Abstract
This study aimed to determine lamivudine disposition in infants and to construct an appropriate dose adjustment for age, given the widespread use of lamivudine for both the prevention of mother-to-child transmission of human immunodeficiency virus (HIV) and the treatment of HIV-infected infants. Using a pooled-population approach, the pharmacokinetics of lamivudine in HIV-exposed or -infected infants from four Pediatric AIDS Clinical Trials Group studies were assessed. Ninety-nine infants provided 559 plasma samples for measurement of lamivudine concentrations. All infants received combination antiretroviral therapy including lamivudine dosed at 2 mg/kg of body weight every 12 h (q12h) for the first 4 to 6 weeks of life and at 4 mg/kg q12h thereafter. Lamivudine's apparent clearance was 0.25 liter/h/kg at birth, doubling by 28 days. In the final model, age and weight were the only significant covariates for lamivudine clearance. While lamivudine is predominantly renally eliminated, the serum creatinine level was not an independent covariate in the final model, possibly because it was confounded by age. Inclusion of interoccasion variability for bioavailability improved the individual subject clearance prediction over the age range studies. Simulations based on the final model predicted that by the age of 4 weeks, 90% of infant lamivudine concentrations with the standard 2 mg/kg dose of lamivudine fell below the adult median concentration. This population pharmacokinetic analysis affirms that adjusting the dose of lamivudine from 2 mg/kg to 4 mg/kg q12 h at the age of 4 weeks for infants with normal maturation of renal function will provide optimal lamivudine exposure, potentially contributing to more successful therapy.
Lamivudine is widely used for infants and children in combination antiretroviral therapy for human immunodeficiency virus type 1 (HIV-1) infection and in the prevention of mother-to-infant HIV transmission (13, 14, 19). As a nucleoside reverse transcriptase inhibitor, lamivudine, along with zidovudine, forms the nucleoside backbone of highly active antiretroviral therapy for HIV-infected infants (23). The combination of lamivudine and zidovudine administered prepartum, peripartum, and postpartum to both the HIV-infected mother and the exposed infant has reduced transmission to the infants by more than two-thirds compared to a placebo (20). In resource-limited countries, the World Health Organization recommends that lamivudine and/or zidovudine be used in combination with single-dose nevirapine administered to the mother at the time of delivery in order to reduce nevirapine resistance (24).
Lamivudine is primarily renally eliminated (9). Consistent with the known postnatal maturation of renal function, initial assessments of lamivudine pharmacokinetics in newborns exposed to HIV at birth suggested that the oral clearance of lamivudine in the first week of life was half that of older children (11, 17). While lamivudine pharmacokinetics have been well described for children, limited pharmacokinetic data exist describing how lamivudine clearance changes in the first months of life (11, 19). In the absence of these data, current standard lamivudine dosing recommendations are to treat neonates in the first few weeks of life with 2 mg/kg of body weight every 12 h (q12h) and then to increase to the standard pediatric dose of 4 mg/kg q12h some time after the first month of life (23). While differences in clearance are anticipated based on the immaturity of renal function during the first year of life, limited data exist characterizing age-related lamivudine pharmacokinetics to ensure that infants in the first year of life receive both a safe and an efficacious dose of lamivudine.
Several Pediatric AIDS Clinical Trials Group (PACTG) protocols have incorporated lamivudine in the treatment of HIV-exposed or -infected infants. However, standard intensive pharmacokinetic evaluations during the first months of life were not included in these protocols. The aim of this study was to use a population pharmacokinetic approach to collect limited samples from a large number of infants and to develop a pharmacokinetic model to describe lamivudine disposition from the first week of life through the age of 2 years. Pooling of lamivudine pharmacokinetic data across these studies of infants enabled (i) the characterization of the developmental aspects of lamivudine elimination during the first year of life with a unified analysis and (ii) the derivation of a pharmacokinetics-based dosing regimen for lamivudine in infants by using simulation techniques.
MATERIALS AND METHODS
Subjects and samples.
We prospectively collected samples for measurement of lamivudine concentrations from 99 term infants participating in four PACTG studies (PACTG 353, 358, 386, and 356). All infants received postnatal antiretroviral therapy with either stavudine, abacavir, nevirapine, or nelfinavir in combination with lamivudine and zidovudine. The results of these PACTG studies have been presented separately elsewhere (1, 6, 12, 15). The human-subject committee at each participating clinical site approved these studies. Written informed consent was obtained from the children's legal guardians.
