Abstract
This study compared the ability of telavancin to the ability of cefazolin and vancomycin to eliminate staphylococci from peritoneal dialysis fluid by using a static in vitro model to simulate the conditions of peritoneal dialysis. The results showed that telavancin exhibited statistically significantly better kill (P < 0.05) against both methicillin-susceptible and methicillin-resistant Staphylococcus aureus.
Peritoneal dialysis-associated peritonitis (PDAP) is the most common complication resulting from peritoneal dialysis. Repeated attacks of peritonitis may render the peritoneal membrane unsuitable for dialysis, due to scarring and thickening (8).
Current empirical therapy for PDAP includes gram-positive coverage with a cephalosporin, such as cefazolin or vancomycin (if beta-lactam resistance or allergy is known or suspected), plus an agent, such as an aminoglycoside, to cover gram negatives (7). A critical issue in the use of beta-lactams or vancomycin is that the drugs require bacteria to be in exponential-phase growth for the antibiotic to be effective (2, 10). However, peritoneal dialysis fluid (PDF) is bacteriostatic, which may compromise the efficacy of these agents (5, 6, 11). Previous experiments performed by Hermsen et al. have shown that the activities of vancomycin and cefazolin are negatively affected in the setting of PDF-induced growth suppression (4).
Telavancin is a lipoglycopeptide antibiotic which, due to multiple mechanisms of action, should not fall victim to PDF-induced growth suppression. In addition, since telavancin is ∼95% protein bound, the low protein concentration in PDF will provide high free-drug concentrations to maximize killing (3).
The purpose of this investigation was to compare the abilities of telavancin, cefazolin, and vancomycin to kill Staphylococcus aureus, a common cause of PDAP, in the context of PDF-induced growth suppression.
Sixteen duplicate concentration-time-kill curve experiments were conducted using a static in vitro model in order to simulate the conditions of PDF during an intraperitoneal dwell. The studies were performed using cation-adjusted Mueller-Hinton broth (CAMHB; Becton Dickinson Microbiology Systems, Sparks, MD) as a control and commercially available PDF (Baxter Dianeal low calcium; Baxter Healthcare Corp., Deerfield, IL) supplemented to contain final concentrations of 2.5% dextrose and 257 mg of calcium/liter. The solution was adjusted to a pH of 7.4, corresponding to the pH after a 4-h intraperitoneal dwell (4). The models were inoculated at time zero and allowed to incubate for 2 h at 37°C. Antibiotic was added at the 2-h time point. Each experiment was performed for 24 h at 37°C.
American Type Culture Collection (ATCC) isolates of methicillin-susceptible S. aureus (MSSA; ATCC 29213) and methicillin-resistant S. aureus (MRSA; ATCC 33592) were used in the experiments. For all experiments, the starting inoculum was approximately 106 CFU/ml, which is comparable to the bacterial load associated with PDAP (5).
Stock solutions of cefazolin (Sigma-Aldrich, St. Louis, MO) and vancomycin (Fisher Scientific, Pittsburgh, PA) were prepared and stored at −80°C. Telavancin stock solutions were prepared by the manufacturer (Theravance, South San Francisco, CA) and stored at −20°C. All antibiotics were administered as bolus injections.
Telavancin concentrations of 10 μg/ml and 50 μg/ml were used in this study. There are reports of telavancin being used safely in human studies at concentrations as high as 150 to 200 μg/ml (9). The concentrations of cefazolin and vancomycin used, 125 μg/ml and 50 μg/ml, respectively, correspond to the intraperitoneal doses that are typically used clinically (4, 7). Antibiotic concentrations were not corrected for protein binding, as PDF has negligible protein content (4, 5). Six samples were processed for bacterial enumeration during each experiment. In the experiments requiring antibiotic administration, antibiotic was not added until the 2-h time point, in order to allow the PDF to exert its growth-suppressive effect on the organisms. Fifty microliters of the correct dilution was processed using a WASP2 spiral plater (Don Whitley Scientific Ltd., West Yorkshire, England). After 18 to 24 h of incubation, the number of CFU per ml was calculated using an aCOLyte automated colony counter (Synbiosis, Cambridge, United Kingdom). Concentration-time-kill curves were constructed by plotting the log10 number of CFU/ml versus time. Slopes for each curve were compared using 95% confidence intervals to elucidate any differences in antibiotic performance.
Susceptibilities to cefazolin, vancomycin, and telavancin were determined for each isolate prior to concentration-time-kill experiments and for all postexposure isolates. Broth dilution MIC testing was performed, following CLSI guidelines for Staphylococcus spp. (1). MSSA (ATCC 29213) was used as a quality control organism.
