Abstract
This study evaluates the effect of renal impairment on the pharmacokinetics of telbivudine. Thirty-six subjects were assigned, on the basis of creatinine clearance (CLCR), to 1 of 5 renal function groups with 6 to 8 subjects per group: normal renal function; mild, moderate, or severe renal impairment; or end-stage renal disease [ESRD] requiring hemodialysis. Subjects received a single oral dose of telbivudine at 600 mg (normal function and mild impairment), 400 mg (moderate impairment), or 200 mg (severe impairment and ESRD); plasma and/or urine samples were collected over a 48-h period for pharmacokinetic analyses. Telbivudine was well tolerated by all subjects. The pharmacokinetics of 600 mg of telbivudine were comparable for subjects with mild renal impairment and normal renal function. Likewise, for subjects with moderate to severe impairment, including ESRD, reduced doses from 200 to 400 mg produced plasma exposure similar to that for subjects with normal renal function. These results indicate that the pharmacokinetics of telbivudine were dependent on renal function, especially for subjects with moderate to severe renal impairment or ESRD. Apparent total plasma clearance, renal clearance (CLR), and urinary excretion of telbivudine decreased as renal function deteriorated. A linear relationship was established between CLR and CLCR. In ESRD subjects, a routine 3.5- to 4-h hemodialysis session removed telbivudine from plasma at an extraction ratio of ∼45%, representing a ∼23% reduction in total exposure. These results suggest that while no adjustment of the telbivudine dose appears necessary for subjects with mild renal impairment, dose adjustment is warranted for those with moderate to severe renal impairment or ESRD in order to achieve optimal plasma exposure.
Telbivudine, an orally bioavailable l-nucleoside with potent anti-hepatitis B virus (HBV) activity (8), has received regulatory approval in the United States and other countries for the treatment of chronic hepatitis B. Clinical trials with adults with chronic hepatitis B have demonstrated that telbivudine is well tolerated, with marked dose-related antiviral activity (6, 7). The approved telbivudine dose of 600 mg/day reduced serum HBV DNA levels by 3.6 log10 copies/ml after 4 weeks of treatment and by >6 log10 copies/ml after 6 to 12 months (6, 7).
Telbivudine is absorbed rapidly following oral administration, reaching maximum concentrations in plasma at ∼3 h (5, 9-12). Telbivudine exhibits dose-proportional pharmacokinetics with respect to the maximum concentration of the drug in plasma (Cmax) and the area under the plasma concentration-time curve (AUC), and a biphasic plasma disposition (5, 10). Concentrations in blood at steady state are approximately 50% higher than those obtained after single dosing, owing to a second-phase elimination that starts more than 12 h after dosing (5, 11, 12). Telbivudine has a long terminal half-life (t1/2) of approximately 40 to 50 h during this second elimination phase, indicating a sustained exposure to the drug that supports once-daily dosing (5).
Telbivudine undergoes minimal hepatic metabolism and is eliminated primarily by renal clearance as the unchanged drug (1, 12). Hepatic impairment has no effect on telbivudine pharmacokinetics (12). In vitro, telbivudine is converted efficiently into high concentrations of its active 5′-triphosphate derivative in the HepG2 cell line and in human hepatocytes in primary culture (4, 8). Twenty-four hours after exposure of HepG2 cells to 10 μM telbivudine, the active triphosphate derivative reaches peak concentrations of nearly 30 μM, with monophosphate and diphosphate forms present at much lower levels (8). In metabolic decay experiments, telbivudine triphosphate exhibits a long t1/2 of at least 15 h (8).
The pharmacokinetics of nucleoside and nucleotide antivirals, including those currently used in the treatment of chronic HBV infection (i.e., lamivudine, adefovir, and entecavir), are altered for patients with renal impairment due to diminished renal clearance (product information for Epivir-HBV [GlaxoSmithKline, Research Triangle Park, NC], Hepsera [Gilead Sciences, Foster City, CA], and Baraclude [Bristol-Myers Squibb, Princeton, NJ]). In that context, it is anticipated that the pharmacokinetics of telbivudine would also be dependent on renal function. The objective of this study was to evaluate the effects of renal impairment and hemodialysis on the pharmacokinetics and safety of telbivudine administered orally as a single 200- to 600-mg dose, as a basis for optimizing telbivudine exposure for renally impaired patients with chronic hepatitis B.