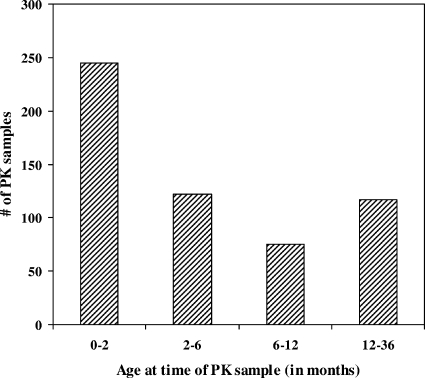
Forty-three HIV-exposed infants were treated with an oral solution of 10 mg/ml of lamivudine at 2 mg/kg q12h for the first 4 to 6 weeks of life as part of PACTG trials 353, 358, and 386. In the fourth study (PACTG 356), 56 HIV-infected infants were treated with lamivudine at 2 to 4 mg/kg q12h initiated within the first 2 years of life and continued for as long as 200 weeks. All infants were healthy and were formula fed by mouth. Three pharmacokinetic samples were collected at multiple study visits from most subjects predose and at 1 to 3 and 4 to 6 h postdose. The timing of the PK samples depended on the antiretrovirals that were studied. For studies with HIV-exposed infants (PACTG 353, 358, and 386), the first sample was taken in the first to third week of life and the second set of pharmacokinetic samples was taken at 6 weeks of life, when prophylaxis therapy was to be discontinued. For study 356, where the subjects were HIV infected, as many as five pharmacokinetic samples were taken over 80 weeks from the time of enrollment. The dose administration times for the three doses preceding and the first dose following the first pharmacokinetic sample were recorded. Forty-two samples were excluded from the analysis due to concentrations that were not quantifiable (<10 ng/ml) (n = 6) or concentration data that were inconsistent with the recorded dose time (n = 36). In total, lamivudine concentrations were analyzed for 559 samples from 99 infants (Table 1). The age distribution at the time of pharmacokinetic sampling is shown in Fig. 1. Of the 245 infants who provided pharmacokinetic samples at the age of 0 to 2 months, 220 were HIV exposed.
TABLE 1.
Pooled patient characteristics
| Patient characteristica | Value
|
|
|---|---|---|
| Median ± SD | Range | |
| Age at first visit (days) | 56 ± 163 | 3-757 |
| Weight at first visit (kg) | 4.2 ± 2.7 | 2.1-16.2 |
| Body surface area at first visit (m2) | 0.25 ± 0.10 | 0.16-0.60 |
| Serum creatinine level at first visit (mg/dl) | 0.40 ± 0.17 | 0.1-0.9 |
| Gender ratio at first visit (M:F) | 39:60 | |
| No. of pharmacokinetic samples in study: | ||
| 353 (ZDV/NFV) | 102 | |
| 356 (ZDV or d4T/NVP with or without ABC and with or without NFV) | 339 | |
| 358 (ZDV) | 55 | |
| 386 (ZDV) | 63 | |
Abbreviations: ZDV, zidovudine; d4T, stavudine; NVP, nevirapine; ABC, abacavir; NFV, nelfinavir.
FIG. 1.
Age distribution of pharmacokinetic (PK) sampling for HIV-exposed and -infected children.
Plasma lamivudine concentrations were determined by a validated enzyme immunoassay method at the University of California, San Diego, Pediatric Pharmacology Laboratory. The lower limit of quantification for lamivudine was 10 ng/ml. The intra- and interassay coefficients of variation (CV) were 5.9% and 13.3%, respectively. The intra- and interassay CV for the controls were <10% and <11.5%, respectively. Accuracy was within an 11.5% deviation from the target.
Pharmacokinetic analysis.
Pharmacokinetic data were analyzed using a population approach and nonlinear mixed-effects modeling software (NONMEM, version V.1; Globomax, Hanover, MD) (2). Structurally, a one-compartment model with first-order absorption and elimination was chosen (ADVAN2 TRANS2). The first-order conditional estimation method was used with ETA-EPS interaction. Pharmacokinetic parameters were scaled by subject weight before evaluation of other potential covariates. Potential covariates were evaluated in a two-step process. First, covariates were added to the model one at a time in a univariate assessment. Covariates that improved the model fitting by a change of 4 in the objective function (P < 0.05) were then evaluated using a multivariate approach. For the multivariate analysis, a backwards elimination process was employed. Covariates were retained if the pharmacokinetic model statistically improved the goodness of fit, represented by a decrease of >6.6 in the minimum value of the objective function (P < 0.01). Empirical Bayesian estimates of individual infant pharmacokinetic parameters were generated from the final model using the post hoc subroutine. Parameters found to have a statistically significant effect on clearance were modeled with linear and nonlinear functions.
