Abstract
Surveillance studies conducted in the United States over the last decade have revealed increasing resistance among community-acquired respiratory pathogens, especially Streptococcus pneumoniae, that may limit future options for empirical therapy. The objective of this study was to assess the scope and magnitude of the problem at the national and regional levels during the 2005-2006 respiratory season (the season when community-acquired respiratory pathogens are prevalent) in the United States. Also, since faropenem is an oral penem being developed for the treatment of community-acquired respiratory tract infections, another study objective was to provide baseline data to benchmark changes in the susceptibility of U.S. respiratory pathogens to the drug in the future. The in vitro activities of faropenem and other agents were determined against 1,543 S. pneumoniae isolates, 978 Haemophilus influenzae isolates, and 489 Moraxella catarrhalis isolates collected from 104 U.S. laboratories across six geographic regions during the 2005-2006 respiratory season. Among S. pneumoniae isolates, the rates of resistance to penicillin, amoxicillin-clavulanate, and cefdinir were 16, 6.4, and 19.2%, respectively. The least effective agents were trimethoprim-sulfamethoxazole (SXT) and azithromycin, with resistance rates of 23.5 and 34%, respectively. Penicillin resistance rates for S. pneumoniae varied by region (from 8.7 to 22.5%), as did multidrug resistance rates for S. pneumoniae (from 8.8 to 24.9%). Resistance to β-lactams, azithromycin, and SXT was higher among S. pneumoniae isolates from children than those from adults. β-Lactamase production rates among H. influenzae and M. catarrhalis isolates were 27.4 and 91.6%, respectively. Faropenem MICs at which 90% of isolates are inhibited were 0.5 μg/ml for S. pneumoniae, 1 μg/ml for H. influenzae, and 0.5 μg/ml for M. catarrhalis, suggesting that faropenem shows promise as a treatment option for respiratory infections caused by contemporary resistant phenotypes.
Community-acquired respiratory tract infections are among the most prevalent and serious infections in the United States and represent the leading cause of physician office visits. The bacterial pathogens most frequently isolated from respiratory tract infection specimens are Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Although many community-acquired respiratory tract infections are treated empirically without culture guidance, the emergence of resistance in respiratory pathogens continues to threaten this practice (12, 13, 18, 19). Of particular concern are the emergence and dissemination of S. pneumoniae strains with high-level resistance to penicillin (MICs, ≥8 μg/ml) (24) and strains that are multidrug resistant (MDR) to several antibiotic classes (7, 15, 22). Among H. influenzae and M. catarrhalis clinical isolates, the production of β-lactamase continues to compromise the use of amoxicillin as a treatment option (8).
The continued prevalence of resistance among respiratory pathogens warrants the search for new, safe, broad-spectrum oral agents that may be used empirically in the community setting. Faropenem is an oral penem with broad-spectrum activity that includes the respiratory pathogens (4, 5, 25). It was previously evaluated in a U.S. surveillance study in 1999 and shown to be active against penicillin-resistant S. pneumoniae and β-lactamase-producing H. influenzae and M. catarrhalis (4). Faropenem medoxomil (formerly faropenem daloxate) is an oral prodrug that has been evaluated as a therapy for community-acquired respiratory tract infections (9, 26, 29), and studies to develop the drug are ongoing. The objective of this study was to continue to benchmark the activity of faropenem and comparator agents against recent clinical isolates of S. pneumoniae, H. influenzae, and M. catarrhalis collected in the United States during the 2005-2006 respiratory season (the season when community-acquired respiratory pathogens are prevalent). These data will serve as a baseline to monitor future changes in the susceptibility of respiratory pathogens to faropenem and other agents at both the regional and the national levels. Since many studies provide data only at the national level, this study was designed to assess regional surveillance data on S. pneumoniae and H. influenzae isolates collected in the 2005-2006 respiratory season.
MATERIALS AND METHODS
Bacterial isolates.
