Abstract
Carbon monoxide (CO) is endogenously produced in the human body, mainly from the oxidation of heme catalyzed by heme oxygenase (HO) enzymes. The induction of HO and the consequent increase in CO production play important physiological roles in vasorelaxation and neurotransmission and in the immune system. The exogenous administration of CO gas and CO-releasing molecules (CO-RMs) has been shown to induce vascular effects and to alleviate hypoxia-reoxygenation injury of mammalian cells. In particular, due to its anti-inflammatory, antiapoptotic, and antiproliferative properties, CO inhibits ischemic-reperfusion injury and provides potent cytoprotective effects during organ and cell transplantation. In spite of these findings regarding the physiology and biology of mammals, nothing is known about the action of CO on bacteria. In the present work, we examined the effect of CO on bacterial cell proliferation. Cell growth experiments showed that CO caused the rapid death of the two pathogenic bacteria tested, Escherichia coli and Staphylococcus aureus, particularly when delivered through organometallic CO-RMs. Of importance is the observation that the effectiveness of the CO-RMs was greater in near-anaerobic environments, as many pathogens are anaerobic organisms and pathogen colonization occurs in environments with low oxygen concentrations. Our results constitute the first evidence that CO can be utilized as an antimicrobial agent. We anticipate our results to be the starting point for the development of novel types of therapeutic drugs designed to combat antibiotic-resistant pathogens, which are widespread and presently a major public health concern.
Carbon monoxide (CO) is a colorless and odorless diatomic gas, chemically inert, that occurs in nature as a product of oxidation or combustion of organic matter. Owing to its lethal effect when present in high concentrations, CO was considered for many years to be only an environmental toxicant that results from air pollution by automobile exhaust. The knowledge that the human body is able to produce small quantities of CO and the evidence that CO derived from heme oxygenase activity contributes to important intracellular functions have modified our perception of CO as a pernicious toxin to include its beneficial effects (15, 16, 22). In consequence, the application of CO gas or CO-releasing molecules (CO-RMs) has emerged as a new therapeutic strategy in medicine (10, 13, 18). The evolution of CO from a toxicant to a molecule of mounting importance in mammals finds a parallel in another diatomic molecule, nitric oxide (NO) (17). NO is produced in the body by the nitric oxide synthase and shares with CO many downstream signaling pathways and regulatory functions, in particular, those associated with the activation of soluble guanylyl cyclase (7, 8, 12). In addition, there is an interplay between the two molecules, since it is proposed that CO is a modulator of nitric oxide synthase (10, 22) and NO up-regulates heme oxygenase (19, 20), which in turn catalyzes the oxidative degradation of free heme into biliverdin, with the concomitant release of iron and CO. NO also constitutes one of the weapons that the mammalian immune system uses to fight pathogens (3, 4). The bactericidal function of NO relies on the deleterious effects caused in the pathogen, e.g., the nitrosylation of iron centers. Although CO is a stable neutral molecule with a long half-life, it shares with NO the high affinity for iron of heme proteins, which is the basis of its toxicity. We therefore set out to explore the possible action of CO on bacterial growth rates. For this purpose, we tested the bioactivity of CO, applied either in the gaseous form or via treatment with CO-RMs, on Escherichia coli and Staphylococcus aureus. These bacteria are major human pathogens that are widespread in the community and are responsible for hospital-acquired infections, exhibiting a concerning degree of antibiotic resistance.
MATERIALS AND METHODS
Reagents.
The different sources or references for CO were as follows: tricarbonyldichlororuthenium(II) dimer (CORM-2), Sigma; tricarbonylchloro(glycinato)ruthenium(II) (CORM-3), reference 6; bromo(pentacarbonyl)manganese (ALF 021), reference 5; and tetraethylammonium molybdenum pentacarbonyl bromide (ALF 062), reference 2. All compounds were freshly prepared as 10 mM stock solutions by dissolution in dimethyl sulfoxide, pure distilled water, or methanol.
Bacterial strains and growth conditions.