MATERIALS AND METHODS
Study design.
This was a phase I, open-label, single-dose, parallel-group study to evaluate the pharmacokinetics and safety of telbivudine (β-l-2′-deoxythymidine) for subjects with normal or impaired renal function. A total of 36 subjects, 6 to 8 subjects in each of the five renal function groups (normal function; mild, moderate, or severe impairment; and end-stage renal disease [ESRD] requiring hemodialysis), were enrolled in the study. Subjects with normal or mild renal impairment were administered a single dose of 600 mg of telbivudine; subjects with moderate or severe renal dysfunction received a single dose of 400 or 200 mg of telbivudine, respectively; and subjects with ESRD were administered two single doses of 200 mg of telbivudine, separated by a washout period of 1 week, either 2 h before the start (predialysis dosing) or within 2 h after the completion (postdialysis dosing) of a 3.5- to 4-h hemodialysis session. All doses were administered on an empty stomach after an overnight fasting period of ∼10 h. Subjects remained in the facility from approximately 10 h prior to the start of dosing until discharge (normal through severe categories) or release for washout (ESRD only) 48 h after dosing. The 200-mg telbivudine tablets were manufactured for Idenix Pharmaceuticals, Inc., by Quintiles Limited, Scotland.
Study population.
Subjects with normal or impaired renal function were required to have a creatinine clearance (CLCR), as estimated using the Cockcroft and Gault method (2), of >80 ml/min (normal function) (n = 8), 50 to 80 ml/min (mild impairment) (n = 8), 30 to 49 ml/min (moderate impairment) (n = 8), or <30 ml/min (severe impairment) (n = 6) or to have ESRD requiring hemodialysis (n = 6). Subjects with ESRD were required to have been on chronic hemodialysis for at least 3 months, with interdialysis intervals of 48 h or more.
Eligible subjects were men and women 18 to 75 years old, with a body weight of ≥50 kg for males and ≥45 kg for females but within 35% of normal body weight relative to height and frame size. Subjects were required to have had stable renal function for at least 4 weeks prior to study entry, without a significant medical history or abnormal laboratory findings other than renal disease appropriate to their treatment assignment. Female subjects who were not postmenopausal were required to have a negative pregnancy test upon study entry. Exclusion criteria included the following: a history of cystic fibrosis, uncontrolled congestive heart failure, myocardial infarction within 6 months, cancer (other than resolved skin cancer) within 5 years or stroke within 6 months prior to the study; uncontrolled insulin-dependent diabetes mellitus; a positive screen for hepatitis B surface antigen (HBsAg), antibodies against HCV, or antibodies against human immunodeficiency virus; clinical evidence of an infection; positive prestudy alcohol breath test or positive screen for other drugs of abuse; a clinically significant decrease in gut motility; hematocrit of <33% or hemoglobin level of <11 g/dl for subjects with normal renal function or mild renal impairment; hematocrit of <28% or hemoglobin level of <9.5 g/dl for subjects with moderate or severe renal impairment; or a requirement for comedication known to affect renal tubular function, except for the use of diuretics. Those with normal renal function agreed to avoid prescription medications from 7 days prior to dosing until after the final blood draw and to take no over-the-counter medications from 3 days prior to dosing until after the final blood draw. Those with renal impairment were allowed to continue over-the-counter and prescription medications, except for 8 h before and 4 h after dosing. All subjects were instructed to avoid caffeine, methylxanthine-containing products, and alcohol from 3 days prior to dosing until after the final blood draw. Within 3 weeks prior to dosing, subjects underwent a screening visit that included assessment of CLCR, medical history, physical examination, vital signs, clinical laboratory testing, and an electrocardiogram (ECG).
All subjects gave written, informed consent prior to participation in the study. The trial was conducted at the Orlando Clinical Research Center (Orlando, FL), the New Orleans Center for Clinical Research (New Orleans, LA), and DaVita Clinical Research (Minneapolis, MN). The ethics committee of each center approved the trial. The first subject was dosed on 6 August 2002, and the last subject completed the trial on 10 April 2003.
Blood and urine sample collection.