Respiratory tract isolates were prospectively collected from 104 participating institutions distributed across the United States; the time period of collection was from October 2005 to April 2006. The geographic and regional distributions of the participating sites are shown in Table 1. The isolates were limited to one per patient and were collected from clinical samples derived from various upper and lower respiratory tract sites, blood, ears, and eyes. In addition, subject demographic information, such as age, gender, and patient location (inpatient/outpatient), was collected for each isolate. All isolates were shipped to the central laboratory of CMI, Inc. (Wilsonville, OR), where each isolate was subcultured and reidentified by standard methods. A total of 1,543 isolates of S. pneumoniae were collected, and of these, 69.1% (1,066 isolates) originated from respiratory specimens, 25.2% (389 isolates) were from blood specimens, 5.6% (86 isolates) originated from ear/eye specimens, and 0.1% (2 isolates) were from other or unknown specimen sources. Of the 978 isolates of H. influenzae that were collected, 89.4% (882 isolates) were derived from respiratory specimens, 4.2% (41 isolates) were from blood specimens, 6% (59 isolates) were from ear/eye specimens, and 0.5% (5 isolates) were from unknown/other specimen sources. A total of 489 isolates of M. catarrhalis were collected for susceptibility testing, and of these, 95.3% (466 isolates) were derived from respiratory specimen sources, 0.8% (4 isolates) were from blood specimens, 2.7% (13 isolates) were from ear/eye specimens, and 1.2% (6 isolates) were obtained from other/unknown sources.
TABLE 1.
Geographic distribution of 104 participating sites across six U.S. regions in the 2005-2006 respiratory season
| Geographic region (total no. of sites) and state | No. of participating sites |
|---|---|
| North central (23) | |
| Iowa | 2 |
| Illinois | 4 |
| Minnesota | 3 |
| Missouri | 4 |
| North Dakota | 1 |
| Nebraska | 3 |
| South Dakota | 3 |
| Wisconsin | 3 |
| Northeast (34) | |
| Connecticut | 2 |
| District of Columbia | 1 |
| Indiana | 4 |
| Massachusetts | 2 |
| Michigan | 4 |
| New Hampshire | 1 |
| New York | 9 |
| Ohio | 6 |
| Pennsylvania | 3 |
| Rhode Island | 1 |
| Vermont | 1 |
| Northwest (6) | |
| Idaho | 1 |
| Montana | 1 |
| Oregon | 2 |
| Washington | 2 |
| South central (15) | |
| Kansas | 3 |
| Oklahoma | 3 |
| Tennessee | 2 |
| Texas | 7 |
| Southeast (19) | |
| Alabama | 3 |
| Arkansas | 1 |
| Florida | 2 |
| Georgia | 1 |
| Kentucky | 2 |
| North Carolina | 4 |
| Virginia | 4 |
| West Virginia | 2 |
| Southwest (7) | |
| California | 4 |
| Colorado | 2 |
| Utah | 1 |
Antimicrobial susceptibility testing.
The isolates were tested for their susceptibilities to faropenem, meropenem, amoxicillin-clavulanate, cefdinir, cefuroxime, penicillin (S. pneumoniae only), ampicillin (H. influenzae and M. catarrhalis only), azithromycin, telithromycin, levofloxacin, and trimethoprim-sulfamethoxazole (SXT). Antimicrobial susceptibility testing was conducted by broth microdilution using frozen panels that were prepared at CMI, Inc., in accordance with Clinical and Laboratory Standards Institute (CLSI) guidelines (2). Panels were incubated at 35°C for 20 to 24 h in ambient air before the MICs were determined. S. pneumoniae ATCC 49619 and H. influenzae ATCC 49247 were used as daily panel quality control strains. For S. pneumoniae and H. influenzae, breakpoint interpretations were conducted according to CLSI recommendations (3), with the exception of faropenem, for which no CLSI breakpoints are available. Although CLSI has proposed increasing the breakpoints for penicillin G and nonmeningitis isolates of S. pneumoniae, the breakpoints used to interpret the susceptibility results in this study were those described in CLSI document M100 S-16 (3). All H. influenzae and M. catarrhalis isolates were tested for the production of β-lactamase by the DrySlide nitrocefin test (Difco Laboratories, Detroit, MI).
An analysis of single-drug-resistant (SDR) and MDR S. pneumoniae.
Regional prevalence of phenotypic characteristics of SDR and MDR S. pneumoniae were analyzed for all isolates collected in the study. SDR was defined as resistance to penicillin, cefuroxime, azithromycin, SXT, or levofloxacin, and MDR was defined as concurrent resistance to three or more of the above agents.