The final model was cross-validated to assess pharmacokinetic model performance. Twenty data sets were generated in a process that randomly removed five subjects from each data set. Model parameters were reestimated using each data set and were then used to predict the concentrations for the five subjects excluded from the parameter estimation. This approach was repeated, removing a different five subjects with each iteration until every subject's concentrations were predicted with a model developed independently of his or her own data. Median absolute error and median error were calculated as measures of precision and bias. Both of these measures were expressed as percentages of population-predicted concentrations.
Monte Carlo simulations were performed using the final model to generate concentration profiles of 2,000 virtual infants with demographic characteristics similar to those of the study population. The resulting lamivudine concentration profiles of 2 and 4 mg/kg q12h during the first 2 years of life were compared to standard adult lamivudine exposure following 150 mg of lamivudine twice a day (25).
RESULTS
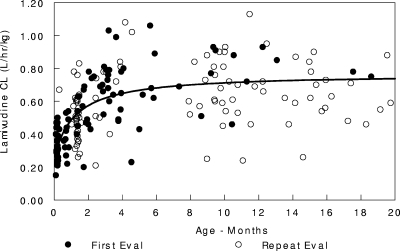
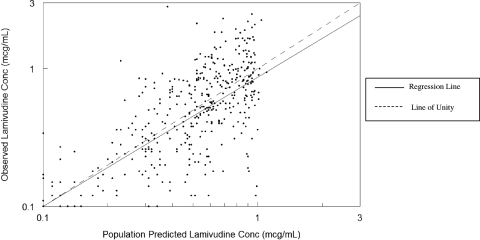
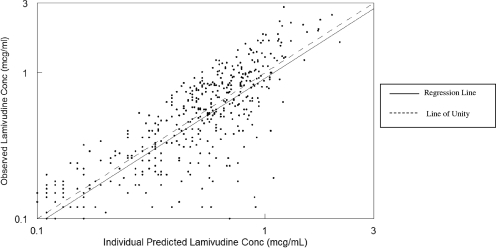
Pharmacokinetic data were available for a total of 99 infants, most of whom were female (62%). The demographic characteristics of the study population are described in Table 1. Overall, the subjects had a median postnatal age of 56 days (range, 3 to 757 days), a median weight of 4.2 kg, and a median body surface area of 0.25 m2 at the first pharmacokinetic evaluation. None of the infants had a significantly elevated serum creatinine level at the first visit (median, 0.4 mg/dl; range 0.1 to 0.9 mg/dl). In the univariate analysis, after scaling for weight, the covariates gender, serum creatinine level, elevated bilirubin level, age, and HIV infection had an impact on clearance. Based on the multivariate analysis, only the effects of age on oral clearance (clearance divided by the bioavailability of the oral dose [CL/F]) and volume of distribution of the oral dose (Vd/F) were retained. Infection status and concomitant medication did not influence lamivudine disposition. Lamivudine clearance, expressed in liters per hour per kilogram, was best described by the following equation: CL/F = (0.251 + 0.516)·age/(36 + age), where “age” is the postnatal age in days (Fig. 2). The estimate for the typical Vd/F was 3.34 liters/kg. The intersubject variabilities in CL and Vd were 34% and 31%, respectively. The residual variability estimated by the model was 45%. The goodness-of-fit plots for the final model are shown in Fig. 3 and 4. The line of unity demonstrates that the model describes the data without bias. The r2 values for Fig. 3 and 4 are 0.624 and 0.773, respectively. Accounting for interoccasion variability in bioavailability improved the individual subject clearance prediction over the span of the study.
FIG. 2.
Lamivudine clearance.
FIG. 3.
Population goodness of fit.
FIG. 4.
Individual goodness of fit.
All of the cross-validation data sets successfully converged with similar parameter and variance estimates as the full data set (CV, <15% across data sets for all parameters and variances). The cross-validation of the model demonstrated it to be unbiased, with a median error of 3.8% (25th to 75th percentile, −36 to 53%). The precision was also acceptable given the large range of concentrations (>200-fold), with a median absolute error of 40% (25th to 75th percentile, 18 to 73%), which was similar to the estimated residual error from the final model (45%).