Concentration-time-kill curve slopes were determined using regression analysis, and 95% confidence intervals on mean slope values were determined. Significance was defined as a P value of <0.05. All graphing and statistical analyses were performed using Microsoft Excel (Microsoft Corp., Redmond, WA).
Liquid chromatography mass spectrometry (LC-MS) testing for measurement of telavancin levels was performed at Alturas Analytics (Moscow, ID). The method was linear over a range of 5 μg/ml to 100 μg/ml, with a lower limit of quantification of 5 μg/ml. Overall accuracy ranged from 75.9% to 107%. These levels were verified using an agar diffusion microbiological assay adapted from a procedure developed at the Antibiotic Research Unit in Uppsala, Sweden (I. Odenholt, personal communication). Vancomycin and cefazolin assays for verification of concentrations were not performed.
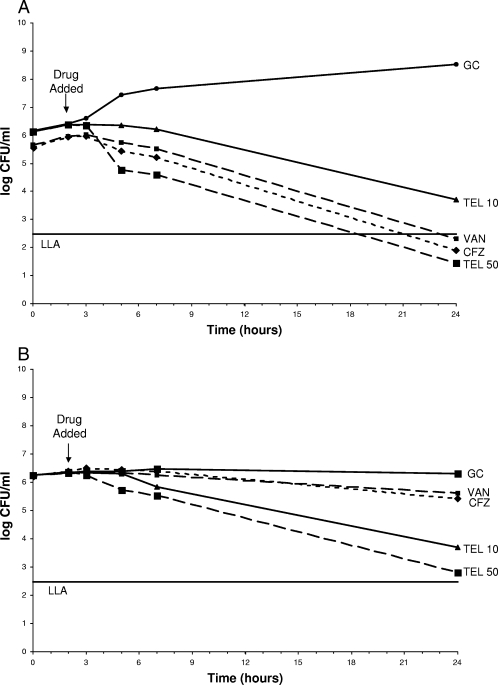
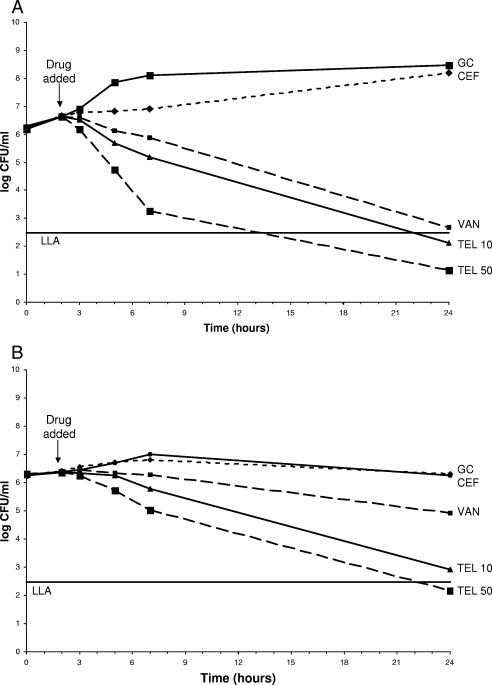
The results showed that telavancin at both 10 μg/ml and 50 μg/ml exhibits statistically significantly better kill (P < 0.05) than cefazolin and vancomycin against MSSA and MRSA in PDF. A telavancin concentration of 50 μg/ml killed more rapidly than a telavancin concentration of 10 μg/ml (Fig. 1 and 2). The preexposure MICs for MSSA were 0.5 μg/ml for cefazolin, 1 μg/ml for vancomycin, and 0.25 μg/ml for telavancin. The MICs for MRSA were >16 μg/ml for cefazolin, 1 μg/ml for vancomycin, and 0.125 μg/ml for telavancin. There were no changes in preexposure versus postexposure MICs. The telavancin levels measured by LC-MS were somewhat higher than desired for both the 10-μg/ml (115 to 155% of the expected level) and the 50-μg/ml (102 to 123% of the expected level) experiments. The microbiological assay verified that the LC-MS levels were accurate (92 to 123% correlation).
FIG. 1.
(A) Telavancin, vancomycin, and cefazolin against MSSA in CAMHB. Antibiotic was added at the 2-h time point. Plotted data represent the means for duplicate simulations. (B) Telavancin, vancomycin, and cefazolin against MSSA in PDF. Antibiotic was added at the 2-h time point. Plotted data represent the means for duplicate simulations. GC, growth control; TEL 10, telavancin peak of 10 μg/ml; TEL 50, telavancin peak of 50 μg/ml; VAN, vancomycin; CFZ, cefazolin; LLA, lower limit of accuracy.