RESULTS
The results in Table 2 show the comparative activities of faropenem and other agents against S. pneumoniae according to penicillin susceptibility status. Of the 1,543 isolates of S. pneumoniae, 958 (62.1%) were penicillin susceptible, 338 (21.9%) were penicillin intermediate, and 247 (16%) were penicillin resistant. Against all S. pneumoniae isolates, faropenem and meropenem were the most active β-lactam agents, with an MIC for 90% of the isolates tested (MIC90) of 0.5 μg/ml compared with MIC90s of 2 μg/ml for penicillin and amoxicillin-clavulanate and 8 μg/ml for both cefdinir and cefuroxime. Telithromycin, levofloxacin, and SXT were active against all S. pneumoniae isolates, with MIC90s of 0.5, 1, and 8 μg/ml, respectively, and the least active agent tested was azithromycin, with an MIC90 of >16 μg/ml. Like that of other β-lactams, the activity of faropenem was affected by penicillin susceptibility status, with MIC90s ranging from 0.015 μg/ml for penicillin-susceptible isolates to 2 μg/ml for penicillin-resistant strains. Although the MICs for faropenem were higher against penicillin-resistant strains, it was more active than any of the other oral β-lactams tested, including amoxicillin-clavulanate, cefdinir, and cefuroxime (MIC90s of 8, >8, and 16 μg/ml, respectively). An analysis of antimicrobial susceptibility data using CLSI interpretive criteria shows that penicillin, azithromycin, and SXT were the least effective agents against all S. pneumoniae isolates, with percent susceptible rates of 62.1%, 65.1%, and 69%, respectively. None of the penicillin-resistant S. pneumoniae isolates were considered susceptible to cefdinir and cefuroxime, and only 51.4% were susceptible to amoxicillin-clavulanate. Telithromycin and levofloxacin were active against penicillin-resistant S. pneumoniae, with percent susceptible rates of 97.2% and 98%, respectively. Although the numbers of isolates were small, there was a small but noticeable increase in levofloxacin resistance in S. pneumoniae, ranging from 0.2% among penicillin-susceptible isolates to 1.6% among penicillin-resistant strains.
TABLE 2.
Antimicrobial susceptibilities of S. pneumoniae, H. influenzae, and M. catarrhalis collected during the 2005-2006 respiratory season in the United States
| Organism, antimicrobial, and phenotype | MIC (μg/ml)
|
% of isolates that werea:
|
|||||
|---|---|---|---|---|---|---|---|
| Range | Modef | 50% | 90% | S | I | R | |
| S. pneumoniaeb | |||||||
| Faropenem | |||||||
| All | ≤0.004-2 | 0.008 | 0.015 | 0.5 | |||
| Penicillin susceptible | ≤0.004-0.06 | 0.008 | 0.008 | 0.015 | |||
| Penicillin intermediate | 0.008-0.5 | 0.03 | 0.06 | 0.25 | |||
| Penicillin resistant | 0.12-2 | 1 | 1 | 2 | |||