Blood samples (∼7 ml at each time point) were collected into heparinized Vacutainer blood collection tubes immediately before (0 h) and at 0.5, 0.75, 1, 2, 3, 4, 8, 12, 16, 20, 24, 28, 32, 36, and 48 h after dosing from all subjects, including those with ESRD with postdialysis dosing. Blood samples for subjects with predialysis dosing were collected immediately before dosing (0 h) and at 0.5, 0.75, 1, and 2 (start of dialysis), 3.75 to 4 (mid-dialysis), 5.5 to 6 (end of dialysis), 8, 12, 16, 20, 24, 28, 32, 36, and 48 h after dosing. At these start-, middle-, and end-of-dialysis time points, paired pre- and postdialyzer samples were collected to assess the effect of hemodialysis in removing telbivudine.
Blood samples were centrifuged at 1,500 × g for 15 min, and plasma was collected and frozen at −20°C until analysis. Urine samples were collected from all subjects except for those with ESRD, at −2 to 0 h (predose) and at 0 to 4, 4 to 8, 8 to 12, 12 to 24, and 24 to 48 h postdose.
Plasma and urine sample analysis.
Plasma and urine telbivudine concentrations were measured using validated high-performance liquid chromatographic methods with tandem mass spectrometric detection. The plasma telbivudine assay had a lower limit of quantitation of 10 ng/ml, a calibration curve from 10 to 5,000 ng/ml, and intra- and interday precision (coefficient of variation [CV]) and accuracy (percent deviation) from 2.3% to 5.6% and 4.2% to 1.4%, respectively, based on quality control samples of 30 to 4,000 ng/ml (12). The sample preparation procedures and the chromatographic and mass spectrometric conditions used in the urine assay were similar to those for the plasma assay. The urine telbivudine assay had a lower limit of quantitation of 30 ng/ml, a calibration curve from 30 to 5,000 ng/ml, and intra- and interday precision (CV) and accuracy (percent deviation) from 4.0% to 6.5% and −5.0% to −1.4%, respectively, based on quality control samples of 90 to 4,000 ng/ml. In both assays, telbivudine was monitored at a mass transition of 243.0→127.1 m/z.
Pharmacokinetic analysis.
Noncompartmental analysis was employed to obtain pharmacokinetic parameters using the Kinetica computer program (version 4.3; Thermo Electron Corporation, Waltham, MA). Cmax and the time to reach Cmax (Tmax) were obtained directly from the concentration-time data. The t1/2 of the observed terminal, initial decline, and absorption phases were calculated as 0.693/k, where k, the corresponding rate constant, was derived using stripping and linear regression analyses. The AUC from time zero to time t (AUC0-t), where t is the time of the last measurable concentration (Ct), was calculated according to the linear trapezoidal rule. The AUC from time zero to infinity (AUC0-∞) was estimated as AUC0-t + Ct/kel, where kel is the rate constant of the observed terminal phase. The apparent total oral plasma clearance (CL/F) was calculated as dose/AUC0-∞. Renal clearance (CLR) was estimated as Au0-t/AUC0-t, where Au0-t is the cumulative amount of unchanged drug excreted into the urine from time zero to time t. Dialytic clearance during hemodialysis (CLHD) was calculated as [(QA × CA) − (QV × CV)]/CA, where QA and QV are the blood flow entering and exiting the dialyzer, respectively, and CA and CV are the paired plasma drug concentrations pre- and postdialyzer. The extraction ratio by hemodialysis with respect to the plasma telbivudine concentration was approximated as (CA − CV)/CA × 100%.
Statistical analysis.
The relationship between telbivudine clearance (CLR) and renal function as measured by CLCR was delineated by linear regression analysis.
Safety analysis.
Safety assessments included adverse event reports, vital sign evaluations, physical examinations, clinical laboratory tests, and ECGs. Adverse event records included spontaneous subject reports, investigator observations, and responses to open-ended questioning by the investigators during scheduled visits. Clinical laboratory samples (hematology, blood chemistry, and urinalysis) were collected on the day prior to dosing and at the time of discharge. Vital signs were measured on the day prior to dosing, on the day of dosing, and at the time of discharge. Physical examination and a 12-lead ECG were performed at the screening visit and at discharge. Safety assessments were performed at the same intervals with respect to administration of the second telbivudine dose for subjects with ESRD. Descriptive statistics were used to summarize safety parameters by the degree of renal impairment.
