Abstract
Combination therapy is the most effective strategy to prevent emergence of resistance during tuberculosis (TB) treatment. Another strategy, albeit theoretical, is to limit the time that drug concentrations fall in the “mutant selection window” (MSW) between the MIC and the mutant prevention concentration (MPC). Drug concentrations above the MPC prevent selective amplification of resistant mutants in vitro even with a single drug exposure. The MSW concept has been validated using fluoroquinolones against Mycobacterium tuberculosis in vitro but not in vivo. Using a mouse model in which serum moxifloxacin (MXF) concentrations were maintained above the MPC, we tested whether this strategy prevents selection of MXF-resistant mutants. Beginning 2 weeks after aerosol infection with M. tuberculosis, when the mean lung log10 CFU count was 7.9 ± 0.2, mice received either no treatment or MXF in the diet at 0.25% to approximate the conventional human dose or 1.5% to maintain serum concentrations above the MPC (8 μg/ml). After 56 days of treatment, lung CFU counts were 3.5 ± 0.8 and 0.9 ± 0.6 in 0.25% and 1.5% of the MXF-treated mice, respectively. In mice given 0.25% MXF, MXF-resistant mutants were selected by day 28 and detected in 16% (3/19) of mice tested on day 56. No selection of MXF-resistant mutants was detected in mice given 1.5% MXF. We conclude that maintaining serum concentrations of MXF above the MPC prevents selection of MXF-resistant mutants. Although this target cannot be achieved clinically with MXF, it might be possible with new fluoroquinolones with more potent activity and/or improved pharmacokinetics.
Proper provision of and adherence to recommended 6-month treatment regimens for tuberculosis (TB) remain unmet goals in many countries of the world, resulting in continued TB transmission, excess morbidity and mortality, and increasing incidence of drug resistance. The new threat of extensively drug-resistant TB (XDR-TB) is the latest consequence of the failure of health care systems around the world to properly diagnose and treat patients with TB (2, 6, 11, 16, 37).
Drug resistance emerges in Mycobacterium tuberculosis through selection of spontaneously preexisting drug-resistant mutants (16). In a wild-type bacillary population, such mutants are present at a predictable frequency of between 10−6 and 10−8 and are selectively amplified by monotherapy or inadequate combination therapy (16). The usual approach to prevent the emergence of drug resistance is to use combination therapy. This is effective due to the rule of independence of mutation, each drug being active on preexisting mutants resistant to other drugs (16). Another theoretical approach proposed by Karl Drlica and colleagues is to administer the drug at doses that produce blood concentrations that continuously exceed the resistance level of all spontaneous drug-resistant mutants and thereby prevent the selective amplification of any mutant population. The drug concentration capable of inhibiting all spontaneous mutants has been termed the “mutant prevention concentration” (MPC). It is defined experimentally as the lowest drug concentration that prevents the emergence of resistant mutants when a large number of organisms (up to 1010 bacilli) are exposed to the drug (10, 35). In addition, Drlica and colleagues posit that the selective amplification of spontaneous drug-resistant mutants from among the susceptible population is most pronounced at concentrations below the MPC yet above the MIC against the susceptible population, a concentration range that defines the “mutant selection window.” This concept has clinical relevance only for drugs to which the level of first-step mutational resistance is relatively low, as is the case for the fluoroquinolones (9), including moxifloxacin (MXF). Recent work has shown that maintaining concentrations outside the mutant selection window for all or part of the dosing interval prevents the selection of resistant mutants from M. tuberculosis in vitro (9, 15), but this concept has never been validated in vivo.
In a previous study, we demonstrated that the MPC of MXF against M. tuberculosis was between 4 and 8 μg/ml and that treatment of infected mice with MXF mixed in the diet at concentrations ranging from 0. 125 to 1% resulted in the selection of drug-resistant mutants (13). Although the latter concentration produced serum MXF concentrations above the MPC (8 μg/ml), we were unable to maintain such concentrations throughout the dosing interval. Therefore, the objectives of the present study were, first, to establish a dosing strategy in which serum MXF concentrations were consistently maintained well above 8 μg/ml and, second, to test whether such a strategy would prevent the selection of MXF-resistant mutants.
MATERIALS AND METHODS
Dosing strategy to maintain serum MXF concentrations above 8 μg/ml.