| Amoxicillin-clavulanate | |||||||
| All | ≤0.12-16 | ≤0.12 | ≤0.12 | 2 | 92.2 | 1.4 | 6.4 |
| Penicillin susceptible | ≤0.12-0.25 | ≤0.12 | ≤0.12 | ≤0.12 | 100 | 0 | 0 |
| Penicillin intermediate | ≤0.12-2 | ≤0.12 | ≤0.12 | 1 | 100 | 0 | 0 |
| Penicillin resistant | 0.5-16 | 8 | 2 | 8 | 51.4 | 8.5 | 40.1 |
| Cefdinir | |||||||
| All | ≤0.06->8 | ≤0.06 | 0.12 | 8 | 76.8 | 4.0 | 19.2 |
| Penicillin susceptible | ≤0.06-1 | ≤0.06 | ≤0.06 | 0.12 | 99.9 | 0.1 | 0 |
| Penicillin intermediate | ≤0.06->8 | 0.12 | 0.25 | 2 | 67.5 | 17.7 | 14.8 |
| Penicillin resistant | 2->8 | 8 | 8 | >8 | 0 | 0 | 100 |
| Cefuroxime | |||||||
| All | ≤0.5-32 | ≤0.5 | ≤0.5 | 8 | 78.2 | 3.1 | 18.7 |
| Penicillin susceptible | ≤0.5-2 | ≤0.5 | ≤0.5 | ≤0.5 | 99.9 | 0.1 | 0 |
| Penicillin intermediate | ≤0.5-16 | ≤0.5 | ≤0.5 | 4 | 74.0 | 13.3 | 12.7 |
| Penicillin resistant | 2-32 | 8 | 8 | 16 | 0 | 0.8 | 99.2 |
| Meropenem | |||||||
| All | ≤0.004-4 | 0.015 | 0.015 | 0.5 | 83.2 | 7.3 | 9.5 |
| Penicillin susceptible | ≤0.004-0.06 | 0.015 | 0.015 | 0.015 | 100 | 0 | 0 |
| Penicillin intermediate | 0.015-0.5 | 0.03 | 0.06 | 0.25 | 93.2 | 6.8 | 0 |
| Penicillin resistant | 0.25-4 | 1 | 1 | 1 | 4.5 | 36.0 | 59.5 |
| Penicillin | |||||||
| All | ≤0.03->4 | ≤0.03 | ≤0.03 | 2 | 62.1 | 21.9 | 16.0 |
| Penicillin susceptible | ≤0.03-0.06 | ≤0.03 | ≤0.03 | ≤0.03 | 100 | 0 | 0 |
| Penicillin intermediate | 0.12-1 | 0.12 | 0.25 | 1 | 0 | 100 | 0 |
| Penicillin resistant | 2->4 | 4 | 4 | 4 | 0 | 0 | 100 |
| Azithromycin | |||||||
| All | ≤0.12->16 | ≤0.12 | ≤0.12 | >16 | 65.5 | 0.5 | 34.0 |
| Penicillin susceptible | ≤0.12->16 | ≤0.12 | ≤0.12 | 4 | 87.9 | 0.8 | 11.3 |
| Penicillin intermediate | ≤0.12->16 | ≤0.12 | 4 | >16 | 35.5 | 0 | 64.5 |
| Penicillin resistant | ≤0.12->16 | 32 | 32 | >16 | 19.4 | 0 | 80.6 |
| Telithromycin | |||||||
| All | ≤0.12->16 | ≤0.12 | ≤0.12 | 0.5 | 99.4 | 0.5 | 0.1 |
| Penicillin susceptible | ≤0.12->16 | ≤0.12 | ≤0.12 | ≤0.12 | 99.8 | 0.1 | 0.1 |
| Penicillin intermediate | ≤0.12-1 | ≤0.12 | ≤0.12 | 0.5 | 100 | 0 | 0 |
| Penicillin resistant | ≤0.12-2 | ≤0.12 | 0.5 | 1 | 97.2 | 2.8 | 0 |
| Levofloxacin | |||||||
| All | 0.25->8 | 1 | 1 | 1 | 99.2 | 0.2 | 0.6 |
| Penicillin susceptible | 0.25->8 | 1 | 1 | 1 | 99.7 | 0.1 | 0.2 |
| Penicillin intermediate | 0.5->8 | 1 | 1 | 1 | 98.8 | 0.3 | 0.9 |
| Penicillin resistant | 0.5->8 | 1 | 1 | 1 | 98.0 | 0.4 | 1.6 |
| SXT | |||||||
| All | ≤0.06->8 | 0.25 | 0.25 | 8 | 69.0 | 7.5 | 23.5 |
| Penicillin susceptible | ≤0.06->8 | 0.25 | 0.25 | 1 | 89.1 | 5.8 | 5.1 |
| Penicillin intermediate | ≤0.06->8 | 0.25 | 1 | 8 | 49.7 | 13.0 | 37.3 |
| Penicillin resistant | 0.25->8 | 8 | 8 | >8 | 17.4 | 6.5 | 76.1 |
| H. influenzaec | |||||||
| Faropenem | |||||||