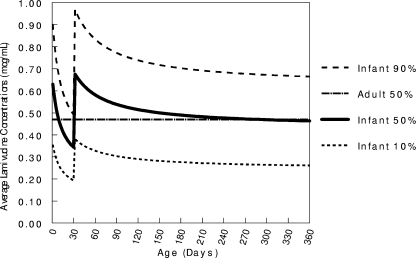
Simulated lamivudine concentration profiles showed that by the age of 4 weeks, 90% of neonates receiving the newborn dose of 2 mg/kg q12h would have average lamivudine concentrations below the adult median concentration (Fig. 5). Increasing the dose to 4 mg/kg q12h at the age of 4 weeks increased the median lamivudine concentration to 0.7 μg/ml. While this is a bit higher than the adult median of 0.47 μg/ml, the vast majority (∼90%) of infant concentrations still remain within a factor of 2 of the adult median.
FIG. 5.
Comparison of infant and adult lamivudine exposure based on an infant dose of 2 mg/kg q12h increased to 4 mg/kg q12h at the age of 4 weeks. The lines represent the 10%, 50%, and 90% lamivudine concentrations for infants and the 50% concentration for adults.
DISCUSSION
Given its safety profile and clinical success record, lamivudine is widely used for infants and children as an integral part of the nucleoside backbone of combination antiretroviral therapy (13, 14). In the PACTG 300 study, zidovudine-plus-lamivudine therapy was found to decrease the progression of HIV disease from that with didanosine monotherapy and was safe for children older than 1 month (14). In a number of trials, lamivudine has been used in combination with zidovudine and/or nevirapine during the first 1 to 6 weeks of life to reduce transmission and limit the development of resistance (13, 16, 20). In the Petra Study, oral lamivudine and zidovudine administered at 36 weeks gestation, intrapartum, and 1 week postpartum to African breastfeeding women and their infants reduced the HIV-1 transmission rate from that with a placebo (5.7% versus 15.3%) (20). Given the growing concerns about resistance with single-dose nevirapine, the World Health Organization recommends that lamivudine and zidovudine be used in combination with single-dose nevirapine administered to the mother at the time of delivery (24). Longer administration of lamivudine-containing regimens to reduce transmission due to breastfeeding is being studied; an understanding of lamivudine pharmacokinetics beyond the first few weeks of life is required. Maintenance of adequate lamivudine concentrations is essential to prevent the development of resistance, since one mutation to valine in the reverse transcriptase at position 184 can lead to a >50-fold decrease in sensitivity to lamivudine (21, 22). Despite the widespread use of lamivudine in pediatrics worldwide, limited pharmacokinetic data exist to ensure that the optimal dose is currently being used for infants.
For adults, the pharmacokinetics of lamivudine in normal, HIV-infected, and hepatitis B-infected subjects have been well studied. The drug is rapidly absorbed after oral administration, with an absolute bioavailability of 86 to 88% for the oral solution, capsule, and tablet (26). Lamivudine exhibits low protein binding, and its pharmacokinetic parameters can be reasonably well described by a one-compartment model. Because lamivudine is primarily renally excreted, CL/F and Vd/F decrease while the half-life and area under the curve increase with increasing renal impairment in adults (9). Extrapolating this finding to the immature renal function of infants, one would predict a lower CL/F and Vd/F in infants than in older children and adults with normal renal function. As renal function matures, it would be predicted that CL/F would also increase, requiring a dose adjustment during this time.
Overall, our pharmacokinetic parameters are consistent with the findings of previous, smaller pediatric studies and with expected maturational changes in renal function. Our post hoc data from the current model are compared to previous mean lamivudine pharmacokinetic estimates for various age groups in Table 2. From neonatal data, oral clearance is seen rapidly increasing between days 1 and 7 of life to a value similar to that for our youngest infants. Previous pediatric studies have reported apparent clearance means from 0.39 to 0.83 liter/h/kg (8, 10). While previous studies indicate that infant clearance increases during the first week of life, our study shows that rapid maturation continues during the subsequent few weeks of life. Our findings are supported by a recent study published by Burger et al., which showed the age-dependent oral clearance of lamivudine in a group of 51 HIV-infected children from the ages of 1.7 to 17 years (5). Though tested in different cohorts, the lower oral clearance of lamivudine in our 29-day- to 3-year-old group compared to their 1.7- to 6-year-old group (0.66 versus 1.03 liters/h/kg) demonstrates age-dependent renal function maturation. This suggests that early infancy and childhood are critical periods for renal development, which may lead to the need for dosing modifications to maintain adequate drug exposure of renally excreted drugs.