MXF powder was graciously provided by Bayer (Leverkusen, Germany). Its half-life in mice is approximately 1 to 2 h (21, 24). In order to maintain relatively constant serum concentrations, MXF was mixed into a powdered mouse diet (Harlan Teklad, Madison, WI). However, the bitter taste of MXF required the addition of sugar in a 10:1 ratio with MXF. Through a series of pilot studies, it was established that 1.5% (wt/wt) MXF in the mixed diet produced consistent serum concentrations ≥8 μg/ml at steady state (i.e., after 48 h). Additional supplementation with MXF in the diet, in the drinking water, and by gavage was necessary during the first 48 h when mice were not yet accustomed to the MXF-containing diet as a loading dose to establish concentrations ≥8 μg/ml as quickly as possible. The final dosing regimen (test regimen) consisted of administration of 2% MXF in the diet, 0.3% (wt/vol) MXF in the water (mixed with the sweetener Aspartame), and 75 mg/kg of body weight MXF twice daily by gavage for the first 24 h. The same regimen was continued in the second 24 h, except that the MXF concentration in drinking water was reduced to 0.15%. For the third day of treatment, dosing in the water and by gavage was discontinued and the diet concentration was reduced to 1.5%, the concentration maintained to the end of treatment. Details of the dosing regimen are given in Table 1.
TABLE 1.
Dosing regimen for rapid attainment of MXF concentrations above the MPC
| Day | Regimen |
|---|---|
| 0 | Start of treatment at 5 p.m., 2% MXF in diet, 0.3% MXF in water |
| 1 | 75 mg/kg MXF by gavage in morning and evening, 2% MXF in diet, 0.3% MXF in water replaced by 0.15% MXF in water in evening |
| 2 | 75 mg/kg MXF by gavage in morning and evening, 2% MXF in diet, 0.15% MXF in water replaced with plain water in evening |
| 3 | 2% MXF in diet replaced with 1.5% MXF in diet in evening |
| 4-53 | Continued on 1.5% MXF in diet |
Aerosol infection model.
One hundred forty-six Swiss (CD-1) mice (6 to 7 weeks of age; weight, 22 ± 2 g [Charles River Laboratories, Wilmington, MA]) were aerosol infected with Mycobacterium tuberculosis using the Middlebrook inhalation exposure system (Glas-Col, Terre Haute, IN). M. tuberculosis H37Rv (MXF MIC, 0.5 μg/ml) was cultivated in Middlebrook 7H9 broth (Fisher, Pittsburgh, PA) supplemented with 10% oleic acid-albumin-dextrose-catalase. When the optical density at 600 nm surpassed 1, the broth culture was used for aerosol infection. Mice were infected in two successive runs, and representative mice from each run were sacrificed to confirm that similar infections were achieved. All animal procedures were approved by the institutional animal care and use committee.
After infection, mice were randomized into three groups: (i) untreated negative controls (n = 26); (ii) positive controls (n = 60) treated with 0.25% MXF in the diet, which produces serum MXF concentrations of 0.5 to 2 μg/ml, consistent with the average serum concentrations produced in humans by the conventional daily oral dose of 400 mg (22); and (iii) test mice (n = 60) treated with the test regimen described in the preceding section. Treatment began 14 days after infection and was continued for 8 weeks.
Assessment of serum MXF concentrations.
To demonstrate that the desired MXF concentrations were achieved and maintained in both groups, serum was sampled at predetermined time points throughout the course of treatment. Three mice per treatment group were sacrificed at 9 a.m. and at 5 p.m. on days 1, 2, 3, and 7 of treatment and at 5 p.m. on days 14, 28, 42, 53, and 56. Because mice consume more diet during the night, the 5 p.m. time point approximates daily trough values. Mice were anesthetized with chloroform and exsanguinated by cardiac puncture. Serum was separated and stored at −70°C before being shipped overnight on dry ice to the Infectious Disease Pharmacokinetics Laboratory, National Jewish Medical and Research Center, Denver, CO. MXF concentrations were determined using a validated assay on a ThermoFinnigan P4000 high-performance liquid chromatography pump (Thermo-Finnigan, San Jose, CA) with a model AS1000 fixed-volume autosampler, a model FL3000 fluorescence detector (Thermo Electron Corporation, Waltham, MA), a Gateway E-Series computer (Gateway, Poway, CA), and the Chromquest high-performance liquid chromatography data management system (Thermo Electron Corporation). The six-point standard curves ranged from 0.2 to 15 g/ml, with linearity extending well above this range (17).
Assessment of treatment efficacy.