| All | 0.008-4 | 0.25 | 0.5 | 1 | |||
| β-Lactamase positive | 0.06-4 | 0.25 | 0.25 | 1 | |||
| β-Lactamase negative | 0.008-4 | 0.25 | 0.5 | 1 | |||
| Amoxicillin-clavulanate | |||||||
| All | ≤0.12-8 | 0.5 | 0.5 | 1 | 99.6 | ||
| β-Lactamase positive | 0.25-4 | 0.5 | 0.5 | 2 | 99.4 | ||
| β-Lactamase negative | ≤0.12-8 | 0.5 | 0.5 | 1 | 100 | ||
| Cefdinir | |||||||
| All | ≤0.06-4 | 0.25 | 0.25 | 1 | 94.8 | ||
| β-Lactamase positive | ≤0.06-4 | 0.25 | 0.25 | 1 | 94.4 | ||
| β-Lactamase negative | ≤0.06-4 | 0.25 | 0.25 | 1 | 95.0 | ||
| Cefuroxime | |||||||
| All | ≤0.5-32 | ≤0.5 | 1 | 2 | 97.6 | 2.1 | 0.3 |
| β-Lactamase positive | ≤0.5-16 | ≤0.5 | 1 | 2 | 96.3 | 3.0 | 0.7 |
| β-Lactamase negative | ≤0.5-32 | ≤0.5 | 1 | 2 | 98.0 | 1.8 | 0.2 |
| Meropenem | |||||||
| All | ≤0.004-8 | 0.03 | 0.03 | 0.12 | 99.7 | ||
| β-Lactamase positive | 0.008-0.25 | 0.03 | 0.03 | 0.06 | 100 | ||
| β-Lactamase negative | ≤0.004-8 | 0.03 | 0.06 | 0.12 | 99.6 | ||
| Ampicillin | |||||||
| All | ≤0.12->16 | 0.25 | 0.25 | >16 | 71.9 | 0.5 | 27.6 |
| β-Lactamase positive | 2->16 | >16 | >16 | >16 | 0 | 0.4 | 99.6 |
| β-Lactamase negative | ≤0.12-4 | 0.25 | 0.25 | 0.5 | 99.0 | 0.6 | 0.4 |
| Azithromycin | |||||||
| All | ≤0.12->16 | 1 | 1 | 2 | 98.7 | ||
| β-Lactamase positive | ≤0.12-16 | 1 | 1 | 2 | 98.1 | ||
| β-Lactamase negative | ≤0.12->16 | 1 | 1 | 2 | 98.9 | ||
| Telithromycin | |||||||
| All | ≤0.12->16 | 2 | 2 | 4 | 96.4 | 2.9 | 0.7 |
| β-Lactamase positive | ≤0.12-16 | 2 | 2 | 4 | 97.0 | 2.2 | 0.8 |
| β-Lactamase negative | ≤0.12->16 | 2 | 2 | 4 | 96.1 | 3.2 | 0.7 |
| Levofloxacin | |||||||
| All | ≤0.06->8 | ≤0.06 | ≤0.06 | ≤0.06 | 99.9 | ||
| β-Lactamase positive | ≤0.06-0.12 | ≤0.06 | ≤0.06 | ≤0.06 | 100 | ||
| β-Lactamase negative | ≤0.06->8 | ≤0.06 | ≤0.06 | ≤0.06 | 99.7 | ||
| SXT | |||||||
| All | ≤0.06->8 | 0.25 | 0.5 | 8 | 64.9 | 16.8 | 18.3 |
| β-Lactamase positive | ≤0.06->8 | 0.12 | 0.5 | 8 | 63.7 | 14.1 | 22.2 |
| β-Lactamase negative | ≤0.06->8 | 0.25 | 0.5 | 8 | 65.4 | 17.9 | 16.7 |
| M. catarrhalisd,e | |||||||
| Faropenem | 0.03-1 | 0.5 | 0.25 | 0.5 | |||
| Amoxicillin-clavulanate | ≤0.12-1 | ≤0.12 | ≤0.12 | 0.25 | |||
| Cefdinir | ≤0.06-1 | 0.25 | 0.25 | 0.5 | |||
| Cefuroxime | ≤0.5-8 | 2 | 1 | 2 | |||
| Meropenem | ≤0.004-0.12 | ≤0.004 | ≤0.004 | 0.008 | |||
| Ampicillin | 0.25->16 | 4 | 4 | 16 | |||
| Azithromycin | ≤0.12-4 | ≤0.12 | ≤0.12 | ≤0.12 | |||
| Telithromycin | ≤0.12-4 | ≤0.12 | ≤0.12 | ≤0.12 | |||
| Levofloxacin | ≤0.06-0.25 | ≤0.06 | ≤0.06 | ≤0.06 | |||
| SXT | ≤0.06-4 | 0.25 | 0.25 | 0.5 | |||
Percentages of isolates that were susceptible (S), intermediate (I), and resistant (R) according to CLSI breakpoints. Breakpoints are not available for faropenem.
