-l-2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 42:3179-3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kock, J., and H. J. Schlicht. 1993. Analysis of the earliest steps of hepadnavirus replication: genome repair after infectious entry into hepatocytes does not depend on viral polymerase activity. J. Virol. 67:4867-4874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ladner, S. K., M. J. Otto, C. S. Barker, K. Zaifert, G. H. Wang, J. T. Guo, C. Seeger, and R. W. King. 1997. Inducible expression of human hepatitis B virus (HBV) in stably transfected hepatoblastoma cells: a novel system for screening potential inhibitors of HBV replication. Antimicrob. Agents Chemother. 41:1715-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai, C. L., J. Dienstag, E. Schiff, N. W. Leung, M. Atkins, C. Hunt, N. Brown, M. Woessner, R. Boehme, and L. Condreay. 2003. Prevalence and clinical correlates of YMDD variants during lamivudine therapy for patients with chronic hepatitis B. Clin. Infect. Dis. 36:687-696. [DOI] [PubMed] [Google Scholar]
- 32.Lai, C. L., S. G. Lim, N. A. Brown, X. J. Zhou, D. M. Lloyd, Y. M. Lee, M. F. Yuen, G. C. Chao, and M. W. Myers. 2004. A dose-finding study of once-daily oral telbivudine in HBeAg-positive patients with chronic hepatitis B virus infection. Hepatology 40:719-726. [DOI] [PubMed] [Google Scholar]
- 33.Lee, W. M. 1997. Hepatitis B virus infection. N. Engl. J. Med. 337:1733-1745. [DOI] [PubMed] [Google Scholar]
- 34.Lenhoff, R. J., and J. Summers. 1994. Coordinate regulation of replication and virus assembly by the large envelope protein of an avian hepadnavirus. J. Virol. 68:4565-4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liaw, Y. F. 2001. Impact of YMDD mutations during lamivudine therapy in patients with chronic hepatitis B. Antivir. Chem. Chemother. 12(Suppl. 1):67-71. [PubMed] [Google Scholar]
- 36.Liu, M. C., M. Yu, N. L. Zhang, W. B. Gong, Y. Wang, W. H. Piao, Q. H. Wang, and G. Q. Wang. 2004. Dynamic analysis of hepatitis B virus DNA and its antigens in 2.2.15 cells. J. Viral Hepat. 11:124-129. [DOI] [PubMed] [Google Scholar]
- 37.London, W. T. 1981. Primary hepatocellular carcinoma—etiology, pathogenesis, and prevention. Hum. Pathol. 12:1085-1097. [DOI] [PubMed] [Google Scholar]
- 38.Mailliard, M. E., and J. L. Gollan. 2006. Emerging therapeutics for chronic hepatitis B. Annu. Rev. Med. 57:155-166. [DOI] [PubMed] [Google Scholar]
- 39.Mangold, C. M., and R. E. Streeck. 1993. Mutational analysis of the cysteine residues in the hepatitis B virus small envelope protein. J. Virol. 67:4588-4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mangold, C. M., F. Unckell, M. Werr, and R. E. Streeck. 1997. Analysis of intermolecular disulfide bonds and free sulfhydryl groups in hepatitis B surface antigen particles. Arch. Virol. 142:2257-2267. [DOI] [PubMed] [Google Scholar]
- 41.Mangold, C. M., F. Unckell, M. Werr, and R. E. Streeck. 1995. Secretion and antigenicity of hepatitis B virus small envelope proteins lacking cysteines in the major antigenic region. Virology 211:535-543. [DOI] [PubMed] [Google Scholar]
- 42.Mehta, A. S., B. Gu, B. Conyers, S. Ouzounov, L. Wang, R. M. Moriarty, R. A. Dwek, and T. M. Block. 2004. α-Galactosylceramide and novel synthetic glycolipids directly induce the innate host defense pathway and have direct activity against hepatitis B and C viruses. Antimicrob. Agents Chemother. 48:2085-2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menne, S., C. A. Roneker, B. E. Korba, J. L. Gerin, B. C. Tennant, and P. J. Cote. 2002. Immunization with surface antigen vaccine alone and after treatment with 1-(2-fluoro-5-methyl-β-l-arabinofuranosyl)-uracil (l-FMAU) breaks humoral and cell-mediated immune tolerance in chronic woodchuck hepatitis virus infection. J. Virol. 76:5305-5314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menne, S., C. A. Roneker, B. C. Tennant, B. E. Korba, J. L. Gerin, and P. J. Cote. 2002. Immunogenic effects of woodchuck hepatitis virus surface antigen vaccine in combination with antiviral therapy: breaking of humoral and cellular immune tolerance in chronic woodchuck hepatitis virus infection. Intervirology 45:237-250. [DOI] [PubMed] [Google Scholar]