FIG. 2.
(A) Telavancin, vancomycin, and cefazolin against MRSA in CAMHB. Antibiotic was added at the 2-h time point. Plotted data represent the means for duplicate simulations. (B) Telavancin, vancomycin, and cefazolin against MRSA in PDF. Antibiotic was added at the 2-h time point. Plotted data represent the means for duplicate simulations. For abbreviations, see the legend to Fig. 1.
Based on these data, telavancin seems to be a promising agent for treatment of PDAP. Telavancin may shorten the treatment period and thus reduce scarring and inflammation, extending the viability of the peritoneal membrane. In addition, a shorter course of therapy decreases the potential for induced resistance or disturbance of normal flora at remote sites.
Acknowledgments
This study was funded by Theravance and kindly supported by the Melendy Summer Research Scholarship.
Footnotes
Published ahead of print on 1 October 2007.
REFERENCES
- 1.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility testing. Sixteenth informational supplement. CLSI document M100-A16. Clinical and Laboratory Standards Institute, Wayne, PA.
- 2.Cozens, R. M., E. Tuomanen, W. Tosch, O. Zak, J. Suter, and A. Tomasz. 1986. Evaluation of the bactericidal activity of beta-lactam antibiotics on slowly growing bacteria cultured in the chemostat. Antimicrob. Agents Chemother. 29:797-802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hegde, S. S., N. Reyes, T. Wiens, N. Vanasse, R. Skinner, J. McCullough, K. Kaniga, J. Pace, R. Thomas, J. Shaw, G. Obedencio, and J. K. Judice. 2004. Pharmacodynamics of telavancin (TD-6424), a novel bactericidal agent, against gram-positive bacteria. Antimicrob. Agents Chemother. 48:3043-3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hermsen, E. D., L. B. Hovde, J. R. Hotchkiss, and J. C. Rotschafer. 2003. Increased killing of staphylococci and streptococci by daptomycin compared with cefazolin and vancomycin in an in vitro peritoneal dialysate model. Antimicrob. Agents Chemother. 47:3764-3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McDonald, W. A., J. Watts, and M. I. Bowmer. 1986. Factors affecting Staphylococcus epidermidis growth in peritoneal dialysis solutions. J. Clin. Microbiol. 24:104-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morton, A. R., G. Evas, A. Shannon, and D. Zoutman. 1994. Growth of coagulase negative staphylococci in peritoneal dialysate fluid: effect of calcium concentration. ASAIO J. 40:M431-M434. [DOI] [PubMed] [Google Scholar]
- 7.Piraino, B., G. Bailie, J. Bernardini, E. Boeschoten, A. Gupta, C. Holmes, E. Kuijper, P. Li, W. Lye, S. Mujais, D. Paterson, M. Fontan, A. Ramos, F. Schaefer, and L. Uttley. 2005. Peritoneal dialysis-related infections recommendations: 2005 update. Perit. Dial. Int. 25:107-131. [PubMed] [Google Scholar]
- 8.Selgas, R., M. J. Fernandez-Reyes, E. Bosque, M. A. Bajo, F. Borrego, C. Jimenez, G. Del Peso, and F. De Alvaro. 1994. Functional longevity of the human peritoneum: how long is continuous peritoneal dialysis possible? Results of a prospective medium long-term study. Am. J. Kidney Dis. 23:64-73. [DOI] [PubMed] [Google Scholar]
- 9.Shaw, J. P., J. Seroogy, K. Kaniga, D. L. Higgins, M. Kitt, and S. Barriere. 2005. Pharmacokinetics, serum inhibitory and bactericidal activity, and safety of telavancin in healthy subjects. Antimicrob. Agents Chemother. 49:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tuomanen, E., R. Cozens, W. Tosch, O. Zak, and A. Tomasz. 1986. The rate of killing of Escherichia coli by beta-lactam antibiotics is strictly proportional to the rate of bacterial growth. J. Gen. Microbiol. 132:1297-1304. [DOI] [PubMed] [Google Scholar]
- 11.Zelenitsky, S., C. Franczuk, A. Fine, R. Ariano, and G. Harding. 2002. Antibiotic tolerance of peritoneal bacterial isolates in dialysis fluids. J. Antimicrob. Chemother. 49:863-866. [DOI] [PubMed] [Google Scholar]