E. coli K-12 ATCC 23716 and S. aureus NCTC8325 were grown in minimal salts (MS) medium (1.3% [wt/vol] Na2HPO4, 0.3% [wt/vol] KH2PO4, 0.05% [wt/vol] NaCl, and 0.1% [wt/vol] NH4Cl supplemented with 20 mM glucose, 2 mM MgSO4, 100 μM CaCl2, and 0.25% [wt/vol] Casamino Acids) and in Luria-Bertani (LB) medium (1% [wt/vol] tryptone, 0.5% [wt/vol] yeast extract, and 1% [wt/vol] NaCl), respectively, under different oxygen supply conditions. Aerobic experiments were undertaken with flasks filled to one-fifth of their volume, microaerobic tests were conducted with closed flasks filled to one-half of their volume, and anaerobic conditions were produced in rubber-sealed flasks that, once filled with medium and closed, were extensively fluxed with nitrogen gas.
CO gas and CO-RM treatment.
Overnight cultures of E. coli or S. aureus grown in LB or tryptic soy broth, respectively, were used to inoculate fresh MS medium (E. coli) or LB medium (S. aureus), and the cultures on fresh medium were incubated at 37°C under the required aeration conditions to an optical density at 600 nm of 0.3. At this point, cells were exposed to a flux of CO gas for 15 min or to CO-RMs. Untreated cells were bubbled with nitrogen gas or treated with dimethyl sulfoxide, water, or methanol, depending on the solvent used to dissolve the CO-RM.
The inactive form of ALF 062 was prepared by mixing vigorously with 20% methanol in a closed flask over 2 to 3 h. The counterion of ALF 062, tetraethyl ammonium bromide, and one of the products of ALF 062 decomposition, sodium molybdate, were used at the same concentration as ALF 062 (50 μM).
Viability assays.
The number of viable cells was evaluated by measuring the CFU per milliliter upon plating serial dilutions of the various cultures onto agar plates. The percent survival was calculated as the number of colonies originated by treated cultures divided by the number of colonies formed upon the plating of untreated cultures. Sensitivity tests were conducted by plating 5-μl serial dilutions of cultures grown for 4 h and treated with CO-RMs, with or without the CO scavenger hemoglobin (Hb [bovine form used at 20 μM; Sigma]), onto agar. The experiments were performed with a minimum of three independent cultures, and the results are presented in the figures as averaged values with error bars representing one standard deviation.
The investigation of MICs and minimal bactericidal concentrations (MBCs) was carried out by the tube dilution test. Briefly, 2.5 ml of minimal medium was inoculated with an overnight culture of E. coli or S. aureus to give an optical density at 600 nm of 0.005 to 0.01. Different concentrations of CORM-2, between 150 μM and 2 mM, were added to the diluted suspensions in the wells of 24-well plates, and the plates were incubated for at least 18 h at 37°C and 90 rpm. The concentration of CORM-2 in the first well in the series with no sign of visible growth was reported as the MIC. All the cultures that exhibited a lack of cell growth were then subsequently plated onto agar devoid of any drug. After incubation at 37°C for 24 h, the lowest concentration of CORM-2 in a culture with no growth was assumed to be the MBC.
CO release kinetics.
CO-RMs were mixed with MS or LB medium in sealed vessels, and the vessels were incubated at room temperature under constant stirring and protected from light. Gas samples were collected after 30 min and 4 h and analyzed using a gas chromatograph (Thermo Finnigan Trace) equipped with a CTRI column (Alltech) and a thermal conductivity detector. The CO released was quantified using a calibration curve recorded prior to the reaction course.
Inductively coupled plasma mass spectrometry analysis.
E. coli cells cultured in MS medium with or without 50 μM ALF 062 were collected after 1 h of growth, and the cellular metal content was analyzed at Instituto de Investigação das Pescas e do Mar, Lisbon, Portugal. The intracellular concentration of Mo in E. coli cultures was assayed on a quadropole inductively coupled plasma mass spectrometer (X series; Thermo Elemental) equipped with a Peltier impact bead spray chamber and a concentric Meinhard nebulizer. The experimental parameters were as follows: 790 W of forward power, peak jumping mode, and 150 sweeps per replicate (dwell time, 10 ms; dead time, 30 ns). A seven-point calibration within a range of 1 to 100 μg liter−1 was used to quantify metal concentrations. Coefficients of variation for determinations of metal content (n = 5) ranged between 0.5 and 2%. The precision and accuracy of metal concentration measurements, as determined through the repeated analysis of reference materials (TORT-1, TORT-2, DORM-2, and DORM-3 from the National Research Council of Canada) by using indium as an internal standard, were within 1 to 2%. Procedural blanks always accounted for less than 1% of the total molybdenum concentrations in the samples.