TABLE 2.
Comparison of mean lamivudine pharmacokinetic values for HIV-exposed and -infected infants, children, and adults
| Age group (sample size) | Oral dose (q12h) | Pharmacokinetic parameter (95% CI)a
|
Source or reference | |||
|---|---|---|---|---|---|---|
| CL/F (liters/h/kg) | Vd/F (liters/kg) | t1/2 (h) | AUC (μg·h/ml) | |||
| 3-28 days (n = 40) | 2 mg/kg | 0.37 (0.25-0.48) | 3.12 (2.29-3.96) | 6.2 (4.6-7.8) | 6.0 (4.0-8.0) | This study (post hoc) |
| 1 day (n = 16) | 2 mg/kg | 0.19 (0.14-0.26) | NA | 6.2 (5.3-8.0) | 9.8 (7.6-14.1) | 17 |
| 7 days (n = 16) | 2 mg/kg | 0.32 (0.26-0.40) | NA | 7.9 (7.0-9.0) | 6.3 (5.0-7.8) | 17 |
| 29 days-3 yr (n = 59) | 4 mg/kg | 0.66 (0.46-0.86) | 3.44 (2.53-4.35) | 3.8 (2.8-4.8) | 6.8 (3.9-9.7) | This study (post hoc) |
| 3 yr-14 yr (n = 21) | 4 mg/kg | 0.39 (0.25-0.50) | NA | 2.1 (1.9-2.5) | 8.8 (7.9-15.4) | 7 |
| 3 mo-17 yr (n = 8) | 4 mg/kg | NA | NA | 1.8 (0.9-4.2) | 5.2 | 11 |
| 2-13 yr (n = 19) | 4 mg/kg | 0.90 (0.78-1.04) | NA | NA | 8.9 (7.7-10.3) | 3 |
| 1.7-6 yr (n = 17)b | 4 mg/kg | 1.03 (0.67-1.22) | 4.31 (2.59-6.93) | 3.3 (2.5-4.3) | 3.7 (3.4-6.2) | 5 |
| 7-18 yr (n = 34)b | 4 mg/kg | 0.57 (0.45-0.74) | 2.29 (1.64-4.10) | 3.1 (2.6-3.7) | 6.5 (5.2-8.6) | 5 |
| ≥18 yr (n = 394) | 150-300 mg (2-4 mg/kg) | 0.34 (0.32-0.36) | 1.73 (1.58-1.87) | NA | NA | 18 |
| ≥18 yr (n = 13) | 150 mg (4 mg/kg) | NA | NA | 6.1 (4.2-8.0) | 17.1 (10.6-23.6) | 4 |
AUC, total area under the serum concentration curve; NA, not available; t1/2, half-life; 95% CI, 95% confidence interval.
Values for this study are reported as median (interquartile range).
In our study, after scaling for weight, the univariate analysis demonstrated that gender, serum creatinine levels, elevated bilirubin levels, age, and HIV infection had an impact on clearance. However, only age was found to affect the model, and was consequently retained, in the multivariate analysis. In infant populations, several clinical characteristics can be highly correlated and can lead to a high number of covariates being excluded during the multivariate analysis. Therefore, one must be cautious in constructing only mechanistically plausible models to prevent erroneous inferences from significant covariates. Our data support the observation that lamivudine clearance increases in parallel with the maturation of renal function, as expected in view of the predominantly renal elimination of lamivudine. In adults, impaired renal function demonstrates a significant relationship with lamivudine disposition after a single oral dose of 300 mg (9). In an adult population pharmacokinetic model, creatinine clearance and weight were significant covariates (18). Although serum creatinine did not improve our model's clearance predictability in the multivariate analysis, this finding may be confounded by the correlation between age and serum creatinine levels. Our evaluation may also be limited by the fact that we measured only serum creatinine levels and did not measure creatinine clearance. Also, there were no subjects with significant renal dysfunction, and the granularity of the serum creatinine results was limited (rounded to the nearest 0.1 mg/dl, with a median of 0.4 mg/dl). The lack of an association may be due to lamivudine's substantial renal excretion, which may not be predicted by serum creatinine levels or glomerular filtration. In addition, the transporter(s) responsible for lamivudine secretion may mature at a different rate than the glomerulus. Finally, in our population, while one would expect serum creatinine levels to stay stable, the serum creatinine samples in which the reported values were measured and the lamivudine pharmacokinetic samples were not always collected at the same time, possibly explaining the discordance between serum creatinine and clearance.