Three untreated mice from each run were sacrificed 1 day after infection to determine the number of CFU implanted and 14 days after infection (day 0) to determine the baseline CFU count at the initiation of treatment. Three mice from each treatment group were sacrificed on days 14, 28, 42, and 53 of treatment. Drug-containing diet was discontinued on day 53 to provide for a 3-day washout period. On day 56, all remaining mice from the positive control group (0.25% MXF) and four mice from the test group were sacrificed for CFU counts at completion of treatment. Because of the prospect that mice in the test group could have been culture negative on completion of therapy, the remaining 20 mice in the test group were kept for an additional 8 weeks without treatment to allow regrowth of viable bacilli and amplification of small numbers of resistant mutants (if present). Quantitative lung CFU counts were performed as previously described, except that Middlebrook 7H11 agar was used (23, 34). In brief, mouse lungs were homogenized in 5 ml phosphate-buffered saline using glass homogenizers and 0.5 ml aliquots were plated onto 7H11 agar plates in duplicate at appropriate dilutions. The lowest detection limit therefore was 5 CFU/lung when plated undiluted.
Detection of MXF-resistant mutants.
MXF-resistant mutants were detected on 7H11 agar by adapting the standard methods used for TB drug susceptibility testing (5, 18). Two methods were used.
(i) Direct proportion method.
In parallel with the quantitative CFU counts for assessment of treatment efficacy, 0.5-ml aliquots of lung homogenates were plated undiluted in duplicate on 7H11 plates containing MXF at concentrations of 0.5, 2, and 8 μg/ml.
(ii) Indirect proportion method.
After counting CFU on drug-free plates to determine treatment efficacy, an attempt was made to scrape together all colonies from the most heavily populated plates. This material was resuspended in 7 ml phosphate-buffered saline to obtain an approximate cell density of 1 × 108 cells/ml and replated on 7H11 agar plates with and without MXF at 0.5, 2, and 8 μg/ml. Samples were considered to be enriched for drug-resistant mutants if the proportion of resistant CFU observed was ≥1 log10 greater than the baseline proportion observed in untreated mice at day 0.
Analysis of resistant mutants.
All CFU isolated on 0.5, 2, and 8 μg/ml of MXF by the direct method and 8 randomly selected resistant isolates from those obtained by the indirect method were further analyzed for mutations in the quinolone resistance-determining regions (QRDRs) of gyrA and gyrB. Genomic DNA was extracted from the resistant colonies by the cetyltrimethylammonium bromide-NaCl procedure (31). A 320-bp region of gyrA and a 428-bp region of gyrB were amplified by PCR using primers and conditions described previously (19, 30). The PCR products were then purified and sequenced to detect mutations.
RESULTS
Assessment of serum MXF concentrations.
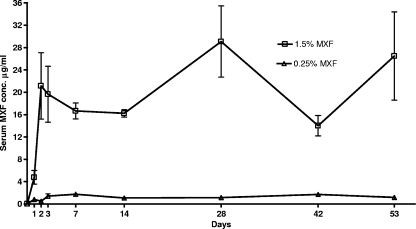
The serum MXF concentrations observed during the course of the experiment are provided in Fig. 1. Concentrations in the positive controls (0.25% MXF) ranged from 0.41 to 2.2 μg/ml (mean, 1.14 ± 0.68 μg/ml), for an average area under the concentration-time curve from 0 to 24 h (AUC0-24) of 27.4 μg h/ml. These data are consistent with the serum concentrations obtained in humans with the conventional 400-mg daily oral dose where the Cmax and AUC are 2.5 to 5.0 μg/ml and 26.9 to 39.0 μg h/ml, respectively (27, 28, 32). Concentrations in test mice reached 8 μg/ml during the first 24 h and were maintained above this level throughout the entire course of treatment (mean, 17.51 ± 11.12 μg/ml). Despite the high sustained-MXF concentrations, there was no outward evidence of drug toxicity.
FIG. 1.
Observed MXF serum concentrations during treatment.
Assessment of treatment efficacy.
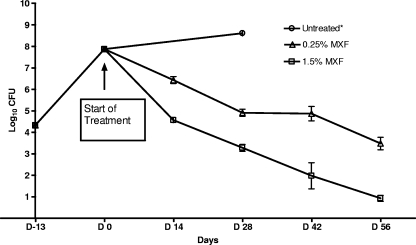
Group mean CFU counts are displayed in Fig. 2. Mean lung CFU counts 1 day after infection were 4.27 ± 0.05 and 4.40 ± 0.09 log10 CFU (mean, 4.33 ± 0.10 log10 CFU) in mice infected in aerosol runs 1 and 2, respectively. At the initiation of treatment (day 0), the mean CFU count had increased to 7.87 ± 0.18 log10 CFU. All untreated mice, except one, died during the first month after infection; the remaining mouse was sacrificed at day 28 and had a lung CFU count of 8.61 log10 CFU. During the course of treatment, there was a progressive, dose-dependent decrease in the CFU counts in mice receiving MXF. By the end of treatment (day 53 and day 56 results combined) the CFU counts in positive controls (i.e., mice treated with 0.25% MXF in the diet), had fallen by 4.39 logs to 3.48 ± 0.76 log10 CFU. In mice treated with the test regimen, the CFU count was reduced by almost 7 logs to 0.93 ± 0.62 log10 CFU. These results demonstrate the remarkable dose-dependent bactericidal activity of MXF against M. tuberculosis.