RESULTS AND DISCUSSION
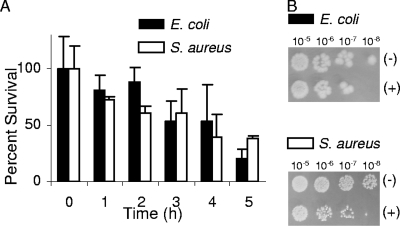
The effect of CO on the viability of bacteria was investigated first by the direct delivery of CO gas. The administration of CO gas, fluxed into the growing cultures, led to a significant growth impairment of E. coli and S. aureus (Fig. 1).
FIG. 1.
Effects of CO gas on E. coli and S. aureus viability. (A) E. coli and S. aureus cells were grown under microaerobic conditions in MS and LB media, respectively, and exposed to a flux of CO gas for 15 min. (B) Sensitivity tests were conducted by plating the indicated serial dilutions of the cultures collected after 4 h of exposure to CO gas (+) or to nitrogen gas (−).
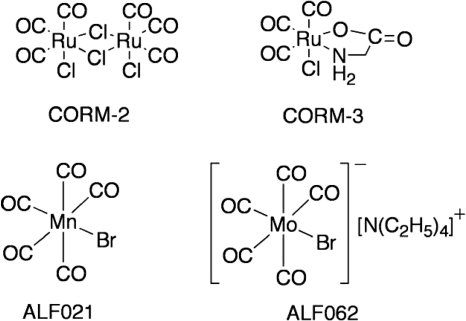
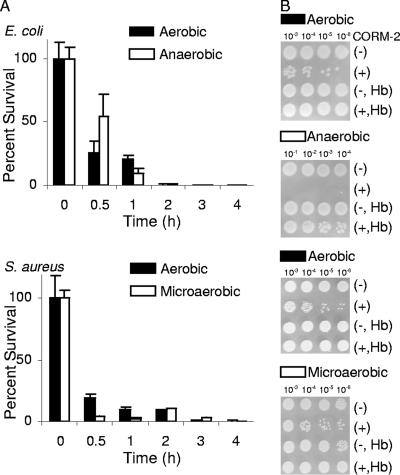
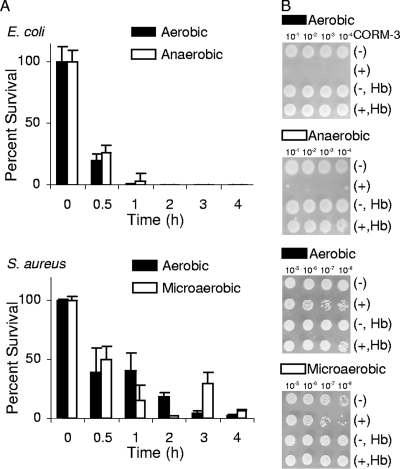
To evaluate the potential of CO-RMs, the compounds indicated in Fig. 2 were selected. CORM-2 and CORM-3 are active in a variety of CO-mediated biological processes, both in vitro and in vivo (9). In the first series of experiments, the effect of CO released from CORM-2 on the growth of E. coli and S. aureus was studied with bacteria cultured under different levels of oxygen supply. Shortly after the exposure to CORM-2, the percentage of surviving cells significantly diminished (Fig. 3). Experiments using water-soluble CORM-3 revealed that, albeit requiring higher concentrations than CORM-2 due to its chemical composition, the compound also strongly decreased the viability of E. coli and S. aureus cells (Fig. 4). However, while the addition of CORM-3 resulted in a strong inhibition of E. coli cell growth, S. aureus was more resistant to CORM-3 (Fig. 4A), particularly under aerobic conditions. In general, the action of the two compounds was rapid and extended over time, as cells did not resume growth over the subsequent 4 h (Fig. 3 and 4) or after 8 h (data not shown).
FIG. 2.
Chemical structures of CO-RMs used in this study.
FIG. 3.
