Abstract
This study describes the pattern and extent of drug resistance in 1,774 strains of Salmonella enterica serovar Typhi isolated across Asia between 1993 and 2005 and characterizes the molecular mechanisms underlying the reduced susceptibilities to fluoroquinolones of these strains. For 1,393 serovar Typhi strains collected in southern Vietnam, the proportion of multidrug resistance has remained high since 1993 (50% in 2004) and there was a dramatic increase in nalidixic acid resistance between 1993 (4%) and 2005 (97%). In a cross-sectional sample of 381 serovar Typhi strains from 8 Asian countries, Bangladesh, China, India, Indonesia, Laos, Nepal, Pakistan, and central Vietnam, collected in 2002 to 2004, various rates of multidrug resistance (16 to 37%) and nalidixic acid resistance (5 to 51%) were found. The eight Asian countries involved in this study are home to approximately 80% of the world's typhoid fever cases. These results document the scale of drug resistance across Asia. The Ser83→Phe substitution in GyrA was the predominant alteration in serovar Typhi strains from Vietnam (117/127 isolates; 92.1%). No mutations in gyrB, parC, or parE were detected in 55 of these strains. In vitro time-kill experiments showed a reduction in the efficacy of ofloxacin against strains harboring a single-amino-acid substitution at codon 83 or 87 of GyrA; this effect was more marked against a strain with a double substitution. The 8-methoxy fluoroquinolone gatifloxacin showed rapid killing of serovar Typhi harboring both the single- and double-amino-acid substitutions.
There are approximately 21 million cases of typhoid fever worldwide, with a particularly high incidence in Asia. An estimated 220,000 deaths per year occur as a consequence of the disease (11).
This article describes the extent and pattern of drug resistance of Salmonella enterica serovar Typhi across Asia. This information is vital for guiding treatment and is also important for helping policy makers to plan vaccination campaigns. The emergence and spread of drug resistance have limited treatment options for typhoid fever in many countries.
Since the isolation of multidrug-resistant (MDR) serovar Typhi strains which show resistance to all first-line antibiotics (chloramphenicol, ampicillin, and trimethoprim-sulfamethoxazole) in the 1980s, the fluoroquinolone class of antibiotics has become the treatment of choice for enteric fever (4, 38). Unfortunately, outbreaks of serovar Typhi strains that were resistant to nalidixic acid (the prototype quinolone, which is used for in vitro screening tests) and showed reduced susceptibility to the fluoroquinolones have been reported subsequently in a number of countries (25). Vietnam and particularly the Mekong Delta region of Vietnam faced a series of typhoid fever epidemics over the last decade, imposed on a background of endemic disease, that reflected changes in resistance patterns and pointed to a serious problem of drug resistance (24). MDR is associated with a transferable plasmid (36), while reduced susceptibility to the fluoroquinolones in serovar Typhi is usually associated with point mutations in the bacterial target genes encoding DNA gyrase and/or DNA topoisomerase IV.
This study describes the magnitude and patterns of drug resistance in 1,393 serovar Typhi strains isolated from 1993 to 2005 in Vietnam and from a cross-sectional sample set of 381 serovar Typhi strains isolated in 2002 to 2004 in eight Asian countries (Bangladesh, China, India, Indonesia, Laos, Nepal, Pakistan, and central Vietnam). These countries are home to more than 80% of the world's typhoid fever cases (11). We defined the molecular mechanism of nalidixic acid resistance and performed in vitro bacterial time-kill experiments with isolates that harbored the common mutations in the gyrA gene. The time-kill experiments allowed us to model the impact of the gyrA mutations on the time course of the antimicrobial effects of older (ofloxacin) and newer-generation (gatifloxacin) fluoroquinolones.
(This work was presented in part at the American Meeting of Hygiene and Tropical Medicine, Atlanta, GA, December 2005.)
MATERIALS AND METHODS
Bacterial isolates. (i) Serovar Typhi strains isolated in southern Vietnam from 1993 to 2005.
One thousand three hundred ninety-three serovar Typhi isolates were collected consecutively from patients with uncomplicated typhoid fever during prospective hospital-based clinical studies between 1993 and 2005 conducted at Dong Thap Provincial Hospital, Dong Nai Peadiatric Hospital, An Giang Provincial Hospital, and the Hospital for Tropical Diseases, Ho Chi Minh City, all located in southern Vietnam. These studies have been described previously (7, 8, 23, 26, 32-35).
(ii) Serovar Typhi isolates from eight Asian countries in 2002 to 2004.
One hundred forty-nine serovar Typhi isolates were collected in March and April 2003 during a hospital-based descriptive study at Patan Hospital, Kathmandu, Nepal. Fifty isolates were collected consecutively during a clinical trial in 2002 and 2003 at the Wellcome Trust-Mahosot Hospital-Oxford Tropical Medicine Research Collaboration, Lao People's Democratic Republic, Laos (27). One hundred eighty-two serovar Typhi isolates were collected as part of population-based prospective surveillance studies conducted by multiple teams in collaboration with the International Vaccine Institute (IVI), Seoul, South Korea (1). These surveillance sites included whole townships (China and Vietnam), specific slum areas (Bangladesh, Pakistan, and India), and an impoverished urban subdistrict (Indonesia). Forty isolates were collected from February till December 2003 in an urban slum in Dhaka, Bangladesh; the setting has been described (5); 21 isolates were collected during 2002 in Hechi city, Guang Xi, China; 23 strains were collected from May to July 2003 in slum areas in Kolkata, West Bengal, India; 17 isolates were collected from July to September 2002 in North Jakarta, Indonesia; 34 strains were isolated between January 2002 and March 2003 in one slum area in Karachi, Pakistan; and 47 isolates were collected between July 2002 and September 2004 in Hue city, central Vietnam.
All serovar Typhi isolates were collected consecutively from febrile patients during the indicated periods and came from geographically contiguous areas. The isolates were unselected and were representative of the population they came from.
Identification and antimicrobial susceptibilities.
Isolates were identified using the API20E biochemical identification system (bioMerieux, Paris, France). Serology was carried out using specific antisera (polyvalent O, O9, Hd, and Vi) (Murex, Dartford, United Kingdom).