In our model, accounting for interoccasion variability in bioavailability improved the individual subject clearance prediction over the span of the study. This could reflect differences in absorption based on diet or other physiological processes that affect absorption and other pharmacokinetic parameters. Clinical adverse event reporting from these four PACTG trials found no associated gastrointestinal illness that could have accounted for the differences in absorption. The intervals between pharmacokinetic evaluations were long enough for significant changes in lamivudine disposition to occur. Thus, this study represents not only day-to-day variability but variability in maturation as well. For infants, accounting for interoccasion variability is especially important, since renal maturation and changes in clearance and volume of distribution are occurring as the individual ages. Given the importance of maintaining consistent lamivudine levels to prevent the emergence of resistance, it is important to recognize the variability within a single subject as well as between subjects.
Given the changes in clearance in infants and children within the first 2 years of life, it is important that dosing reflect these changes. Through our simulation we found that transitioning children from 2 mg/kg q12 h to 4 mg/kg q12 h at the age of 4 weeks maintains lamivudine concentrations near adult concentrations. While exact lamivudine levels necessary to optimize therapy are not known, it is clear that the risk of HIV progression is inversely related to age (23). With such a risk of progression and the good safety profile of lamivudine in children, a dosing regimen that maintains plasma drug concentrations equal to or higher than those for adults would be reasonable given the potential benefit of decreasing viremia more rapidly in this susceptible population.
While it is the intracellular triphosphate anabolite of lamivudine that is the active moiety, we are currently limited to measuring lamivudine plasma concentrations and extrapolating the clinical effects from these measurements. The volume of blood needed to measure intracellular lamivudine triphosphate concentrations with current technology makes serial evaluations impractical for infants and children. While newer technologies are currently being developed to measure intracellular concentrations, these methodologies, which include the use of a radiolabeled drug in microdosing quantities, have not yet been evaluated for antiretrovirals in pediatrics.
Lamivudine is a widely used, well-tolerated, effective medication for the interruption of mother-to-child transmission of HIV and the treatment of HIV-infected children worldwide. This pharmacokinetic analysis indicates early increases in lamivudine clearance during infancy, requiring a dose increase to maintain adequate lamivudine exposure. Using a population pharmacokinetic analysis, this study has demonstrated that the variability in infant lamivudine pharmacokinetics requires a dose transition from 2 mg/kg q12 h to 4 mg/kg q12 h at the age of 28 days for infants with normal maturation of renal function in order to provide adequate lamivudine exposure for the majority of infants. As future studies help define sources of lamivudine pharmacokinetic variability and its exposure-response surface, further refinement of lamivudine use will be possible to ensure optimal drug concentrations and improve the control of viremia in pediatric HIV worldwide.
Acknowledgments
This project was supported in part by NIH grants UO1-AI32907 and UO1-AI32391 (to K. Luzuriaga), UO1-AI42845 (to the University of Massachusetts Center for AIDS Research), and UO1-AI41089 (to E. P. Acosta).
We do not have any financial disclosures or other conflicts of interest. We are indebted to the children and their guardians who made this work possible.
Footnotes
Published ahead of print on 24 September 2007.
REFERENCES
- 1.Acosta, E. P., A. Bardeguez, C. D. Zorrilla, R. Van Dyke, M. D. Hughes, S. Huang, L. Pompeo, A. M. Stek, J. Pitt, D. H. Watts, E. Smith, E. Jimenez, and L. Mofenson. 2004. Pharmacokinetics of saquinavir plus low-dose ritonavir in human immunodeficiency virus-infected pregnant women. Antimicrob. Agents Chemother. 48:430-436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beal, S. L., and A. J. Boeckmann. 1994. NONMEM users guide. NONMEM Project Group, University of California, San Francisco.