Effects of CORM-2 on E. coli and S. aureus cell viability. (A) E. coli cells were grown in MS under aerobic and anaerobic conditions and treated with 250 μM CORM-2. S. aureus cells were grown aerobically and microaerobically in LB medium and exposed to 250 μM CORM-2. (B) Results of tests of the sensitivity of cultures to CORM-2 (see Materials and Methods). The indicated dilutions of cultures were treated with CORM-2 (+; 250 μM) or left untreated (−) and assayed in the absence or in the presence of Hb.
FIG. 4.
Effects of CORM-3 on E. coli and S. aureus cell viability. (A) E. coli cells were grown in MS medium either aerobically or anaerobically and treated with 400 μM CORM-3. S. aureus cells were grown aerobically or microaerobically in LB medium to which 500 or 400 μM CORM-3 was added, respectively. (B) Sensitivity tests were conducted by plating dilutions of cultures grown as described in Materials and Methods after exposure to CORM-3 (+) or no treatment (−) in the absence or in the presence of Hb. The concentrations of CORM-3 used were the same as those indicated in the legend to panel A.
In order to examine whether the bactericidal effect of CO-RMs was due to CO, cell growth experiments with CO-RMs were also performed in the presence of Hb, a high-affinity CO scavenger. In all cases, the bactericidal effect on E. coli and S. aureus was completely lost (Fig. 3B and 4B), thus demonstrating that the antimicrobial action of CO-RMs is dependent on their release of CO.
Bactericidal activity has been defined as a ratio of the MBC to the MIC of <4 (14). The determination of the CORM-2 MBC/MIC ratios for E. coli and S. aureus to be 1.5 and 1.0, respectively, revealed the bactericidal character of CORM-2.
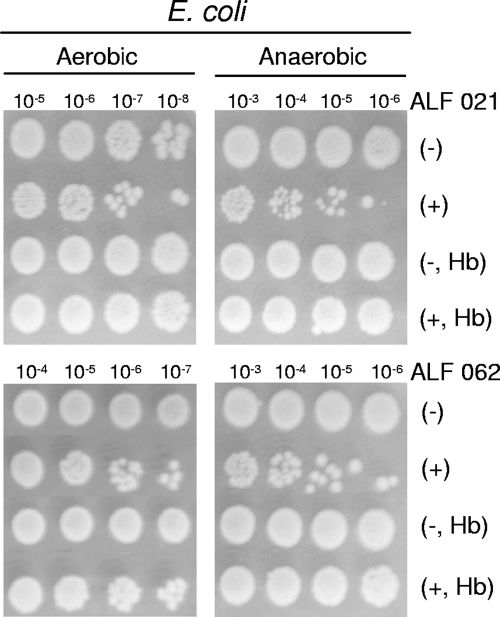
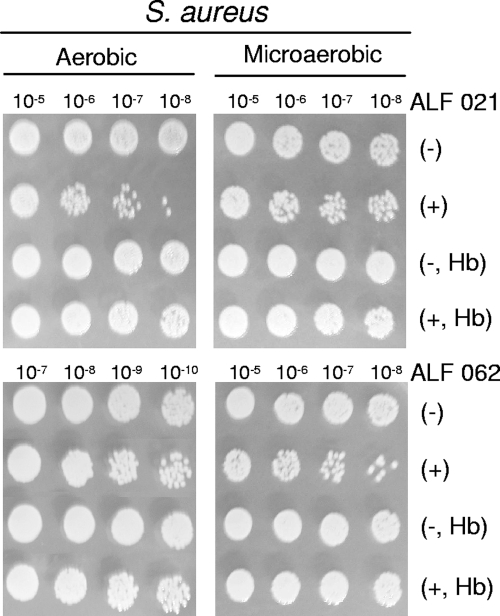
The two other CO-RMs used to investigate the bactericidal effect of CO, namely, manganese carbonyl ALF 021 and molybdenum carbonyl ALF 062, were also seen to be capable of strongly reducing the viability of E. coli and S. aureus (Fig. 5 and 6). Again, the addition of Hb completely eliminated the harmful action of ALF compounds on the two bacteria (Fig. 5 and 6). Furthermore, to ensure that the activity of ALF 062 was not related to its decomposition products, we tested the effects of tetraethyl ammonium bromide, sodium molybdate, and a solution of inactivated ALF 062, obtained after the cessation of CO release (see Materials and Methods), on bacterial growth. None of these compounds had bactericidal properties or altered growth kinetics (data not shown). Therefore, the bactericidal effects of ALF 062 are due to its capacity to release CO.