- 45.Michalak, T. I., P. D. Hodgson, and N. D. Churchill. 2000. Posttranscriptional inhibition of class I major histocompatibility complex presentation on hepatocytes and lymphoid cells in chronic woodchuck hepatitis virus infection. J. Virol. 74:4483-4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miyakawa, Y., H. Okamoto, and M. Mayumi. 1997. The molecular basis of hepatitis B e antigen (HBeAg)-negative infections. J. Viral Hepat. 4:1-8. [DOI] [PubMed] [Google Scholar]
- 47.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 48.Ou, J. H. 1997. Molecular biology of hepatitis B virus e antigen. J. Gastroenterol. Hepatol 12:S178-S187. [DOI] [PubMed] [Google Scholar]
- 49.Patzer, E. J., G. R. Nakamura, and A. Yaffe. 1984. Intracellular transport and secretion of hepatitis B surface antigen in mammalian cells. J. Virol. 51:346-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Robinson, W. S., L. Klote, and N. Aoki. 1990. Hepadnaviruses in cirrhotic liver and hepatocellular carcinoma. J. Med. Virol. 31:18-32. [DOI] [PubMed] [Google Scholar]
- 51.Seeger, C., and W. S. Mason. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seeger, C., J. Summers, and W. S. Mason. 1991. Viral DNA synthesis. Curr. Top. Microbiol. Immunol. 168:41-60. [DOI] [PubMed] [Google Scholar]
- 53.Sells, M. A., M. L. Chen, and G. Acs. 1987. Production of hepatitis B virus particles in Hep G2 cells transfected with cloned hepatitis B virus DNA. Proc. Natl. Acad. Sci. USA 84:1005-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shin, M. S., E. H. Kang, and Y. I. Lee. 2005. A flavonoid from medicinal plants blocks hepatitis B virus-e antigen secretion in HBV-infected hepatocytes. Antivir. Res. 67:163-168. [DOI] [PubMed] [Google Scholar]
- 55.Simsek, E., A. Mehta, T. Zhou, R. A. Dwek, and T. Block. 2005. Hepatitis B virus large and middle glycoproteins are degraded by a proteasome pathway in glucosidase-inhibited cells but not in cells with functional glucosidase enzyme. J. Virol. 79:12914-12920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stray, S. J., C. R. Bourne, S. Punna, W. G. Lewis, M. G. Finn, and A. Zlotnick. 2005. A heteroaryldihydropyrimidine activates and can misdirect hepatitis B virus capsid assembly. Proc. Natl. Acad. Sci. USA 102:8138-8143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tenney, D. J., S. M. Levine, R. E. Rose, A. W. Walsh, S. P. Weinheimer, L. Discotto, M. Plym, K. Pokornowski, C. F. Yu, P. Angus, A. Ayres, A. Bartholomeusz, W. Sievert, G. Thompson, N. Warner, S. Locarnini, and R. J. Colonno. 2004. Clinical emergence of entecavir-resistant hepatitis B virus requires additional substitutions in virus already resistant to lamivudine. Antimicrob. Agents Chemother. 48:3498-3507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terrault, N. 2000. Management of hepatitis B virus infection in liver transplant recipients: prospects and challenges. Clin. Transplant 14(Suppl. 2):39-43. [PubMed] [Google Scholar]
- 59.Tipples, G. A., M. M. Ma, K. P. Fischer, V. G. Bain, N. M. Kneteman, and D. L. Tyrrell. 1996. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology 24:714-717. [DOI] [PubMed] [Google Scholar]
- 60.Wang, J., and T. I. Michalak. 2006. Inhibition by woodchuck hepatitis virus of class I major histocompatibility complex presentation on hepatocytes is mediated by virus envelope pre-S2 protein and can be reversed by treatment with gamma interferon. J. Virol. 80:8541-8553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wong, T., and S. S. Lee. 2006. Hepatitis C: a review for primary care physicians. Can. Med. Assoc. J. 174:649-659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yuen, M. F., and C. L. Lai. 2005. Telbivudine: an upcoming agent for chronic hepatitis B. Expert Rev. Anti-Infect Ther. 3:489-494. [DOI] [PubMed] [Google Scholar]
- 63.Zhou, T., H. Guo, J. T. Guo, A. Cuconati, A. Mehta, and T. M. Block. 2006. Hepatitis B virus e antigen production is dependent upon covalently closed circular (ccc) DNA in HepAD38 cell cultures and may serve as a cccDNA surrogate in antiviral screening assays. Antivir. Res. 72:116-124. [DOI] [PubMed] [Google Scholar]
- 64.Zlotnick, A., P. Ceres, S. Singh, and J. M. Johnson. 2002. A small molecule inhibits and misdirects assembly of hepatitis B virus capsids. J. Virol. 76:4848-4854. [DOI] [PMC free article] [PubMed] [Google Scholar]