Antimicrobial susceptibility testing with ampicillin, chloramphenicol, trimethoprim-sulfamethoxazole, nalidixic acid, ofloxacin, ciprofloxacin, gatifloxacin, and ceftriaxone was performed by disc diffusion according to Clinical and Laboratory Standards Institute (CLSI) methods (10) and interpreted following CLSI guidelines (9). The control strains used for all susceptibility tests were Escherichia coli ATCC 25922, Pseudomonas aeruginosa ATCC 27853, and Staphylococcus aureus ATCC 29213. MICs were determined by using the E-test (AB Biodisk, Solna, Sweden). MDR of isolates was defined as resistance to chloramphenicol (MIC ≥ 32 μg/ml), ampicillin (MIC ≥ 32 μg/ml), and trimethoprim-sulfamethoxazole (MIC ≥ 8/152 μg/ml). Nalidixic acid resistance was defined as a MIC of ≥32 μg/ml. The breakpoints for ofloxacin and gatifloxacin were ≤2 μg/ml (susceptible) and ≥8 μg/ml (resistant), and for ciprofloxacin, ≤1 μg/ml (susceptible) and ≥4 mg/ml (resistant) (9). All tests were performed at the Hospital for Tropical Diseases (HTD), Ho Chi Minh City, Vietnam, except for the isolates from Nepal, which were tested at Patan Hospital, Kathmandu, Nepal, using identical methods.
DNA isolation.
A single colony was inoculated in 6 ml of LB broth (Sigma) and incubated overnight at 37°C. DNA was extracted using the Qiagen Genomic-tip 100/G and Genomic DNA buffer set (Qiagen, Ltd., Hilden, Germany) or the cetyltrimethylammonium bromide method of DNA extraction (2). DNA stock was stored at −20 and −80°C. Four hundred nanograms of DNA was used for each PCR.
PCR and sequencing.
Oligonucleotide primer pairs are shown in Table 1. PCR amplifications of gyrA (347 bp), gyrB (345 bp), parC (270 bp), and parE (240 bp) were performed with 30 cycles of denaturation at 92°C for 1 min, annealing at 62°C for 1 min, and extension at 74°C for 2 min, followed by a final extension step at 74°C for 1 min.
TABLE 1.
Oligonucleotide primer sequences used for PCR amplification
| Gene | Primer | Primer sequence (5′→3′) | Reference |
|---|---|---|---|
| GyrA | GYRA/P1 | TGTCCGAGATGGCCTGAAGC | 16 |
| GYRA/P2 | TACCGTCATASGTTATCCACG | ||
| GyrB | StygyrB1 | CAAACTGGCGGACTGTCAGG | 20 |
| StygyrB1 | TTCCGGCATCTGACGATAGA | ||
| parC | StmparC1 | CTATGCGATGTCAGAGCTGG | 13 |
| StmparC2 | TAACAGCAGCTCGGCGTATT | ||
| parE | StmparE1 | TCTCTTCCGATGAAGTGCTG | 13 |
| StmparE2 | ATACGGTATAGCGGCGGTAG | ||
| qnrS | QnrS1 | ATGGAAACCTACAATCATAC | —a |
| QnrS2 | AAAAACACCTCGACTTAAGT | ||
| QnrA | QP1 | GATAAAGTTTTTCAGCAAGAGG | 19 |
| QP2 | ATCCAGATCGGCAAAGGTTA |
Sequences for the qnrS primers were designed based on the sequence of Shigella flexneri (17).
PCR products were purified using the QIAquick PCR purification kit (Qiagen GmbH, Hamburg, Germany) and used directly as templates for sequencing, which was performed with the CEQ DTCS-Quick Start kit and analyzed using an automated sequencer, the CEQ8000 genetic analysis system (Beckman Coulter, Inc., Fullerton, CA).
Selected strains were screened for the presence of the qnrA and qnrS genes by PCR. The PCR conditions for the amplification of qnrS were as follows: 94°C for 2 min; 34 cycles of 94°C for 45 s, 48°C for 45 s, and 72°C for 45 s; final extension at 74°C for 5 min. PCR conditions for qnrA were identical except for the annealing temperature, which was 53°C. The positive control used was a Citrobacter sp. isolate (identified by API20E) harboring both the qnrA and qnrS genes, as confirmed by sequencing of PCR products.
In vitro time-kill analysis.
All time-kill experiments were determined in duplicate. Ofloxacin powder was purchased from Sigma, Steinheim, Germany, and gatifloxacin powder was provided from Bristol-Myers Squibb, New Brunswick, NJ. Three serovar Typhi colonies were taken and inoculated in 10 ml Mueller-Hinton broth (Oxoid, Basingstoke, United Kingdom) at 37°C for 15 to 18 h. Two drops of this broth were inoculated into 10 ml of Mueller-Hinton broth and incubated at 37°C for 1 h to give 2 × 106 CFU/ml. Ten milliters of Mueller-Hinton broth containing ofloxacin or gatifloxacin at 32× MIC was added at time zero to give a final concentration of 16× MIC; serial twofold dilutions were used to obtain 8×, 4×, 2×, and 1× MIC. The growth control contained no antibiotic. The cultures were incubated at 35 to 37°C for 24 h. Viable counts were measured immediately prior to the addition of the antibiotic and at 30 min and 1, 2, 4, 6, 8, and 24 h after the addition of the antibiotic. Viable counts were performed by using the Miles and Misra technique on nutrient agar plates following serial dilution in maximum-recovery diluents (Oxoid, United Kingdom). The lower limit of detection was 101 CFU/ml.
Nucleotide sequence accession numbers.
The partial DNA sequences of the gyrA gene of serovar Typhi AG 152 and DT 18 have been registered in the GenBank nucleotide sequence database under the accession numbers EF680460 and EF680461, respectively.
RESULTS
Antimicrobial susceptibility testing. (i) Serovar Typhi isolated in southern Vietnam from 1993 to 2005.