FIG. 2.
Lung CFU counts during treatment. *, all mice in the negative control except for one died within 4 weeks of infection. D−13, 1 day after infection.
Detection of MXF-resistant mutants.
In untreated mice, the baseline proportions of resistant mutants were 1 × 10−7 at 0.5 μg/ml MXF, between 1 × 10−9 and 1 × 10−8 at 2 μg/ml MXF, and undetectable (i.e., <10−9) at 8 μg/ml MXF. The proportion of resistant mutants isolated over the course of treatment is displayed in Table 2. Using the direct method, mutants resistant to MXF were detected at day 28 among mice receiving 0.25% MXF in the diet, as 1 of 3 mice yielded isolates resistant to 0.5 μg/ml MXF at a frequency of 1.0 × 10−3. At day 42, 1 out of 2 mice treated with 0.25% MXF in the diet yielded isolates resistant to 0.5 μg/ml (5.3 × 10−5) and 2 μg/ml (5.3 × 10−5) of MXF. Both these findings were confirmed by the indirect method. On treatment completion, 1 out of 19 mice treated with 0.25% MXF in the diet yielded CFU resistant to 0.5 μg/ml (2.5 × 10−3) and 2 μg/ml of MXF (1.3 × 10−3) by the direct method. Two additional mice in this group were found to harbor an increased proportion of resistant mutants by the indirect method. The proportion of resistant colonies seen in these mice was estimated to be between 10−4 and 10−5, at least 100 times greater than the baseline proportion established in untreated mice. The mice had counts of only 4,500 and 17,500 CFU per lung at treatment completion. This coupled with the fact that only 60% of the lung homogenate was plated on MXF-containing plates may explain why the resistant mutants were not detected by the direct method but an increased proportion of resistant mutants were identified by the indirect method.
TABLE 2.
Proportion of mice harboring MXF-resistant mutants
| Drug regimen | Method of estimation | Proportion of mice harboring mutant strains on day:
|
||||
|---|---|---|---|---|---|---|
| 14 | 28 | 42 | 56 | 56 + 8 wka | ||
| 0.25% MXF | Direct | 0/3 | 1/3 | 1/2 | 1/19 | |
| Indirect | 0/3 | 1/3 | NAb | 3/19 | ||
| 1.5% MXF | Direct | 0/3 | 0/3 | 0/3 | 0/7 | 0/20 |
| Indirect | 0/3 | 0/3 | 0/3 | 0/7 | 0/20 | |
Mice sacrificed after being kept without treatment for an additional 8 weeks.
Not available due to overgrowth of contaminants.
No MXF-resistant mutant was detected by either method in any mouse treated with the test regimen (1.5% MXF in the diet) at any point during treatment. Even among the 20 mice held for 8 additional weeks after treatment completion, the proportion of resistant mutants was similar to that observed in untreated animals, indicating that the treatment of infected mice with MXF at concentrations above the MPC level completely prevented the selective amplification of MXF-resistant mutants.
Analysis of MXF-resistant mutants.
All MXF-resistant mutants detected in mice treated with 0.25% MXF in the diet had mutations in the QRDR of gyrA or gyrB (Table 3). All resistant CFU isolated from the day 42 mouse showed an E512D mutation in gyrB, while all resistant CFU isolated from day 56 mouse 1 showed a D94N mutation in gyrA. The same mutations were confirmed in the isolates obtained by the indirect method. For the day 28 mouse, mutation analysis of resistant isolates from direct plates was not possible; however, isolates from indirect plates showed the D94N mutation in gyrA. For day 56 mice 2 and 3, resistant isolates were only detected by the indirect method. Sequence analysis from these plates showed only the D94N mutation in gyrA in isolates from mouse 2 and only the E512 mutation in gyrB in isolates from mouse 3.
TABLE 3.