RESULTS
Baseline characteristics.
Twenty-seven male and nine female subjects participated in the study (Table 1). Subject demographic parameters were similar across the five renal function groups. Renal function as measured by CLCR fell within the defining ranges of the corresponding categories of renal impairment. All enrolled subjects completed the study.
TABLE 1.
Baseline characteristics of subjects
| Characteristic | Value for patients in the following renal function category:
|
|||||
|---|---|---|---|---|---|---|
| Normal (n = 8) | Mild impairment (n = 8) | Moderate impairment (n = 8) | Severe impairment (n = 6) | ESRD (n = 6) | Total (n = 36) | |
| Sex (no. of males/females) | 5/3 | 8/0 | 6/2 | 3/3 | 5/1 | 27/9 |
| Race (no. of Caucasians/ African-Americans/others) | 3/4/1 | 5/3/0 | 4/3/1 | 2/3/1 | 2/4/0 | 16/17/3 |
| Age (yr) (mean ± SD) | 48.3 ± 9.2 | 68.9 ± 5.5 | 59.6 ± 12.6 | 57.3 ± 17.7 | 51.7 ± 11.3 | 57.4 ± 13.2 |
| Weight (kg) (mean ± SD) | 79.7 ± 12.4 | 81.4 ± 10.8 | 88.4 ± 21.1 | 81.1 ± 22.6 | 81.9 ± 15.2 | 82.6 ± 16.1 |
| CLCR (ml/min)a | ||||||
| Mean ± SD | 114.4 ± 27.1 | 66.2 ± 8.3 | 43.8 ± 3.7 | 20.2 ± 6.8 | 10.6 ± 3.8 | NA |
| Range | 80.0-153.2 | 56.0-79.0 | 37.0-49.0 | 11.0-29.0 | 5.0-15.0 | |
Estimated by the Cockcroft and Gault method (2). NA, not applicable.
Pharmacokinetics.
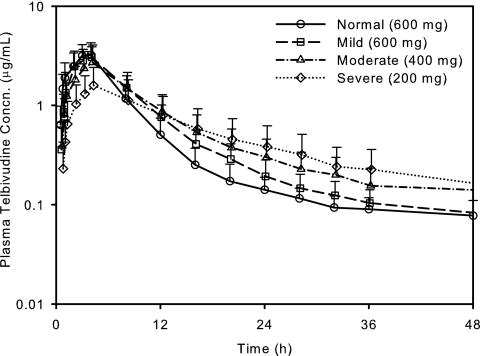
Compared with subjects with normal renal function, the plasma concentration-time courses of telbivudine were altered for subjects with impaired renal function, especially for those with moderate to severe renal impairment (Fig. 1) and those with ESRD requiring hemodialysis (Fig. 2). After oral administration of a single 600-mg dose of telbivudine, plasma exposure was similar for subjects with normal renal function or mild impairment with respect to the mean Cmax (3.4 versus 3.2 μg/ml, respectively) and AUC0-t (26.5 versus 29.2 μg·h/ml) (Table 2). For subjects with moderate renal impairment, the prospectively reduced dose of 400 mg also produced exposure (mean Cmax, 2.8 μg/ml; mean AUC0-t, 30.1 μg·h/ml) comparable to that with the 600-mg dose for subjects with normal renal function. For subjects with severe renal impairment, including ESRD, the prospectively reduced dose of 200 mg produced a comparable AUC (mean, 25.3 to 41.8 μg·h/ml) but a lower Cmax (mean, 1.2 to 2.1 μg/ml). Irrespective of the doses administered, telbivudine exhibited a consistent mean absorption t1/2 of ∼1 h and a median Tmax of 4.0 h across renal function groups, including ESRD subjects with postdialysis dosing.
FIG. 1.
Mean plasma telbivudine concentration-time profiles following oral administration of a single dose of 600 mg to subjects with normal renal function or mild renal impairment, or a single dose of 400 or 200 mg, respectively, to subjects with moderate or severe renal impairment. Error bars, standard deviations.