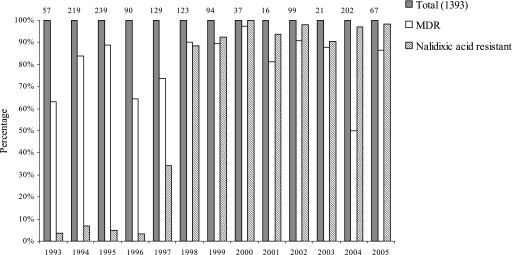
Between 1993 and 2005, 1,393 isolates of serovar Typhi were collected (Fig. 1). The proportion of MDR serovar Typhi was 63.2% (36/57 strains) in 1993 and increased to more than 80% in the late 1990s and early 2000. During the same period, there was a dramatic increase in nalidixic acid resistance. In 1993, 2 out of 57 (3.5%) serovar Typhi isolated from patients in southern Vietnam were nalidixic acid resistant (respective MICs of ofloxacin, 0.250 and 0.125 μg/ml) (37). Nalidixic acid resistance surged to 88.6% (109/123) in 1998. It has remained at high levels since then, with 97% (196/202) of isolates in 2004. Since 1998, a high proportion of strains show the combination of MDR and nalidixic acid resistance (Fig. 1).
FIG. 1.
Antimicrobial drug resistance of serovar Typhi strains isolated during clinical studies in southern Vietnam from 1993 to 2005. Percentages of MDR and nalidixic acid-resistant serovar Typhi isolates. The number of isolates from each year is shown on top of the bars.
The antimicrobial susceptibility data of 202 serovar Typhi isolated in 2004 in southern Vietnam are shown in more detail in Table 2.
TABLE 2.
Antimicrobial drug resistance of serovar Typhi isolates in 2002 to 2004 across eight Asian countriesa
| Country | % Nalidixic acid-resistant isolatesa | MIC of ciprofloxacin (μg/ml)
|
% Ciprofloxacin-resistant isolatesa | MIC of gatifloxacin (μg/ml)
|
% Chloramphenicol-resistant isolatesa | % MDR Isolatesa | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| Range | 50% | 90% | Range | 50% | 90% | |||||
| China | 4.8 (1/21) | 0.008-0.25 | 0.015 | 0.03 | 0 | 0.012-0.190 | 0.023 | 0.023 | 0 (0/21) | 0 (0/21) |
| Indonesia | 0 (0/17) | 0.002-0.03 | 0.015 | 0.015 | 0 | 0.012-0.023 | 0.016 | 0.023 | 0 (0/17) | 0 (0/17) |
| Laos | 0 (0/50) | 0.006-0.023 | 0.012 | 0.016 | 0 | 0.012-0.047 | 0.016 | 0.023 | 18 (9/50) | 16 (8/50) |
| Bangladesh | 40 (16/40) | 0.006-0.38 | 0.025 | 0.38 | 0 | 0.012-0.19 | 0.016 | 0.19 | 40 (16/40) | 37.5 (15/40) |
| India | 47.8 (11/23) | 0.006-0.25 | 0.094 | 0.25 | 0 | 0.012-0.19 | 0.125 | 0.19 | 26 (6/23) | 26 (6/23) |
| Nepal | 51 (76/149) | 0.002-32 | 0.125 | 0.5 | 4 (6/149) | 0.012-1.500 | 0.094 | 0.25 | 19 (28/149) | NAb |
| Pakistan | 38.3 (13/34) | 0.004-0.25 | 0.012 | 0.25 | 0 | 0.012-0.190 | 0.023 | 0.19 | 26.5 (9/34) | 26.5 (9/34) |
| Central Vietnam (IVI) | 50 (23/47) | 0.006-0.5 | 0.023 | 0.38 | 0 | 0.008-0.250 | 0.016 | 0.19 | 21.3 (10/47) | 21.3 (10/47) |
| Southern Vietnam (HTD) | 97 (196/202) | 0.008-0.75 | 0.38 | 0.5 | 0 | 0.006-0.250 | 0.125 | 0.19 | 50 (101/202) | 50 (101/202) |
Parenthetical numbers indicate no. of resistant isolates/no. tested.
NA, not available.
(ii) Serovar Typhi strains isolated in eight Asian countries in 2002 to 2004.
The antimicrobial susceptibilities of 381 serovar Typhi isolates collected in 2002 to 2004 from eight Asian countries were analyzed (Table 2). There were various rates of MDR across the sites, ranging from 16% (8/50) of isolates from Laos to 37.5% (15/40) from Bangladesh. China and Indonesia were exceptions, with no MDR serovar Typhi identified.
The percentages of nalidixic acid-resistant serovar Typhi isolates ranged from 0% in Indonesia and Laos and 4.8% (1/21) in China to 51% (76/149) in Nepal (Table 2). The combination of MDR and nalidixic acid resistance was found in 4.3% (2/47) of serovar Typhi isolates from central Vietnam, 8.7% (2/23) of isolates from India, 23.5% (8/140) of isolates from Pakistan, and 30% (12/40) of isolates from Bangladesh. In Nepal, 18.1% (27/149) of serovar Typhi isolates were resistant to chloramphenicol and nalidixic acid.
However, using current CLSI breakpoints, all isolates remained susceptible in vitro to ciprofloxacin and ofloxacin, with the exception of one isolate from southern Vietnam, AG 152, with intermediate susceptibility (MIC, 3.0 μg/ml) to ofloxacin (Table 3) and six isolates (4%) from Nepal that were ciprofloxacin resistant. The highest MICs of gatifloxacin at which 50% and 90% of serovar Typhi isolates were inhibited were 0.125 μl/ml and 0.25 μl/ml, respectively (Table 2). All isolates were susceptible to ceftriaxone.
TABLE 3.