- 3.Bergshoeff, A., D. Burger, C. Verweij, L. Farrelly, J. Flynn, M. Le Prevost, S. Walker, V. Novelli, H. Lyall, S. Khoo, and D. Gibb. 2005. Plasma pharmacokinetics of once- versus twice-daily lamivudine and abacavir: simplification of combination treatment in HIV-1-infected children (PENTA-13). Antivir. Ther. 10:239-246. [PubMed] [Google Scholar]
- 4.Bruno, R., M. B. Regazzi, V. Ciappina, P. Villani, P. Sacchi, M. Montagna, R. Panebianco, and G. Filice. 2001. Comparison of the plasma pharmacokinetics of lamivudine during twice and once daily administration in patients with HIV. Clin. Pharmacokinet. 40:695-700. [DOI] [PubMed] [Google Scholar]
- 5.Burger, D. M., G. Verweel, N. Rakhmanina, C. P. Verwey-Van Wissen, C. J. La Porte, A. S. Bergshoeff, H. Lyall, N. G. Hartwig, H. Green, S. Soldin, D. M. Gibb, and R. de Groot. 2007. Age-dependent pharmacokinetics of lamivudine in HIV-infected children. Clin. Pharmacol. Ther. 81:517-520. [DOI] [PubMed] [Google Scholar]
- 6.Capparelli, E. V., J. L. Sullivan, L. Mofenson, E. Smith, B. Graham, P. Britto, M. I. Becker, D. Holland, J. D. Connor, and K. Luzuriaga. 2001. Pharmacokinetics of nelfinavir in human immunodeficiency virus-infected infants. Pediatr. Infect. Dis. J. 20:746-751. [DOI] [PubMed] [Google Scholar]
- 7.Fletcher, C. V., S. P. Kawle, T. N. Kakuda, P. L. Anderson, D. Weller, L. R. Bushman, R. C. Brundage, and R. P. Remmel. 2000. Zidovudine triphosphate and lamivudine triphosphate concentration-response relationships in HIV-infected persons. AIDS 14:2137-2144. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher, C. V., R. Yogev, S. A. Nachman, A. Wiznia, S. Pelton, K. McIntosh, and K. Stanley. 2004. Pharmacokinetic characteristics of ritonavir, zidovudine, lamivudine, and stavudine in children with human immunodeficiency virus infection. Pharmacotherapy 24:453-459. [DOI] [PubMed] [Google Scholar]
- 9.Heald, A. E., P. H. Hsyu, G. J. Yuen, P. Robinson, P. Mydlow, and J. A. Bartlett. 1996. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with renal dysfunction. Antimicrob. Agents Chemother. 40:1514-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, M. A., K. H. Moore, G. J. Yuen, A. Bye, and G. E. Pakes. 1999. Clinical pharmacokinetics of lamivudine. Clin. Pharmacokinet. 36:41-66. [DOI] [PubMed] [Google Scholar]
- 11.Lewis, L. L., D. Venzon, J. Church, M. Farley, S. Wheeler, A. Keller, M. Rubin, G. Yuen, B. Mueller, M. Sloas, L. Wood, F. Balis, G. M. Shearer, P. Brouwers, J. Goldsmith, P. A. Pizzo, et al. 1996. Lamivudine in children with human immunodeficiency virus infection: a phase I/II study. J. Infect. Dis. 174:16-25. [DOI] [PubMed] [Google Scholar]
- 12.Luzuriaga, K., M. McManus, L. Mofenson, P. Britto, B. Graham, and J. L. Sullivan. 2004. A trial of three antiretroviral regimens in HIV-1-infected children. N. Engl. J. Med. 350:2471-2480. [DOI] [PubMed] [Google Scholar]
- 13.Mandelbrot, L., A. Landreau-Mascaro, C. Rekacewicz, A. Berrebi, J. L. Benifla, M. Burgard, E. Lachassine, B. Barret, M. L. Chaix, A. Bongain, N. Ciraru-Vigneron, C. Crenn-Hebert, J. F. Delfraissy, C. Rouzioux, M. J. Mayaux, and S. Blanche. 2001. Lamivudine-zidovudine combination for prevention of maternal-infant transmission of HIV-1. JAMA 285:2083-2093. [DOI] [PubMed] [Google Scholar]
- 14.McKinney, R. E., Jr., G. M. Johnson, K. Stanley, F. H. Yong, A. Keller, K. J. O'Donnell, P. Brouwers, W. G. Mitchell, R. Yogev, D. W. Wara, A. Wiznia, L. Mofenson, J. McNamara, S. A. Spector, et al. 1998. A randomized study of combined zidovudine-lamivudine versus didanosine monotherapy in children with symptomatic therapy-naive HIV-1 infection. J. Pediatr. 133:500-508. [DOI] [PubMed] [Google Scholar]