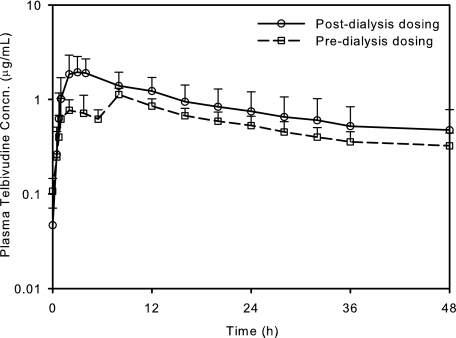
FIG. 2.
Mean plasma telbivudine concentration-time profiles for subjects with ESRD administered a single dose of 200 mg of telbivudine pre- and postdialysis. Error bars, standard deviations.
TABLE 2.
Summary of telbivudine pharmacokinetic parameters following oral administrationa
| Parameter | Valuea for patients in the following renal function category (dose):
|
|||||
|---|---|---|---|---|---|---|
| Normal (600 mg) | Mild impairment (600 mg) | Moderate impairment (400 mg) | Severe impairment (200 mg) | ESRD (200 mg)
|
||
| Pre-dialysis dosing | Post-dialysis dosing | |||||
| Cmax (μg/ml) | 3.4 ± 0.9 | 3.2 ± 0.9 | 2.8 ± 1.3 | 1.6 ± 0.8 | 1.2 ± 0.1 | 2.1 ± 0.9 |
| AUC0-t (μg·h/ml) | 26.5 ± 8.6 | 29.2 ± 8.8 | 30.1 ± 12.0 | 25.3 ± 13.1 | 26.5 ± 4.8 | 41.8 ± 21.5 |
| AUC0-∞ (μg·h/ml) | 28.5 ± 9.6 | 32.5 ± 10.1 | 36.0 ± 13.2 | 32.5 ± 13.2 | 43.4 ± 12.9 | 67.4 ± 36.9 |
| Tmax (h)b | 4.0 (2.0-4.0) | 4.0 (2.0-4.0) | 4.0 (1.0-4.0) | 4.0 (4.0-4.0) | 8.0 (1.0-8.0) | 3.5 (2.0-4.0) |
| t1/2 (h) | ||||||
| Elimination | 20.5 ± 4.5 | 26.7 ± 6.0 | 31.2 ± 13.0 | 32.9 ± 16.3 | 35.2 ± 8.9 | 37.9 ± 8.7 |
| Initial decline | 2.32 ± 0.24 | 3.42 ± 0.57 | 3.82 ± 0.76 | 4.50 ± 1.20 | NA | 4.87 ± 1.66 |
| Absorption | 1.00 ± 0.23 | 1.04 ± 0.17 | 1.12 ± 0.46 | 1.02 ± 0.22 | NA | 0.90 ± 0.05 |
| CL/F (liters/h) | 22.9 ± 6.2 | 20.7 ± 9.1 | 13.6 ± 8.7 | 7.1 ± 2.8 | 4.9 ± 1.2 | 3.6 ± 1.4 |
| CLR (liters/h) | 7.6 ± 2.9 | 5.0 ± 1.2 | 2.6 ± 1.2 | 0.7 ± 0.4 | 13.3 ± 2.5c | NA |
| Ae (% of dose) | 31.5 ± 8.5 | 23.6 ± 8.3 | 19.8 ± 11.9 | 8.0 ± 5.3 | NA | NA |
Subjects with normal renal function and those with mild renal impairment received a single dose of 600 mg, and subjects with moderate or severe renal impairment received a single dose of 400 or 200 mg, respectively. Unless indicated otherwise, values are means ± standard deviations. NA, not applicable.
Tmax values are medians (ranges).
Dialytic clearance.