Of the 1,543 isolates of S. pneumoniae, 958 were penicillin susceptible, 338 were penicillin intermediate, and 247 were penicillin resistant.
Of the 987 isolates of H. influenzae, 270 were β-lactamase positive, and 717 were β-lactamase negative.
CLSI breakpoints are not available for M. catarrhalis.
A total of 489 isolates of M. catarrhalis were tested.
Mode, modal MIC.
The results in Table 3 show the demographic characteristics of subjects from whom S. pneumoniae was isolated during the 2005-2006 respiratory season. An analysis of susceptibility results according to the age of the subject shows that for all β-lactams, the MIC90s were typically twofold higher in isolates from children 5 years old or younger compared with those from subjects who were at least 6 years old. The MIC90 for faropenem against pediatric isolates of S. pneumoniae was 1 μg/ml compared with 8 μg/ml for amoxicillin-clavulanate and >8 μg/ml for cefdinir. An analysis of the interpretive criteria for amoxicillin-clavulanate shows that resistance ranged from 2.9% in isolates from subjects older than 65 years to 17.3% in isolates from children younger than 2 years. Resistance to cefdinir ranged from 15.8% in isolates from subjects older than 65 years to 33.2% in isolates from children younger than 2 years. The least effective agent against pediatric isolates of S. pneumoniae was azithromycin, where 50.5% of isolates from children younger than 2 years were resistant. No levofloxacin-resistant S. pneumoniae isolates were isolated from children 14 years old or younger, and this is likely to be the result of little or no use of fluoroquinolones in this population. There were three levofloxacin-resistant strains (0.4%) isolated from subjects aged 6 to 14 years and six levofloxacin-resistant strains (1.4%) isolated from subjects older than 65 years. An analysis of susceptibility results according to gender or patient location (inpatient versus outpatient) showed no major differences in the susceptibility results of S. pneumoniae to any of the agents tested.
TABLE 3.
Resistance by demographic characteristic for subjects from whom S. pneumoniae was isolated during the 2005-2006 respiratory season
| Patient category | No. of isolates tested | MIC90a (%) of isolates resistant to:
|
||||||
|---|---|---|---|---|---|---|---|---|
| Faropenemb | Cefdinir | Amoxicillin-clavulanate | Penicillin | Azithromycin | SXT | Levofloxacin | ||
| Age, yr | ||||||||
| <2 | 208 | 1 | >8 (33.2) | 8 (17.3) | 4 (30.8) | >16 (50.5) | 8 (42.8) | 1 (0) |
| 3-5 | 99 | 1 | >8 (23.2) | 8 (12.1) | 4 (21.2) | >16 (42.4) | >8 (30.3) | 1 (0) |
| 6-14 | 86 | 0.5 | 8 (19.8) | 2 (7.0) | 2 (15.1) | >16 (36.0) | 8 (19.8) | 1 (0) |
| 15-64 | 700 | 0.5 | 4 (15.4) | 2 (4.3) | 2 (13.0) | >16 (27.6) | 8 (18.3) | 1 (0.4) |
| >65 | 444 | 0.5 | 4 (15.8) | 2 (2.9) | 2 (12.6) | >16 (33.8) | 8 (21.2) | 1 (1.4) |
| Gender | ||||||||
| Male | 888 | 0.5 | 8 (19.8) | 2 (6.0) | 2 (16.0) | >16 (36.0) | 8 (24.8) | 1 (0.8) |
| Female | 651 | 0.5 | 8 (18.3) | 2 (6.9) | 2 (16.0) | >16 (31.2) | 8 (21.4) | 1 (0.3) |
| Location | ||||||||
| Inpatient | 696 | 0.5 | 8 (18.8) | 2 (5.2) | 2 (14.7) | >16 (33.0) | 8 (22.0) | 1 (0.1) |
| Outpatient | 847 | 0.5 | 8 (19.6) | 2 (7.4) | 4 (17.1) | >16 (34.8) | 8 (24.7) | 1 (0.9) |
MIC90s are expressed as micrograms per milliliter.