Results of sequence analysis of the QRDR of gyrA, gyrB, parC, and parE and MICs of antimicrobial agents for 55 selected serovar Typhi strains
| Isolatea | Yr of isolation | Country or provinceb | Amino acid substitution(s) in gyrA | Nucleotide change(s) in gyrA | QRDR profilec
|
Presence of MDR | MIC of drug (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| gyrB | parC | parE | Nalidixic acid | Ciprofloxacin | Ofloxacin | Gatifloxacin | ||||||
| D 43* | 2004 | India | S83Y | TCC→TAC | wt | wt | wt | No | >256 | 0.25 | 0.5 | 0.19 |
| B 111* | 2004 | India | S83Y | TCC→TAC | wt | wt | wt | Yes | >256 | 0.25 | 0.5 | 0.19 |
| E 86* | 2004 | India | S83F | TCC→TTC | wt | wt | wt | No | >256 | 0.25 | 0.5 | 0.19 |
| A 102* | 2004 | India | S83Y | TCC→TAC | wt | wt | wt | Yes | >256 | 0.25 | 0.5 | 0.19 |
| C 152* | 2004 | India | S83Y | TCC→TAC | wt | wt | wt | No | >256 | 0.25 | 0.5 | 0.19 |
| CT 29* | 1994 | Tien Giang | S83F | TCC→TTC | wt | wt | wt | Yes | 256 | 0.5 | 1 | 0.094 |
| CT 61* | 1994 | Tien Giang | S83F | TCC→TTC | wt | wt | wt | Yes | 256 | 0.125 | 1 | 0.064 |
| nar 102* | 1995 | HCMC | S83F | TCC→TTC | wt | wt | wt | Yes | 128 | 0.125 | 1 | 0.094 |
| nar 104* | 1995 | HCMC | S83F | TCC→TTC | wt | wt | wt | Yes | 256 | 0.25 | 1 | 0.125 |
| nar 107* | 1995 | HCMC | S83F | TCC→TTC | wt | wt | wt | Yes | 256 | 0.25 | 1 | 0.125 |
| nar 108 | 1995 | HCMC | S83F | TCC→TTC | wt | wt | wt | No | 256 | 0.25 | 1 | 0.125 |
| ipt 2* | 1995 | HCMC | D87G | GAC→GGC | wt | wt | wt | No | 256 | 0.25 | 2 | 0.125 |
| nar 28 | 1996 | HCMC | S83F | TCC→TTC | wt | wt | wt | Yes | 256 | 0.5 | 1 | 0.19 |
| nar 45 | 1996 | HCMC | S83F | TCC→TTC | wt | wt | wt | Yes | 256 | 0.5 | 0.5 | 0.125 |
| nar 46 | 1996 | HCMC | D87A | GAC→GCC | wt | wt | wt | Yes | 64 | 0.06 | 0.5 | 0.032 |
| nar 50 | 1996 | HCMC | S83F | TCC→TTC | wt | wt | wt | Yes | 256 | 0.5 | 1 | 0.094 |
| nar 51 | 1996 | HCMC | S83F | TCC→TTC | wt | wt | wt | Yes | 256 | 0.5 | 1 | 0.125 |
| ipt 32 | 1997 | Long An | S83F | TCC→TTC | wt | wt | wt | Yes | 256 | 0.5 | 1 | 0.125 |
| ipt 33 | 1997 | Long An | S83F | TCC→TTC | wt | wt | wt | Yes | 256 | 0.5 | 0.5 | 0.125 |
| CT 118* | 2001 | HCMC | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.38 | 1 | 0.094 |
| CT 142* | 2001 | HCMC | S83F | TCC→TTC | wt | wt | wt | No | >256 | 0.38 | 1 | 0.125 |
| CT 144* | 2001 | Can Tho | D87G | GAC→GGC | wt | wt | wt | No | >256 | 0.25 | 1 | 0.094 |
| CT 145* | 2001 | Long An | S83F | TCC→TTC | wt | wt | wt | No | 128 | 0.38 | 0.5 | 0.094 |
| DT 2* | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 2 | 0.125 |
| DT 3* | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 2 | 0.094 |
| DT 9 | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 2 | 0.25 |
| DT 15 | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1 | 0.094 |
| DT 18 | 2002 | Dong Thap | S83F and D87G | TCC→TTC and GAC→GGC | wt | wt | wt | Yes | >256 | 0.5 | 2 | 0.25 |
| DT 37 | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1 | 0.125 |
| DT 40 | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.38 | 0.5 | 0.125 |
| DT 42 | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1 | 0.125 |
| DT 47* | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | 128 | 0.5 | 1 | 0.125 |
| DT 48 | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1 | 0.125 |
| DT 49 | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1 | 0.125 |
| DT 54 | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1 | 0.125 |
| DT 60 | 2002 | Dong Thap | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.25 | 1 | 0.125 |
| AG 3 | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | No | >256 | 0. | 2 | 0.25 |
| AG 5 | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1 | 0.25 |
| AG 6 | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1 | 0.19 |
| AG 7 | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1.5 | 0.19 |
| AG 8 | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1.5 | 0.19 |
| AG 15 | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1.5 | 0.13 |
| AG 16 | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.38 | 1.5 | 0.13 |
| AG 17 | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.5 | 1.5 | 0.19 |
| AG 152* | 2005 | An Giang | S83F and D87N | TCC→TTC and GAC→AAC | wt | wt | wt | Yes | >256 | 0.38 | 3 | 0.25 |
| AG 168 | 2005 | An Giang | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.38 | 1 | 0.13 |
| AG 169 | 2005 | An Giang | S83F | TCC→TTC | wt | wt | wt | No | >256 | 0.38 | 1 | 0.13 |
| AG 176 | 2005 | An Giang | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.25 | 0.75 | 0.09 |
| AG 182 | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | No | >256 | 0.38 | 1 | 0.13 |
| AG 258* | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | No | >256 | 0.5 | 1.5 | 0.19 |
| AG 259* | 2004 | An Giang | S83F | TCC→TTC | wt | wt | wt | No | >256 | 0.38 | 1.5 | 0.13 |
| HTD 798 | 2003 | HCMC | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.38 | 1 | 0.13 |
| BL 21801* | 2004 | Pakistan | S83F | TCC→TTC | wt | wt | wt | No | >256 | 0.25 | 0.5 | 0.19 |
| BL 21095* | 2004 | Pakistan | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.25 | 0.5 | 0.19 |
| BL 3769* | 2004 | Pakistan | S83F | TCC→TTC | wt | wt | wt | Yes | >256 | 0.25 | 0.5 | 0.19 |
Isolate names consist of an abbreviation for the study followed by the isolate number. *, strain screened for presence of qnrA and qnrS genes by PCR.