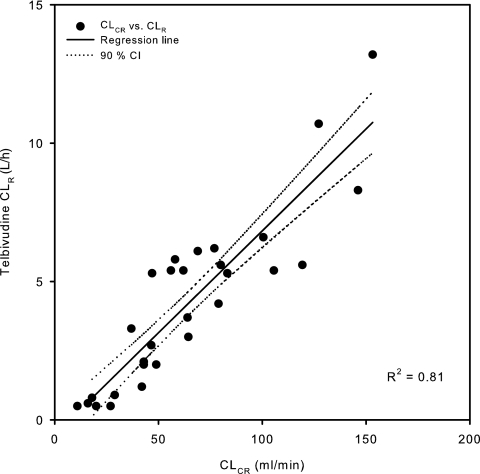
The altered plasma pharmacokinetics of telbivudine for subjects with moderate to severe renal impairment are a result of their reduced renal function. Telbivudine exhibited lower CL/F, CLR, and urine excretion and longer distribution/elimination t1/2 as CLCR decreased (Table 2). The mean CL/F and CLR decreased from 22.9 and 20.7 liters/h and 7.6 and 5.0 liters/h, respectively, for subjects with normal renal function and mild renal impairment to 13.6 and 7.1 liters/h and 2.6 and 0.7 liters/h, respectively, for subjects with moderate and severe renal impairment. Subjects with ESRD were anuric and exhibited a much reduced mean CL/F of 3.6 to 4.9 liters/h. Regression analysis showed that the telbivudine CLR is linearly proportional to renal function as measured by CLCR (R2 = 0.810) (Fig. 3). The mean cumulative urine excretion level (Ae) was 31.5%, 23.6%, 19.8%, and 8.0% of the administered dose for subjects with normal renal function and mild, moderate, and severe renal impairment, respectively. Reduced renal excretion was reflected by shallower initial decline and elimination phases, characterized by an increased t1/2 as the severity of renal impairment increased, from 2.32 h and 20.5 h, respectively, for normal subjects to 4.87 h and 35.2 to 37.9 h for subjects with ESRD (Table 2).
FIG. 3.
Relationship between telbivudine CLR and CLCR. CI, confidence interval.
Telbivudine was efficiently removed by hemodialysis, with a mean extraction ratio of 44.7% ± 9.2%, as estimated from paired plasma samples obtained at the entry and exit of the dialyzer at the start (43.0%) and midpoint (47.1%) of dialysis and upon completion of dialysis (40.1%). Comparison of AUCs following pre- and postdialysis dosing revealed a mean reduction of approximately 23% in overall systemic exposure to telbivudine with 3.5 to 4 h of hemodialysis for ESRD subjects (Fig. 2). During hemodialysis, telbivudine exhibited a mean CLHD of 13.3 liters/h.
Safety results.
There were no study drug-related discontinuations, serious adverse events, or deaths. Fifteen adverse events were reported by eight subjects, with 13 rated as mild and 2 cases of headache rated as moderate in intensity. Two adverse events were considered possibly related to telbivudine, including one instance each of emesis and loose stools. All adverse events resolved by the end of the study, except for one case of sinus congestion. The degree of renal impairment had no effect on the frequency or types of adverse events. There were no changes in vital signs, physical examination findings, clinical laboratory results, or ECG results attributable to telbivudine.
DISCUSSION
The pharmacokinetic parameters of telbivudine for subjects with normal renal function in the current study are consistent with those obtained in previous single-dose studies with comparable sampling durations (5, 9, 11, 12). This study confirms that systemic telbivudine is eliminated predominantly by renal clearance, with pharmacokinetics dependent on renal function, consistent with findings for other anti-HBV nucleoside and nucleotide analogs, including lamivudine, entecavir, and adefovir (Epivir-HBV, Baraclude, and Hepsera product information). Unlike the latter compounds, which exhibit a major active component in their renal clearance (see the product information), the CLR of telbivudine for subjects with normal renal function approximates the normal glomerular filtration rate of 80 to 120 ml/min, indicating that passive diffusion is the main renal clearance mechanism.
For subjects with normal renal function, the total plasma clearance (CL) of telbivudine would be ∼12 liters/h after adjustment using the absolute oral bioavailability (F) of ∼52% (X. J. Zhou et al., unpublished data); therefore, for these subjects, CLR represents the majority (∼63%) of CL. This large proportion predisposes telbivudine pharmacokinetics to CLR, which in turn is linearly dependent on renal function, measured as CLCR (Fig. 3). Similar relationships were reported previously for other agents, e.g., lamivudine (3). For subjects with anuric ESRD, telbivudine had a high CLHD that approximated total plasma CL for subjects with normal renal function, indicating that during hemodialysis, CLHD essentially represents the total clearance of the drug. However, a routine 3.5- to 4-h hemodialysis session had limited impact on overall systemic drug exposure. Of note, since dialysis started after administration and continued over the absorption phase, the drug extracted during this period should represent the maximum possible amount removable. Therefore, dialysis during any post-Cmax interval is expected to have much less impact on overall exposure. Nevertheless, telbivudine should be administered after dialysis to preserve optimal exposure.