FIG. 5.
Sensitivity of E. coli to ALF 021 and ALF 062 compounds. E. coli cells grown under aerobic or anaerobic conditions were treated with 500 or 200 μM ALF 021, respectively, and with 50 μM ALF 062 (see Materials and Methods) in the absence or in the presence of Hb. The indicated dilutions of cultures exposed to CO-RMs (+) or not exposed (−) were subjected to sensitivity tests.
FIG. 6.
Sensitivity of S. aureus to ALF 021 and ALF 062 compounds. S. aureus cells grown under aerobic and microaerobic conditions were treated with 600 μM ALF 021 and 50 μM ALF 062. The indicated dilutions of cultures exposed to CO-RMs (+) or not exposed (−) were subjected to sensitivity tests in the absence or in the presence of Hb, as described in Materials and Methods.
It should be mentioned that neither CORM-2 nor CORM-3 releases CO gas when dissolved in the media utilized, even at concentrations higher than those used in our experiments (Table 1). Furthermore, although ALF 021 and ALF 062 release CO gas upon dissolution in the medium, they do so in rather small amounts within the time scale of the experiment (Table 1). However, inductively coupled plasma mass spectrometry analysis of E. coli cells incubated with ALF 062 revealed a very large increase in the content of Mo (155 μg g−1) compared to that in control cells (2.5 μg g−1), confirming that the Mo from ALF 062 accumulates inside the E. coli cells, where it releases CO to the cellular targets.
TABLE 1.
CO released into medium by CO-RMsa
| CO-RM (concn, mM) | CO equivalent in MS medium at:
|
CO equivalent in LB medium at:
|
||
|---|---|---|---|---|
| 30 min | 240 min | 30 min | 240 min | |
| CORM-2 (5) | 0 | 0 | 0 | 0.1 |
| CORM-3 (12) | 0 | 0 | 0 | 0 |
| ALF 021 (6) | 0 | 0.5 | 0 | 0.5 |
| ALF 062 (6) | 1.4 | 3.8 | 0.2 | 1.6 |
Amounts of CO are expressed as CO equivalents (number of CO groups released per CO-RM molecule).
Since the bactericidal effect of the CO-RMs does not require the release of CO gas to the extracellular medium (Table 1), we must conclude that CO has to be delivered to the cellular targets directly from the CO-RMs. Because Mo from bactericidally active (CO-loaded) ALF 062 is found to accumulate rapidly within cells, we infer that it transports CO and delivers it into the intracellular space, where it reaches the cellular targets and causes the decrease of bacterial cell viability. If Hb is present in the medium, the high affinity of Hb for CO results in a fast transfer (or abstraction) of the active CO from the CO-RMs (or from gas) to the protein hemes and the effective scavenging of CO as CO-Hb (see below). Under these conditions, no CO will be available for intracellular delivery and the cells remain alive.
Albeit with some minor deviations, the general pattern of our results shows that CO-RM toxicity is enhanced when growth occurs under lower oxygen concentrations. For example, ALF 021 was more effective in reducing the viability of E. coli cells grown anaerobically (200 μM ALF 021) than that of cells grown aerobically (500 μM ALF 021). The augmentation of the effect of CO at low oxygen concentrations may be explained by the preferential binding of CO to the ferrous form of heme proteins, which are predominant under reducing environments. More importantly, the bactericidal effect of CO-RMs under anaerobic conditions indicates that growth inhibition is not restricted to the impairment of the respiratory chain by the binding of CO to cytochrome oxidase, which is likely to contribute to the bactericidal activity of these compounds under aerobic conditions. This fact is quite important since pathogen colonization occurs in near-anaerobic environments and since many pathogens are anaerobic organisms. On the other hand, the type of bacterial cell wall also seems not to interfere with the action of CO-RMs, as judged by the similar decreases in cell viability observed for the gram-positive (S. aureus) and gram-negative (E. coli) species upon treatment with the same CO-RM. Hence, CO-RMs have the potential for use as bactericides against a wide range of microorganisms independently of the type of bacterial cell wall and oxygen growth requirements.