An Giang Province, Dong Thap Province, Can Tho Province, Tien Giang Province, Long An Province, and Ho Chi Minh City (HCMC) are located in southern Vietnam.
wt, wild type.
DNA sequence analysis of QRDR of DNA gyrase and DNA topoisomerase IV and effect of mutations on fluoroquinolone susceptibility.
One hundred twenty-seven nalidixic acid-resistant serovar Typhi isolates (118 from southern Vietnam, 5 from India, and 4 from Pakistan) with reduced susceptibilities to the fluoroquinolones (MIC of ofloxacin ranging from 0.5 μg/ml to 3 μg/ml) were selected for molecular analysis of the quinolone resistance determining region (QRDR) of gyrA. Six different types of mutations were detected. The most prevalent amino acid substitution was Ser83→Phe (TCC→TTC) in 117/127 (92.1%) strains. Four isolates (3.1%) had an alteration at codon 83 changing Ser to Tyr (TCC→TAC). Two isolates showed the Asp87→Gly (GAC→GGC) substitution and two isolates the Asp87→Ala (GAC→GCC) substitution. Two serovar Typhi isolates had double-amino-acid substitutions in GyrA: isolates DT 18 (Ser83→Phe and Asp87→Gly) and AG 152 (Ser83→Phe and Asp87→Asn), as shown in Table 3.
Fifty-five of these strains were analyzed for mutations in the QRDR of gyrB, parC, and parE (13, 20); no mutations were detected (Table 3). Twenty-five isolates (indicated with an asterisk in Table 3) were screened for the presence of the plasmid-mediated quinolone resistance genes qnrA and qnrS (15); none were detected in these isolates.
In vitro time-kill analysis.
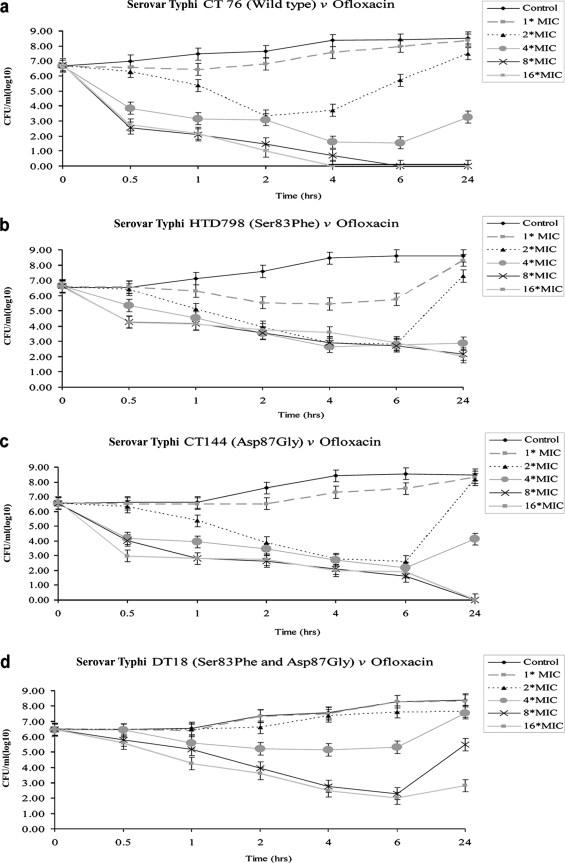
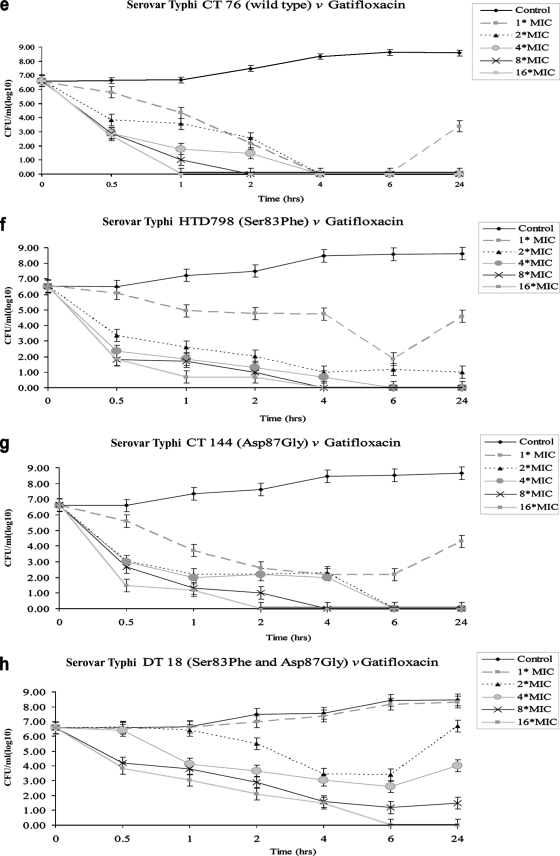
One isolate representing each mutation group was selected for in vitro time-kill experiments: CT 76, wild-type strain (MICs, 0.064 μg/ml for ofloxacin and 0.008 μg/ml for gatifloxacin); HTD 798 (Ser83→Phe; MICs, 1.0 μg/ml for ofloxacin and 0.13 μg/ml for gatifloxacin); CT 144 (Asp87→Gly; MICs, 1 μg/ml for ofloxacin and 0.094 μg/ml for gatifloxacin); and DT 18 (Ser83→Phe and Asp87→Gly; MICs, 2.0 μg/ml for ofloxacin and 0.25 μg/ml for gatifloxacin). The mean changes in log10 CFU/ml are presented in Fig. 2. Ofloxacin showed rapid killing of wild-type strain CT 76 (Fig. 2a); viable counts of serovar Typhi HTD 798 and CT 144 decreased after 4 h at 4× MIC, but complete killing could not be achieved (Fig. 2b and c). No bactericidal activity was achieved against serovar Typhi DT 18 (Fig. 2d). Gatifloxacin at 4× MIC decreased the bacterial population of CT 76, HTD 798, and CT 144 (Fig. 2e, f, and g) in the first 30 min and showed complete killing after 6 h. Viable counts of serovar Typhi DT 18 decreased after 4 h, followed by regrowth; higher concentrations (8× or 16× MIC) showed a more pronounced bactericidal effect against this double mutant (Fig. 2h).