Sequence analysis of QRDR for resistant mutants
| Day of CFU count | No. of resistant mutants isolated at MXF concn (μg/ml) of:
|
Mutation in:
|
|||
|---|---|---|---|---|---|
| 0.5 | 2 | 8 | gyrA | gyrB | |
| 28a | D94N | None | |||
| 42 | 2 | 2 | 0 | None | E512D |
| 56 (mouse 1) | 8 | 4 | 0 | D94N | None |
| 56 (mouse 2)a | D94N | None | |||
| 56 (mouse 3)a | None | E512D | |||
Performed with isolates obtained on plates by the indirect method.
DISCUSSION
In this study, we developed a murine dosing model capable of maintaining MXF serum concentrations above the MPC (i.e., 8 μg/ml) and used this model to demonstrate that this dosing strategy prevents the selective amplification of drug-resistant mutants. On the other hand, monotherapy with a MXF regimen that maintains serum concentrations within the mutant selection window (i.e., between the MIC and the MPC), like the conventional human dose of MXF, selects for resistant mutants. To our knowledge, this is the first study demonstrating the validity of the mutant selection window hypothesis in an animal model of TB.
The emergence of resistance was evident after 4 weeks of monotherapy with 0.25% MXF in the diet and was observed at each time point thereafter. Over the 8-week course of treatment, selective amplification of the MXF-resistant mutant was detected in 5 (18.5%) out of 27 mice treated with 0.25% MXF in the diet. All mutants isolated from these mice had a single mutation in either gyrA (D94N) or gyrB (E512D). The mutation at codon 94 of gyrA is the most frequently reported among fluoroquinolone-resistant clinical isolates (3, 8, 25), so it is not surprising that it was amplified in infected mice treated with human-equipotent doses of MXF. The mutation in gyrB codon 512 has been reported from in vitro studies with other 8-methoxy compounds tested against M. tuberculosis (36), but to our knowledge, this is the first time it has been isolated in vivo, whether from an animal or a human specimen.
What is the significance of these observations for the treatment of tuberculosis? Fluoroquinolones have become the cornerstone oral drugs for treatment of multidrug-resistant TB (MDR-TB). Several observational studies now demonstrate that their use is associated with improved treatment outcomes in this disease (7, 29, 33). As a result, loss of fluoroquinolone susceptibility is a now a criterion for redefining MDR-TB as XDR-TB, a disease that is very difficult to cure with chemotherapy alone. Potent fluoroquinolones such as MXF also are under clinical investigation to replace ethambutol or isoniazid as first-line drugs in novel treatment-shortening regimens (4, 20, 23, 26). Hence, we cannot afford to sacrifice this class of agents to the emerging specter of fluoroquinolone resistance among M. tuberculosis strains. Our results clearly show that MXF concentrations produced by the conventional 400-mg oral dose in humans readily select for drug resistance when the drug is used as monotherapy. This finding is in agreement with a multitude of in vitro studies and clinical observations (12-15). However, we demonstrated here that it is possible to prevent the selection of fluoroquinolone-resistant mutants in vivo if fluoroquinolone exposures are sufficiently high. Unfortunately, none of the currently marketed fluoroquinolones is capable of producing concentrations exceeding the MPC throughout the dosing interval at doses that can be administered safely. However, the development of new fluoroquinolones with more potent anti-TB activity and/or improved pharmacokinetics may introduce new agents capable of meeting this pharmacodynamic target.
Recent work by Gumbo and colleagues suggests that it may not be necessary to maintain concentrations above the MPC for the entire dosing interval to prevent the selection of resistant mutants (15). Using an in vitro pharmacodynamic model with the avirulent H37Ra strain of M. tuberculosis, (MXF MIC, 0.25 μg/ml), Gumbo et al. determined that a free MXF AUC0-24/MIC ratio of 53 may be sufficient to suppress the emergence of resistance. Our findings are consistent with those of Gumbo et al. Assuming 50% of MXF is protein bound, their breakpoint ratio translates into a total MXF AUC0-24/MIC ratio of 106. In our study, the estimated mean total MXF AUC0-24/MIC ratio for the positive control regimen (0.25% MXF) of 54.8 was below this breakpoint, while that for test regimen was much greater than 106.
Although we selected MXF-resistant mutants with our lower, clinically relevant dose of MXF, the proportion of mice harboring such mutants was lower than expected, likely due to the size of our initial inoculum. At the start of treatment, the CFU count was approximately 108 CFU/lung. Given that the frequency of spontaneous mutants resistant to 0.5 μg/ml of MXF in the wild-type population was 1 × 10−7 to 2 × 10−7, there were only a few such mutants present in any given mouse at the onset of treatment. Ideally, a higher initial bacterial burden similar to that used in previous in vitro studies (i.e., 1010) would have been used, but it is not possible to obtain such burdens in the mouse model without causing excessive mortality (13). It is also possible that the steady maintenance of serum MXF concentrations between 0.4 and 2.2 μg/ml suppressed the growth of some first-step mutants with MICs in this range. Prior reports suggest that the MIC of MXF against most single-step mutants is well below 8 μg/ml but is as high as 8 μg/ml against some (1, 8).