The difference between the degrees of action of dissolved molecular CO gas and CO-RMs is striking. When administered as gas, CO had to be present in rather high concentrations (ca. 1 mM) to become effective as a bactericide. The ability of CO-RMs to accumulate inside bacterial cells before they release CO makes these compounds highly effective CO donors to bacterial targets, thereby strongly enhancing the bactericidal efficacy of CO. In fact, the CO-RMs used in this study were able to transfer CO to Hb to form CO-Hb, as judged by the shift of the Hb Soret band from 413 to 418 nm (data not shown) and by the results depicted in Fig. 3B, 4B, 5, and 6. Hence, CO-RMs are capable of delivering CO to heme-containing molecules, as had been shown before for the rapid carbonylation of myoglobin by CORM-3 (11). Likewise, the carbonylation of Hb by CORM-2 and CORM-3 occurs within the mixing time, while that by ALF 021 and ALF 062 takes place in less than 15 min. It is well known that the biological effect of CO on mammalian cells is due mainly to its interaction with iron-containing proteins, such as the above-mentioned cytochrome oxidase. In addition to heme proteins and sensors, CO may bind to almost all transition metal-containing proteins, giving rise to structural modifications and alterations of their biological functions. Hence, in bacteria, there are a large number of likely intracellular targets that can account for the toxic effect of CO revealed in this study.
In spite of the increasing expectations for the use of CO in medicine (10, 13, 18), until now, the role of CO as a bactericidal compound had remained unexplored. Nevertheless, in the early 1970s it was reported that the addition of CO to an aerobic culture of E. coli caused a decrease in DNA replication (21). However, as the authors of the study did not observe any effect of CO on cells growing anaerobically on glucose, they concluded that the inhibition of DNA synthesis in cells grown under aerobic conditions was not due to a direct effect on the replication apparatus but resulted from indirect effects, such as ATP or deoxynucleoside triphosphate depletion (21). In more recent years, in spite of several public concerns, CO has been used by the food industry to generate the bright red color of the dark muscle tissue of meat and fish, which results from the great affinity of CO for the Fe(II) binding site of myoglobin. Interestingly, a very recent study of the influence of different packing systems on meat preservation indicated that packages to which CO gas had been added exhibited less bacterial growth than other packages. These results suggest that CO may be one of the packaging gases responsible for the inhibition of the growth of microorganisms (1). We now show that CO and, in particular, CO-RMs have the ability to kill bacteria under aerobic and anaerobic conditions. We submit that CO-RMs constitute a novel class of antibacterial molecules that may become drug candidates upon the development of safe and controllable methods of CO delivery to bacterial targets that avoid the in vivo scavenging of CO by the red blood cells (10). In particular, nonsystemic bactericides may be a relatively easy application for CO-RMs. Although this is a first visualization of a still very distant goal, bactericides based upon completely new concepts are urgently required, as the emergence and spread of drug-resistant bacterial pathogens reveal a concerning decrease in the effectiveness of currently available antibiotics.
Acknowledgments
This work was supported by Fundação para a Ciência e a Tecnologia (FCT) project POCI/SAU-IMI/56088/2004, and L.S.N. and J.D.S. are recipients of FCT grants SFRH/BD/22425/2005 and SFRH/BDE/15501/2004, respectively.
We thank Werner Haas (Alfama, Lda.) for helpful discussions.
Footnotes
Published ahead of print on 8 October 2007.
REFERENCES
- 1.Brooks, J. C., M. M. Brashears, M. F. Miller, A. R. Hoyle, J. D. Kellermeier, and J. M. Mehaffey. 2006. The spoilage characteristics of ground beef packaged in high-oxygen and low-oxygen modified atmosphere packages, p. 61-65. In Proceedings of the Reciprocal Meat Conference. University of Illinois at Urbana-Champaign, Urbana-Champaign.
- 2.Burgmayer, S. J. N., and J. L. Templeton. 1985. Synthesis and structure of a 7-coordinate molybdenum carbonyl fluoride derivative, Et4N Mo(CO)2(S2CNEt2)2F. Inorg. Chem. 24:2224-2230. [Google Scholar]
- 3.Chakravortty, D., and M. Hensel. 2003. Inducible nitric oxide synthase and control of intracellular bacterial pathogens. Microbes Infect. 5:621-627. [DOI] [PubMed] [Google Scholar]
- 4.Fang, F. C. 2004. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat. Rev. Microbiol. 2:820-832. [DOI] [PubMed] [Google Scholar]
- 5.Herrmann, W. A., and G. Brauer (ed.). 1997. Synthetic methods of organometallic and inorganic chemistry, vol. 7. Georg Thieme Verlag, Stuttgart, Germany.