FIG. 2.
In vitro time-kill experiments of wild-type serovar Typhi and serovar Typhi harboring single and double amino acid substitutions in GyrA. Figure 2a to d shows exposure to ofloxacin, and Fig. 2e to h shows exposure to gatifloxacin at concentrations of 1× to 16× MIC over 24 h. Results represent means of duplicate values; the standard deviation is indicated by error bars.
DISCUSSION
This study describes the trends in antimicrobial drug resistance of serovar Typhi in Vietnam between 1993 and 2005 and across Asia in 2002 to 2004.
In 1993, during the initial outbreak of MDR serovar Typhi in Kien Giang province in the south of Vietnam, the fluoroquinolone antibiotics were introduced for the treatment of typhoid fever (22). Since 1993, the proportion of MDR serovar Typhi has remained at high levels and there has been a dramatic increase in nalidixic acid resistance. In 1998, 5 years after ofloxacin and ciprofloxacin become widely available in an uncontrolled market, 87% of the isolates were resistant to nalidixic acid; this increased to 97% by 2004. The combination of MDR and nalidixic acid resistance is a particular problem in Vietnam, because it severely restricts the therapeutic options for patients with typhoid fever.
Patients infected with nalidixic acid-resistant serovar Typhi show poor clinical response, high failure rates (up to 36%), and prolonged fecal carriage when treated with an older-generation fluoroquinolone, such as ofloxacin (8, 26). The antimicrobial resistance data from southern Vietnam are complemented by the results of a cross-sectional study from eight Asian countries: Bangladesh, China, India, Indonesia, Laos, Nepal, Pakistan, and Vietnam. These countries are home to approximately 80% of the world's typhoid fever cases (11).
While in southern Vietnam the MDR phenotype of serovar Typhi has remained at high levels over the last 13 years, there have been reports of a return to chloramphenicol sensitivity in some regions (12, 21). However, in our study the prevalence of chloramphenicol resistance remained high in many Asian countries (18% in Laos, 19% in Nepal, 26% in India and Pakistan, and 40% in Bangladesh), with the exception of China and Indonesia.
In 2002 to 2004, all countries in the region, with the exception of China and Laos, faced a problem of nalidixic acid resistance, with southern Vietnam as a particular hot spot. Roumagnac et al. recently suggested that fluoroquinolone use has driven the clonal expansion of a nalidixic acid-resistant serovar Typhi haplotype, H58, in Southeast Asia (29). The emergence of resistance of serovar Typhi to ciprofloxacin (6/149 isolates; 4%) in Nepal, together with reports of high-level ciprofloxacin resistance in India and Bangladesh (14, 28, 30), might be the prelude to a worsening drug resistance problem in Asia.
In this study carried out across Asia, mutations associated with nalidixic acid resistance and reduced susceptibility to fluoroquinolones for serovar Typhi were defined only in gyrA, as single-amino-acid substitutions at either codon 83 or 87 (6, 18, 31, 37), with the exception of two isolates from Vietnam, which had double-amino-acid substitutions. There have been two recent reports of serovar Typhi with the Ser83Phe and Asp87Gly double alteration in high-level-ciprofloxacin-resistant serovar Typhi (28, 30). In our study, the isolates with double mutations in gyrA were less susceptible to the fluoroquinolones, and this phenotype may become more widespread in the future if continued drug pressure is applied. This is a particular problem in many parts of Asia, where antibiotics are readily available in an unregulated marketplace and inadequate doses and durations of antibiotics are often used.
Our time-kill experiments suggest that the choice of the fluoroquinolone and the dose used for the treatment of serovar Typhi may be critical and underline that clearly not all the fluoroquinolones are as susceptible to these common mutations. Continued use of the older-generation fluoroquinolones (ofloxacin and ciprofloxacin) may encourage the persistence of resistant isolates and lead to the development of new mutations which might compromise the efficacy of the newer generation. With lower MICs and better responses in the time-kill experiments, it is possible that gatifloxacin (and potentially other newer-generation fluoroquinolones) would prove a better choice for use in typhoid fever. This provides a clear rationale for the clinical assessment of these drugs in randomized controlled trials in typhoid fever. If these in vitro data are supported by clinical results, then this newer generation of fluoroquinolones should be recommended for the treatment of typhoid fever instead of ciprofloxacin and ofloxacin.
In conclusion, the emergence and persistence of MDR and nalidixic acid-resistant serovar Typhi strains constitute a major problem across Asia. No drug has ever been developed specifically for typhoid fever, and there are very few potential targets in Salmonella against which new drugs could be designed (3). We need to use our current drugs better and use the best and most affordable drugs available in order to prevent further resistance. Knowledge of the extent of drug resistance should be an important factor when discussing the implementation of a comprehensive typhoid vaccination strategy.
Acknowledgments
We are grateful to the directors of Dong Thap Provincial Hospital, An Giang Provincial Hospital, and the Hospital for Tropical Diseases, Ho Chi Minh City, Vietnam, for their support.
We thank the microbiology staff and all the doctors and nurses who cared for the patients in these studies.
This work was funded by The Wellcome Trust, United Kingdom. Support came from the Diseases of the Most Impoverished Program (DOMI), funded by the Bill and Melinda Gates Foundation and coordinated by the International Vaccine Institute, Seoul, South Korea.
Footnotes
Published ahead of print on 1 October 2007.