Although renal function influenced telbivudine elimination, the time course of telbivudine absorption was minimally affected. Regardless of renal function, the Tmax and absorption t1/2 remained consistent. For ESRD subjects dosed predialysis, the usual Cmax was not observed, since the 3.5- to 4-h dialysis session starting 2 h after dosing encompassed its typical Tmax. However at the end of dialysis, plasma telbivudine concentrations quickly rebounded to the maximum. The Cmax reached at the shifted Tmax was comparable to that at the same time point after postdialysis dosing, suggesting that there was a large pool of telbivudine, consistent with its large volume of distribution (5).
In a phase I/II trial with HBV-infected patients, telbivudine exhibited dose-proportional pharmacokinetics and dose/exposure-related viral response, and near-maximal antiviral effects were achieved with telbivudine doses of 400 to 800 mg/day (7, 10). No dose-limiting toxicities were observed in the studied dose range of 25 to 800 mg/day (7). The defined dose-response relationship of telbivudine, in conjunction with its renal function-dependent pharmacokinetics, supports a pharmacologic rationale for dose adjustment for patients with renal impairment.
After oral administration of a single 600-mg dose, the plasma exposure of telbivudine was similar for subjects with normal renal function or mild impairment, indicating that the standard dose of 600 mg/day is suitable for both groups. In contrast, for subjects with moderate to severe renal impairment (CLCR, <50 ml/min), including those with ESRD, dose adjustment is warranted to achieve an exposure comparable to that for normal subjects who receive the standard 600-mg/day dose. This can be accomplished by extending the dosing interval or by reducing the daily dose. At present, telbivudine is approved for the treatment of chronic hepatitis B, with a single, nondividable 600-mg tablet as the only available formulation, leaving dose interval adjustment as the interim option. The dose interval adjustment scheme approved by major regulatory authorities for patients with a CLCR of 30 to 49 ml/min or <30 ml/min (nondialytic) or with ESRD requiring hemodialysis is 600 mg every 2, 3, or 4 days, respectively (Tyzeka product information; Idenix Pharmaceuticals, Inc., Cambridge, MA, and Novartis Pharma Stein AG, Stein, Switzerland). Reduction of the daily dose is generally preferred over dose interval adjustment because of anticipated better compliance. Daily dose adjustment can be implemented for patients with a CLCR of <50 ml/min once an oral solution formulation of telbivudine becomes commercially available.
In summary, the degree of renal impairment had no effect on the frequency of adverse events or other safety measures following a single 200- to 600-mg dose of telbivudine. The results indicate that telbivudine pharmacokinetics are dependent on renal function, and they further support dose adjustment for patients with a CLCR of <50 ml/min in order to achieve optimal antiviral exposure.
Acknowledgments
This work was supported by Idenix Pharmaceuticals, Inc. S. Swan (DaVita Clinical Research), W. B. Smith (New Orleans Center for Clinical Research), and T. C. Marbury (Orlando Clinical Research Center) were principal investigators involved in the clinical conduct of this study through a contractual agreement with Idenix Pharmaceuticals, Inc.
We thank the volunteers, patients, and staff of DaVita Clinical Research, the New Orleans Center for Clinical Research, and the Orlando Clinical Research Center. We are also grateful to R. Boehme for critical review of the manuscript and helpful suggestions.
Footnotes
Published ahead of print on 17 September 2007.