In the end, our findings confirm that it is possible to suppress the selective amplification of fluoroquinolone-resistant mutants during MXF monotherapy in vivo provided serum concentrations are maintained above the MPC. Although this may not be the only pharmacodynamic target associated with resistance suppression, it is evident that no currently marketed fluoroquinolone can be expected to meet any such recognized pharmacodynamic target safely. Hence, the development of new fluoroquinolones with greater potency against M. tuberculosis and/or improved pharmacokinetics or safety profiles will be necessary to more effectively suppress the emergence of resistance. Though treatment of TB with monotherapy is never advisable, regimens that include new drugs optimized to limit or prevent selective amplification of resistance should be more effective in controlling the emergence of drug resistance during combination therapy, especially under conditions of suboptimal adherence. A point to note, however, is that the drug exposures that lead to resistance in combination therapy could be different from those observed in monotherapy experiments. Further studies are needed to address this question.
Acknowledgments
We thank Ian Rosenthal for valuable input in planning the study, Kathy Williams and Rokeya Tasneen for technical assistance, and Charles Peloquin for determining the serum concentrations of MXF.
This work was supported by NIAID contract N01-40007.
Footnotes
Published ahead of print on 15 October 2007.
REFERENCES
- 1.Aubry, A., N. Veziris, E. Cambau, C. Truffot-Pernot, V. Jarlier, and L. M. Fisher. 2006. Novel gyrase mutations in quinolone-resistant and -hypersusceptible clinical isolates of Mycobacterium tuberculosis: functional analysis of mutant enzymes. Antimicrob. Agents Chemother. 50:104-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aziz, M. A., A. Wright, A. Laszlo, A. De Muynck, F. Portaels, A. Van Deun, C. Wells, P. Nunn, L. Blanc, and M. Raviglione. 2006. Epidemiology of antituberculosis drug resistance (the Global Project on Anti-tuberculosis Drug Resistance Surveillance): an updated analysis. Lancet 368:2142-2154. [DOI] [PubMed] [Google Scholar]
- 3.Bozeman, L., W. Burman, B. Metchock, L. Welch, and M. Weiner. 2005. Fluoroquinolone susceptibility among Mycobacterium tuberculosis isolates from the United States and Canada. Clin. Infect. Dis. 40:386-391. [DOI] [PubMed] [Google Scholar]
- 4.Burman, W. J., S. Goldberg, J. L. Johnson, G. Muzanye, M. Engle, A. W. Mosher, S. Choudhri, C. L. Daley, S. S. Munsiff, Z. Zhao, A. Vernon, and R. E. Chaisson. 2006. Moxifloxacin versus ethambutol in the first 2 months of treatment for pulmonary tuberculosis. Am. J. Respir. Crit. Care Med. 174:331-338. [DOI] [PubMed] [Google Scholar]
- 5.Canetti, G., S. Froman, J. Grosset, P. Hauduroy, M. Langerova, H. T. Mahler, G. Meissner, D. A. Mitchison, and L. Sula. 1963. Mycobacteria: laboratory methods for testing drug sensitivity and resistance. Bull. W. H. O. 29:565-578. [PMC free article] [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention. 2006. Emergence of Mycobacterium tuberculosis with extensive resistance to second-line drugs—worldwide, 2000-2004. Morb. Mortal. Wkly. Rep. 55:301-305. [PubMed] [Google Scholar]
- 7.Chan, E. D., V. Laurel, M. J. Strand, J. F. Chan, M. L. Huynh, M. Goble, and M. D. Iseman. 2004. Treatment and outcome analysis of 205 patients with multidrug-resistant tuberculosis. Am. J. Respir. Crit. Care Med. 169:1103-1109. [DOI] [PubMed] [Google Scholar]