REFERENCES
- 1.Acosta, C. J., C. M. Galindo, M. Ali, R. A. Elyazeed, R. L. Ochiai, M. C. Danovaro-Holliday, A. L. Page, V. D. Thiem, Y. Jin, J. K. Park, H. Lee, M. K. Puri, B. Ivanoff, M. D. Agtini, R. Soeharno, C. H. Simanjuntak, N. H. Punjabi, D. G. Canh, D. Sur, Q. Nizami, B. Manna, D. Bai-qing, D. D. Anh, Y. Honghui, S. K. Bhattacharya, Z. Bhutta, D. D. Trach, Z. Y. Xu, T. Pang, A. Donner, and J. D. Clemens. 2005. A multi-country cluster randomized controlled effectiveness evaluation to accelerate the introduction of Vi polysaccharide typhoid vaccine in developing countries in Asia: rationale and design. Trop. Med. Int. Health 10:1219-1228. [DOI] [PubMed] [Google Scholar]
- 2.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Preparation and analysis of DNA, p. 2.0.1-2.14.8. In F. M. Ausubel, R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.), Current protocols in molecular biology, vol. 1. Wiley, New York, New York. [Google Scholar]
- 3.Becker, D., M. Selbach, C. Rollenhagen, M. Ballmaier, T. F. Meyer, M. Mann, and D. Bumann. 2006. Robust Salmonella metabolism limits possibilities for new antimicrobials. Nature 440:303-307. [DOI] [PubMed] [Google Scholar]
- 4.Bhan, M. K., R. Bahl, and S. Bhatnagar. 2005. Typhoid and paratyphoid fever. Lancet 366:749-762. [DOI] [PubMed] [Google Scholar]
- 5.Brooks, W. A., A. Hossain, D. Goswami, K. Nahar, K. Alam, N. Ahmed, A. Naheed, G. B. Nair, S. Luby, and R. F. Breiman. 2005. Bacteremic typhoid fever in children in an urban slum, Bangladesh. Emerg. Infect. Dis. 11:326-329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, J. C., P. M. Shanahan, M. V. Jesudason, C. J. Thomson, and S. G. Amyes. 1996. Mutations responsible for reduced susceptibility to 4-quinolones in clinical isolates of multi-resistant Salmonella typhi in India. J. Antimicrob. Chemother. 37:891-900. [DOI] [PubMed] [Google Scholar]
- 7.Cao, X. T., R. Kneen, T. A. Nguyen, D. L. Truong, N. J. White, and C. M. Parry. 1999. A comparative study of ofloxacin and cefixime for treatment of typhoid fever in children. The Dong Nai Pediatric Center Typhoid Study Group. Pediatr. Infect. Dis. J. 18:245-248. [DOI] [PubMed] [Google Scholar]
- 8.Chinh, N. T., C. M. Parry, N. T. Ly, H. D. Ha, M. X. Thong, T. S. Diep, J. Wain, N. J. White, and J. J. Farrar. 2000. A randomized controlled comparison of azithromycin and ofloxacin for treatment of multidrug-resistant or nalidixic acid-resistant enteric fever. Antimicrob. Agents Chemother. 44:1855-1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clinical and Laboratory Standards Institute. 2007. Performance standards for antimicrobial susceptibility testing; 17th informational supplement. MS100-S17. CLSI, Wayne, PA.
- 10.Clinical and Laboratory Standards Institute. 2006. Performance standards for antimicrobial susceptibility tests. Approved standard, 9th ed. CLSI document M2-A9. CLSI, Wayne, PA.
- 11.Crump, J. A., S. P. Luby, and E. D. Mintz. 2004. The global burden of typhoid fever. Bull. World Health Organ. 82:346-353. [PMC free article] [PubMed] [Google Scholar]
- 12.Dutta, S., D. Sur, B. Manna, S. K. Bhattacharya, J. L. Deen, and J. D. Clemens. 2005. Rollback of Salmonella enterica serotype Typhi resistance to chloramphenicol and other antimicrobials in Kolkata, India. Antimicrob. Agents Chemother. 49:1662-1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eaves, D. J., L. Randall, D. T. Gray, A. Buckley, M. J. Woodward, A. P. White, and L. J. Piddock. 2004. Prevalence of mutations within the quinolone resistance-determining region of gyrA, gyrB, parC, and parE and association with antibiotic resistance in quinolone-resistant Salmonella enterica. Antimicrob. Agents Chemother. 48:4012-4015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaind, R., B. Paglietti, M. Murgia, R. Dawar, S. Uzzau, P. Cappuccinelli, M. Deb, P. Aggarwal, and S. Rubino. 2006. Molecular characterization of ciprofloxacin-resistant Salmonella enterica serovar Typhi and Paratyphi A causing enteric fever in India. J. Antimicrob. Chemother. 58:1139-1144. [DOI] [PubMed] [Google Scholar]
- 15.Gay, K., A. Robicsek, J. Strahilevitz, C. H. Park, G. Jacoby, T. J. Barrett, F. Medalla, T. M. Chiller, and D. C. Hooper. 2006. Plasmid-mediated quinolone resistance in non-Typhi serotypes of Salmonella enterica. Clin. Infect. Dis. 43:297-304. [DOI] [PubMed] [Google Scholar]
- 16.Griggs, D. J., K. Gensberg, and L. J. Piddock. 1996. Mutations in gyrA gene of quinolone-resistant Salmonella serotypes isolated from humans and animals. Antimicrob. Agents Chemother. 40:1009-1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hata, M., M. Suzuki, M. Matsumoto, M. Takahashi, K. Sato, S. Ibe, and K. Sakae. 2005. Cloning of a novel gene for quinolone resistance from a transferable plasmid in Shigella flexneri 2b. Antimicrob. Agents Chemother. 49:801-803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirose, K., A. Hashimoto, K. Tamura, Y. Kawamura, T. Ezaki, H. Sagara, and H. Watanabe. 2002. DNA sequence analysis of DNA gyrase and DNA topoisomerase IV quinolone resistance-determining regions of Salmonella enterica serovar Typhi and serovar Paratyphi A. Antimicrob. Agents Chemother. 46:3249-3252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jacoby, G. A., K. E. Walsh, D. M. Mills, V. J. Walker, H. Oh, A. Robicsek, and D. C. Hooper. 2006. qnrB, another plasmid-mediated gene for quinolone resistance. Antimicrob. Agents Chemother. 50:1178-1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ling, J. M., E. W. Chan, A. W. Lam, and A. F. Cheng. 2003. Mutations in topoisomerase genes of fluoroquinolone-resistant salmonellae in Hong Kong. Antimicrob. Agents Chemother. 47:3567-3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mohanty, S., K. Renuka, S. Sood, B. K. Das, and A. Kapil. 2006. Antibiogram pattern and seasonality of Salmonella serotypes in a North Indian tertiary care hospital. Epidemiol. Infect. 134:961-966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nguyen, T. A., K. Ha Ba, and T. D. Nguyen. 1993. Typhoid fever in South Vietnam, 1990-1993. Bull. Soc. Pathol. Exot. 86:476-478. (In French.) [PubMed] [Google Scholar]