REFERENCES
- 1.Cretton-Scott, E., X. J. Zhou, E. G. Bridges, B. Tennant, A. Juodawlkis, G. Grosselin, J. L. Imbach, C. Pierra, D. Dukhan, R. F. Schinazi, J. P. Sommadossi, and E. M. Bryant. 1999. Pharmacokinetics of β-l-thymidine and β-l-2′-deoxycytidine in woodchucks and monkeys. Antivir. Ther. 4:A124. [Google Scholar]
- 2.Fournier, A., and J. M. Achard. 2000. Mnemotechnical note on the use of Cockcroft creatinine clearance formula for the validation of a 24-h urine collection. Nephrol. Dial. Transplant. 15:1677-1678. [DOI] [PubMed] [Google Scholar]
- 3.Heald, A. E., P. H. Hsyu, G. J. Yuen, P. Robinson, P. Mydlow, and J. A. Bartlett. 1996. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with renal dysfunction. Antimicrob. Agents Chemother. 40:1514-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hernandez-Santiago, B., L. Placidi, E. Cretton-Scott, A. Faraj, E. G. Bridges, M. L. Bryant, J. Rodriguez-Orengo, J. L. Imbach, G. Gosselin, C. Pierra, D. Dukhan, and J. P. Sommadossi. 2002. Pharmacology of β-l-thymidine and β-l-2′-deoxycytidine in HepG2 cells and primary human hepatocytes: relevance to chemotherapeutic efficacy against hepatitis B virus. Antimicrob. Agents Chemother. 46:1728-1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu, P., J. Jiang, H. Wang, K. Pietropaolo, G. C. Chao, N. A. Brown, and X. J. Zhou. 2006. Single-dose and multiple-dose pharmacokinetics and safety of telbivudine after oral administration in healthy Chinese subjects. J. Clin. Pharmacol. 46:999-1007. [DOI] [PubMed] [Google Scholar]
- 6.Lai, C. L., N. Leung, E. K. Teo, M. Tong, F. Wong, H. W. Hann, S. Han, T. Poynard, M. Myers, G. Chao, D. Lloyd, N. Brown, and the Telbivudine Phase II Investigator Group. 2005. A 1-year trial of telbivudine, lamivudine, and the combination in patients with chronic hepatitis B e antigen-positive chronic hepatitis B. Gastroenterology 129:528-536. [DOI] [PubMed] [Google Scholar]
- 7.Lai, C. L., S. G. Lim, N. A. Brown, X. J. Zhou, D. M. Lloyd, Y. M. Lee, M. F. Yuen, G. C. Chao, and M. W. Myers. 2004. A dose-finding study of once-daily oral telbivudine in HBeAg-positive patients with chronic hepatitis B virus infection. Hepatology 40:719-726. [DOI] [PubMed] [Google Scholar]
- 8.Standring, D. N., E. G. Bridges, L. Placidi, A. Faraj, A. G. Loi, C. Pierra, D. Dukhan, G. Gosselin, J. L. Imbach, B. Hernandez, A. Juodawlkis, B. Tennant, B. Korba, P. Cote, E. Cretton-Scott, R. F. Schinazi, M. Myers, M. L. Bryant, and J. P. Sommadossi. 2001. Antiviral β-l-nucleosides specific for the hepatitis B virus infection. Antivir. Chem. Chemother. 12:119-129. [PubMed] [Google Scholar]
- 9.Zhou, X. J., B. A. Fielman, D. M. Lloyd, G. C. Chao, and N. A. Brown. 2006. Pharmacokinetics of telbivudine in healthy subjects and absence of drug interaction with lamivudine or adefovir dipivoxil. Antimicrob. Agents Chemother. 50:2309-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhou, X. J., S. G. Lim, D. M. Lloyd, G. C. Chao, N. A. Brown, and C. L. Lai. 2006. Pharmacokinetics of telbivudine following oral administration of escalating single and multiple doses in patients with chronic hepatitis B virus infection: pharmacodynamic implications. Antimicrob. Agents Chemother. 50:874-879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhou, X. J., D. M. Lloyd, C. C. Chao, and N. A. Brown. 2006. Absence of food effect on the pharmacokinetics of telbivudine following oral administration in healthy subjects. J. Clin. Pharmacol. 46:275-281. [DOI] [PubMed] [Google Scholar]
- 12.Zhou, X. J., T. C. Marbury, H. W. Alcorn, W. B. Smith, P. G. Dubuc, G. C. Chao, and N. A. Brown. 2006. Pharmacokinetics of telbivudine in subjects with various degrees of hepatic impairment. Antimicrob. Agents Chemother. 50:1721-1726. [DOI] [PMC free article] [PubMed] [Google Scholar]