- 8.Cheng, A. F. B., W. W. Yew, E. W. C. Chan, M. L. Chin, M. M. M. Hui, and R. C. Y. Chan. 2004. Multiplex PCR amplimer conformation analysis for rapid detection of gyrA mutations in fluoroquinolone-resistant Mycobacterium tuberculosis clinical isolates. Antimicrob. Agents Chemother. 48:596-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong, Y., X. Zhao, B. N. Kreiswirth, and K. Drlica. 2000. Mutant prevention concentration as a measure of antibiotic potency: studies with clinical isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 44:2581-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Drlica, K., and X. Zhao. 2007. Mutant selection window hypothesis updated. Clin. Infect. Dis. 44:681-688. [DOI] [PubMed] [Google Scholar]
- 11.Dye, C., M. Hosseini, and C. Watt. 2007. Did we reach the 2005 targets for tuberculosis control? Bull. W. H. O. 85:364-369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ginsburg, A. S., N. Hooper, N. Parrish, K. E. Dooley, S. E. Dorman, J. Booth, M. Diener-West, W. G. Merz, W. R. Bishai, and T. R. Sterling. 2003. Fluoroquinolone resistance in patients with newly diagnosed tuberculosis. Clin. Infect. Dis. 37:1448-1452. [DOI] [PubMed] [Google Scholar]
- 13.Ginsburg, A. S., R. Sun, H. Calamita, C. P. Scott, W. R. Bishai, and J. H. Grosset. 2005. Emergence of fluoroquinolone resistance in Mycobacterium tuberculosis during continuously dosed moxifloxacin monotherapy in a mouse model. Antimicrob. Agents Chemother. 49:3977-3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ginsburg, A. S., S. C. Woolwine, N. Hooper, W. H. Benjamin, Jr., W. R. Bishai, S. E. Dorman, and T. R. Sterling. 2003. The rapid development of fluoroquinolone resistance in M. tuberculosis. N. Engl. J. Med. 349:1977-1978. [DOI] [PubMed] [Google Scholar]
- 15.Gumbo, T., A. Louie, M. R. Deziel, L. M. Parsons, M. Salfinger, and G. L. Drusano. 2004. Selection of a moxifloxacin dose that suppresses drug resistance in Mycobacterium tuberculosis, by use of an in vitro pharmacodynamic infection model and mathematical modeling. J. Infect. Dis. 190:1642-1651. [DOI] [PubMed] [Google Scholar]
- 16.Iseman, M. D. 1994. Evolution of drug-resistant tuberculosis: a tale of two species. Proc. Natl. Acad. Sci. USA 91:2428-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson, J. L., D. J. Hadad, W. H. Boom, C. L. Daley, C. A. Peloquin, K. D. Eisenach, D. D. Jankus, S. M. Debanne, E. D. Charlebois, E. Maciel, M. Palaci, and R. Dietze. 2006. Early and extended early bactericidal activity of levofloxacin, gatifloxacin and moxifloxacin in pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 10:605-612. [PubMed] [Google Scholar]
- 18.Kent, P. T., and G. P. Kubica. 1985. Public health mycobacteriology. A guide for the level III laboratory. Centers for Disease Control and Prevention, Atlanta, GA.
- 19.Kocagöz, T., C. J. Hackbarth, I. Ünsal, E. Y. Rosenberg, H. Nikaido, and H. F. Chambers. 1996. Gyrase mutations in laboratory-selected, fluoroquinolone-resistant mutants of Mycobacterium tuberculosis H37Ra. Antimicrob. Agents Chemother. 40:1768-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lienhardt, C., R. Rustomjee, J. Allen, T. Mthigane, J. Levin, B. Fourie, G. Davies, J. Horton, T. Kanyok, and D. Mitchison. 2005. Comparison of 2-months sterilizing activities of several quinolone-containing regimens: preliminary results of a phase II trial in South Africa, abstr. LB2-13. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother. American Society for Microbiology, Washington, DC.