- 23.Nguyen, T. C., T. Solomon, X. T. Mai, T. L. Nguyen, T. T. Nguyen, J. Wain, S. D. To, M. D. Smith, N. P. Day, T. P. Le, C. Parry, and N. J. White. 1997. Short courses of ofloxacin for the treatment of enteric fever. Trans. R. Soc. Trop. Med. Hyg. 91:347-349. [DOI] [PubMed] [Google Scholar]
- 24.Parry, C. M. 2004. The treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever in Viet Nam. Trans. R. Soc. Trop. Med. Hyg. 98:413-422. [DOI] [PubMed] [Google Scholar]
- 25.Parry, C. M., T. T. Hien, G. Dougan, N. J. White, and J. J. Farrar. 2002. Typhoid fever. N. Engl. J. Med. 347:1770-1782. [DOI] [PubMed] [Google Scholar]
- 26.Parry, C. M., V. A. Ho, T. Phuong le, P. V. Bay, M. N. Lanh, T. Tung le, N. T. Tham, J. Wain, T. T. Hien, and J. J. Farrar. 2007. Randomized controlled comparison of ofloxacin, azithromycin, and an ofloxacin-azithromycin combination for treatment of multidrug-resistant and nalidixic acid-resistant typhoid fever. Antimicrob. Agents Chemother. 51:819-825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Phongmany, S., R. Phetsouvanh, S. Sisouphone, C. Darasavath, P. Vongphachane, O. Rattanavong, M. Mayxay, A. C. Ramsay, S. D. Blacksell, C. Thammavong, B. Syhavong, N. J. White, and P. N. Newton. 2005. A randomized comparison of oral chloramphenicol versus ofloxacin in the treatment of uncomplicated typhoid fever in Laos. Trans. R. Soc. Trop. Med. Hyg. 99:451-458. [DOI] [PubMed] [Google Scholar]
- 28.Renuka, K., S. Sood, B. K. Das, and A. Kapil. 2005. High-level ciprofloxacin resistance in Salmonella enterica serotype Typhi in India. J. Med. Microbiol. 54:999-1000. [DOI] [PubMed] [Google Scholar]
- 29.Roumagnac, P., F. X. Weill, C. Dolecek, S. Baker, S. Brisse, N. T. Chinh, T. A. Le, C. J. Acosta, J. Farrar, G. Dougan, and M. Achtman. 2006. Evolutionary history of Salmonella typhi. Science 314:1301-1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saha, S. K., G. L. Darmstadt, A. H. Baqui, D. W. Crook, M. N. Islam, M. Islam, M. Hossain, S. El Arifeen, M. Santosham, and R. E. Black. 2006. Molecular basis of resistance displayed by highly ciprofloxacin-resistant Salmonella enterica serovar Typhi in Bangladesh. J. Clin. Microbiol. 44:3811-3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shirakawa, T., B. Acharya, S. Kinoshita, S. Kumagai, A. Gotoh, and M. Kawabata. 2006. Decreased susceptibility to fluoroquinolones and gyrA gene mutation in the Salmonella enterica serovar Typhi and Paratyphi A isolated in Katmandu, Nepal, in 2003. Diagn. Microbiol. Infect. Dis. 54:299-303. [DOI] [PubMed] [Google Scholar]
- 32.Smith, M. D., N. M. Duong, N. T. Hoa, J. Wain, H. D. Ha, T. S. Diep, N. P. Day, T. T. Hien, and N. J. White. 1994. Comparison of ofloxacin and ceftriaxone for short-course treatment of enteric fever. Antimicrob. Agents Chemother. 38:1716-1720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tran, T. H., D. B. Bethell, T. T. Nguyen, J. Wain, S. D. To, T. P. Le, M. C. Bui, M. D. Nguyen, T. T. Pham, A. L. Walsh, et al. 1995. Short course of ofloxacin for treatment of multidrug-resistant typhoid. Clin. Infect. Dis. 20:917-923. [PubMed] [Google Scholar]
- 34.Vinh, H., N. M. Duong, T. Phuong le, N. T. Truong, P. V. Bay, J. Wain, T. S. Diep, V. A. Ho, N. J. White, N. P. Day, and C. M. Parry. 2005. Comparative trial of short-course ofloxacin for uncomplicated typhoid fever in Vietnamese children. Ann. Trop. Paediatr. 25:17-22. [DOI] [PubMed] [Google Scholar]
- 35.Vinh, H., C. M. Parry, V. T. Hanh, M. T. Chinh, D. House, C. T. Tham, N. T. Thao, T. S. Diep, J. Wain, N. P. Day, N. J. White, and J. J. Farrar. 2004. Double blind comparison of ibuprofen and paracetamol for adjunctive treatment of uncomplicated typhoid fever. Pediatr. Infect. Dis. J. 23:226-230. [DOI] [PubMed] [Google Scholar]
- 36.Wain, J., L. T. Diem Nga, C. Kidgell, K. James, S. Fortune, T. Song Diep, T. Ali, P. O. Gaora, C. Parry, J. Parkhill, J. Farrar, N. J. White, and G. Dougan. 2003. Molecular analysis of incHI1 antimicrobial resistance plasmids from Salmonella serovar Typhi strains associated with typhoid fever. Antimicrob. Agents Chemother. 47:2732-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wain, J., N. T. Hoa, N. T. Chinh, H. Vinh, M. J. Everett, T. S. Diep, N. P. Day, T. Solomon, N. J. White, L. J. Piddock, and C. M. Parry. 1997. Quinolone-resistant Salmonella typhi in Viet Nam: molecular basis of resistance and clinical response to treatment. Clin. Infect. Dis. 25:1404-1410. [DOI] [PubMed] [Google Scholar]
- 38.World Health Organization. 2003. Background document: the diagnosis, treatment and prevention of typhoid fever. Department of Vaccines and Biologicals, World Health Organization, Geneva, Switzerland. http://www.who.int/vaccine_research/documents/en/typhoid_diagnosis.pdf.