- 6.Johnson, T. R., B. E. Mann, I. P. Teasdale, H. Adams, R. Foresti, C. J. Green, and R. Motterlini. 2007. Metal carbonyls as pharmaceuticals? [Ru(CO)3Cl(glycinate)], a CO-releasing molecule with an extensive aqueous solution chemistry. Dalton Trans. 15:1500-1508. [DOI] [PubMed] [Google Scholar]
- 7.Kharitonov, V. G., V. S. Sharma, R. B. Pilz, D. Magde, and D. Koesling. 1995. Basis of guanylate cyclase activation by carbon monoxide. Proc. Natl. Acad. Sci. USA 92:2568-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moncada, S., and E. A. Higgs. 2006. The discovery of nitric oxide and its role in vascular biology. Br. J. Pharmacol. 147(Suppl. 1):S193-S201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Motterlini, R., J. E. Clark, R. Foresti, P. Sarathchandra, B. E. Mann, and C. J. Green. 2002. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ. Res. 90:E17-E24. [DOI] [PubMed] [Google Scholar]
- 10.Motterlini, R., B. E. Mann, and R. Foresti. 2005. Therapeutic applications of carbon monoxide-releasing molecules. Expert Opin. Investig. Drugs 14:1305-1318. [DOI] [PubMed] [Google Scholar]
- 11.Motterlini, R., P. Sawle, J. Hammad, S. Bains, R. Alberto, R. Foresti, and C. J. Green. 2005. CORM-A1: a new pharmacologically active carbon monoxide-releasing molecule. FASEB J. 19:284-286. [DOI] [PubMed] [Google Scholar]
- 12.Mungrue, I. N., D. S. Bredt, D. J. Stewart, and M. Husain. 2003. From molecules to mammals: what's NOS got to do with it? Acta Physiol. Scand. 179:123-135. [DOI] [PubMed] [Google Scholar]
- 13.Nakao, A., A. M. Choi, and N. Murase. 2006. Protective effect of carbon monoxide in transplantation. J. Cell. Mol. Med. 10:650-671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pankey, G. A., and L. D. Sabath. 2004. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram-positive bacterial infections. Clin. Infect. Dis. 38:864-870. [DOI] [PubMed] [Google Scholar]
- 15.Piantadosi, C. A. 2002. Biological chemistry of carbon monoxide. Antioxid. Redox Signal. 4:259-270. [DOI] [PubMed] [Google Scholar]
- 16.Ryter, S. W., J. Alam, and A. M. Choi. 2006. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol. Rev. 86:583-650. [DOI] [PubMed] [Google Scholar]
- 17.Ryter, S. W., D. Morse, and A. M. Choi. 2004. Carbon monoxide: to boldly go where NO has gone before. Sci. STKE 2004:RE6. [DOI] [PubMed] [Google Scholar]
- 18.Ryter, S. W., and L. E. Otterbein. 2004. Carbon monoxide in biology and medicine. Bioessays 26:270-280. [DOI] [PubMed] [Google Scholar]
- 19.Ryter, S. W., L. E. Otterbein, D. Morse, and A. M. Choi. 2002. Heme oxygenase/carbon monoxide signaling pathways: regulation and functional significance. Mol. Cell. Biochem. 234-235:249-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Srisook, K., C. Kim, and Y. N. Cha. 2005. Role of NO in enhancing the expression of HO-1 in LPS-stimulated macrophages. Methods Enzymol. 396:368-377. [DOI] [PubMed] [Google Scholar]
- 21.Weigel, P. H., and P. T. Englund. 1975. Inhibition of DNA replication in Escherichia coli by cyanide and carbon monoxide. J. Biol. Chem. 250:8536-8542. [PubMed] [Google Scholar]
- 22.Wu, L., and R. Wang. 2005. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol. Rev. 57:585-630. [DOI] [PubMed] [Google Scholar]