- 21.Miyazaki, E., M. Miyazaki, J. M. Chen, R. E. Chaisson, and W. R. Bishai. 1999. Moxifloxacin (BAY12-8039), a new 8-methoxyquinolone, is active in a mouse model of tuberculosis. Antimicrob. Agents Chemother. 43:85-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nuermberger, E. L., T. Yoshimatsu, S. Tyagi, W. Bishai, R., and J. Grosset. 2003. Assessment of moxifloxacin activity at clinically relevant dosages in the mouse model of tuberculosis. Am. J. Respir. Crit. Care Med. 169:A433. (Abstract.) [Google Scholar]
- 23.Nuermberger, E. L., T. Yoshimatsu, S. Tyagi, R. J. O'Brien, A. N. Vernon, R. E. Chaisson, W. R. Bishai, and J. H. Grosset. 2004. Moxifloxacin-containing regimen greatly reduces time to culture conversion in murine tuberculosis. Am. J. Respir. Crit. Care Med. 169:421-426. [DOI] [PubMed] [Google Scholar]
- 24.Rosenthal, I. M., K. Williams, S. Tyagi, A. A. Vernon, C. A. Peloquin, W. R. Bishai, J. H. Grosset, and E. L. Nuermberger. 2005. Weekly moxifloxacin and rifapentine is more active than the Denver regimen in murine tuberculosis. Am. J. Respir. Crit. Care Med. 172:1457-1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siddiqi, N., M. Shamim, S. Hussain, R. K. Choudhary, N. Ahmed, Prachee, S. Banerjee, G. R. Savithri, M. Alam, N. Pathak, A. Amin, M. Hanief, V. M. Katoch, S. K. Sharma, and S. E. Hasnain. 2002. Molecular characterization of multidrug-resistant isolates of Mycobacterium tuberculosis from patients in north India. Antimicrob. Agents Chemother. 46:443-450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Spigelman, M. K. 2007. New tuberculosis therapeutics: a growing pipeline. J. Infect. Dis. 196:S28-S34. [DOI] [PubMed] [Google Scholar]
- 27.Stass, H., A. Dalhoff, D. Kubitza, and U. Schühly. 1998. Pharmacokinetics, safety, and tolerability of ascending single doses of moxifloxacin, a new 8-methoxy quinolone, administered to healthy subjects. Antimicrob. Agents Chemother. 42:2060-2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sullivan, J. T., M. Woodruff, J. Lettieri, V. Agarwal, G. J. Krol, P. T. Leese, S. Watson, and A. H. Heller. 1999. Pharmacokinetics of a once-daily oral dose of moxifloxacin (Bay 12-8039), a new enantiomerically pure 8-methoxy quinolone. Antimicrob. Agents Chemother. 43:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tahaoglu, K., T. Torun, T. Sevim, G. Atac, A. Kir, L. Karasulu, I. Ozmen, and N. Kapakli. 2001. The treatment of multidrug-resistant tuberculosis in Turkey. N. Engl. J. Med. 345:170-174. [DOI] [PubMed] [Google Scholar]
- 30.Takiff, H. E., L. Salazar, C. Guerrero, W. Philipp, W. M. Huang, B. Kreiswirth, S. T. Cole, W. R. Jacobs, Jr., and A. Telenti. 1994. Cloning and nucleotide sequence of Mycobacterium tuberculosis gyrA and gyrB genes and detection of quinolone resistance mutations. Antimicrob. Agents Chemother. 38:773-780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Soolingen, D., P. W. M. Hermans, P. E. W. de Haas, D. R. Soll, and J. D. A. van Embden. 1991. Occurrence and stability of insertion sequences in Mycobacterium tuberculosis complex strains: evaluation of an insertion sequence-dependent DNA polymorphism as a tool in the epidemiology of tuberculosis. J. Clin. Microbiol. 29:2578-2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wise, R., J. M. Andrews, G. Marshall, and G. Hartman. 1999. Pharmacokinetics and inflammatory-fluid penetration of moxifloxacin following oral or intravenous administration. Antimicrob. Agents Chemother. 43:1508-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yew, W. W., C. K. Chan, C. H. Chau, C. M. Tam, C. C. Leung, P. C. Wong, and J. Lee. 2000. Outcomes of patients with multidrug-resistant pulmonary tuberculosis treated with ofloxacin/levofloxacin-containing regimens. Chest 117:744-751. [DOI] [PubMed] [Google Scholar]
- 34.Yoshimatsu, T., E. Nuermberger, S. Tyagi, R. Chaisson, W. Bishai, and J. Grosset. 2002. Bactericidal activity of increasing daily and weekly doses of moxifloxacin in murine tuberculosis. Antimicrob. Agents Chemother. 46:1875-1879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhao, X., and K. Drlica. 2001. Restricting the selection of antibiotic-resistant mutants: a general strategy derived from fluoroquinolone studies. Clin. Infect. Dis. 33(Suppl. 3):S147-S156. [DOI] [PubMed] [Google Scholar]
- 36.Zhou, J., Y. Dong, X. Zhao, S. Lee, A. Amin, S. Ramaswamy, J. Domagala, J. M. Musser, and K. Drlica. 2000. Selection of antibiotic-resistant bacterial mutants: allelic diversity among fluoroquinolone-resistant mutations. J. Infect. Dis. 182:517-525. [DOI] [PubMed] [Google Scholar]
- 37.Zignol, M., M. S. Hosseini, A. Wright, C. L. Weezenbeek, P. Nunn, C. J. Watt, B. G. Williams, and C. Dye. 2006. Global incidence of multidrug-resistant tuberculosis. J. Infect. Dis. 194:479-485. [DOI] [PubMed] [Google Scholar]