- 15.Mirochnick, M., A. Stek, M. Acevedo, M. Keller, D. Holland, E. Capparelli, J. Connor, S. Huang, M. Hughes, H. Watts, L. Mofenson, and Y. Bryson. 2005. Safety and pharmacokinetics of nelfinavir coadministered with zidovudine and lamivudine in infants during the first 6 weeks of life. J. Acquir. Immune Defic. Syndr. 39:189-194. [PubMed] [Google Scholar]
- 16.Moodley, D., J. Moodley, H. Coovadia, G. Gray, J. McIntyre, J. Hofmyer, C. Nikodem, D. Hall, M. Gigliotti, P. Robinson, L. Boshoff, and J. L. Sullivan. 2003. A multicenter randomized controlled trial of nevirapine versus a combination of zidovudine and lamivudine to reduce intrapartum and early postpartum mother-to-child transmission of human immunodeficiency virus type 1. J. Infect. Dis. 187:725-735. [DOI] [PubMed] [Google Scholar]
- 17.Moodley, D., K. Pillay, K. Naidoo, J. Moodley, M. A. Johnson, K. H. Moore, P. N. Mudd, Jr., and G. E. Pakes. 2001. Pharmacokinetics of zidovudine and lamivudine in neonates following coadministration of oral doses every 12 hours. J. Clin. Pharmacol. 41:732-741. [DOI] [PubMed] [Google Scholar]
- 18.Moore, K. H., G. J. Yuen, E. K. Hussey, G. E. Pakes, J. J. Eron, Jr., and J. A. Bartlett. 1999. Population pharmacokinetics of lamivudine in adult human immunodeficiency virus-infected patients enrolled in two phase III clinical trials. Antimicrob. Agents Chemother. 43:3025-3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mueller, B. U., L. L. Lewis, G. J. Yuen, M. Farley, A. Keller, J. A. Church, J. C. Goldsmith, D. J. Venzon, M. Rubin, P. A. Pizzo, and F. M. Balis. 1998. Serum and cerebrospinal fluid pharmacokinetics of intravenous and oral lamivudine in human immunodeficiency virus-infected children. Antimicrob. Agents Chemother. 42:3187-3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petra Study Team. 2002. Efficacy of three short-course regimens of zidovudine and lamivudine in preventing early and late transmission of HIV-1 from mother to child in Tanzania, South Africa, and Uganda (Petra study): a randomised, double-blind, placebo-controlled trial. Lancet 359:1178-1186. [DOI] [PubMed] [Google Scholar]
- 21.Schuurman, R., M. Nijhuis, R. van Leeuwen, P. Schipper, D. de Jong, P. Collis, S. A. Danner, J. Mulder, C. Loveday, and C. Christopherson. 1995. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC). J. Infect. Dis. 171:1411-1419. [DOI] [PubMed] [Google Scholar]
- 22.Tisdale, M., S. D. Kemp, N. R. Parry, and B. A. Larder. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. USA 90:5653-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Working Group on Antiretroviral Therapy and the Medical Management of HIV-Infected Children. 26 October 2006. Guidelines for the use of antiretroviral agents in pediatric HIV infection. http://aidsinfo.nih.gov/contentfiles/PediatricGuidelines.pdf.
- 24.World Health Organization. 2004. Antiretroviral drugs for treating pregnant women and preventing HIV infection in infants. Guidelines on care, treatment and support for women living with HIV/AIDS and their children in resource-constrained settings. World Health Organization, Geneva, Switzerland. www.who.int/entity/hiv/pub/mtct/en/arvdrugswomenguidelinesfinal.pdf.
- 25.Yuen, G. J., Y. Lou, N. F. Bumgarner, J. P. Bishop, G. A. Smith, V. R. Otto, and D. D. Hoelscher. 2004. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob. Agents Chemother. 48:176-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yuen, G. J., D. M. Morris, P. K. Mydlow, S. Haidar, S. T. Hall, and E. K. Hussey. 1995. Pharmacokinetics, absolute bioavailability, and absorption characteristics of lamivudine. J. Clin. Pharmacol. 35:1174-1180. [DOI] [PubMed] [Google Scholar]