Breakpoints were not available to assess percent resistance for faropenem.
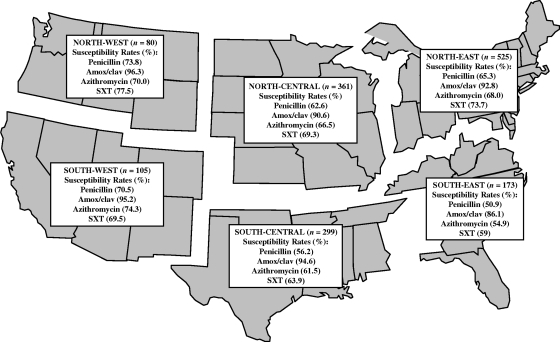
The antimicrobial susceptibility results for S. pneumoniae according to the U.S. geographic region are shown in Fig. 1. The prevalence values of penicillin-susceptible S. pneumoniae ranged from 50.9% in the Southeast to 73.8% in the Northeast. Among the β-lactams, amoxicillin-clavulanate showed the least regional variation, with susceptible rates ranging from 86.1% in the Southeast to 96.3% in the Northwest. The susceptibility rates of S. pneumoniae to azithromycin and SXT also showed significant regional variations that were similar to those observed with penicillin. The incidence of azithromycin-susceptible S. pneumoniae ranged from 54.9% in the Southeast to 74.3% in the Southwest, whereas the incidence of SXT-susceptible S. pneumoniae ranged from 59% in the Southeast to 77.5% on the Northwest.
FIG. 1.
Antimicrobial susceptibility results for S. pneumoniae isolated from subjects in six different U.S. geographic regions during the 2005-2006 respiratory season. Amox/clav, amoxicillin-clavulanate.
The prevalence of SDR and MDR S. pneumoniae was also evaluated among the six U.S. geographic regions (Table 4). There was no major regional variation in SDR, with rates ranging from 15% in the North Central/Northeast to 18.8% in the Northwest. The most prevalent SDR phenotype was resistance to azithromycin, with values of 56.3% for the SDR phenotype in the Southwest and 82.1% for the SDR phenotype in the Southeast. An analysis of the regional prevalence of MDR S. pneumoniae shows rates ranging from 8.8% in the Northwest to 24.9% in the Southeast. The most frequent MDR phenotype encountered in all six regions was resistance to penicillin, azithromycin, and SXT and it accounted for 57.1% of the MDR S. pneumoniae isolates from the Northwest and 81.5% of the MDR strains in the North Central region.
TABLE 4.
Prevalence of SDR and MDR S. pneumoniae in six U.S. geographic regions in 2005-2006
| Region | No. of isolates tested | % of isolates that were SDR | Most prevalent SDR phenotype (% SDR) | % of isolates that were MDR | Most prevalent MDR phenotype (% MDR) |
|---|---|---|---|---|---|
| North Central | 361 | 15 | Azithromycin (79.6) | 15 | Penicillin, azithromycin, SXT (81.5) |
| Northeast | 525 | 15 | Azithromycin (81) | 13 | Penicillin, azithromycin, SXT (70.6) |
| Northwest | 80 | 18.8 | Azithromycin (73.3) | 8.8 | Penicillin, azithromycin, SXT (57.1) |
| South Central | 299 | 16.4 | Azithromycin (71.4) | 17.4 | Penicillin, azithromycin, SXT (67.3) |
| Southeast | 173 | 16.2 | Azithromycin (82.1) | 24.9 | Penicillin, azithromycin, SXT (72.1) |
| Southwest | 105 | 15.2 | Azithromycin (56.3) | 10.5 | Penicillin, azithromycin, SXT (72.7) |
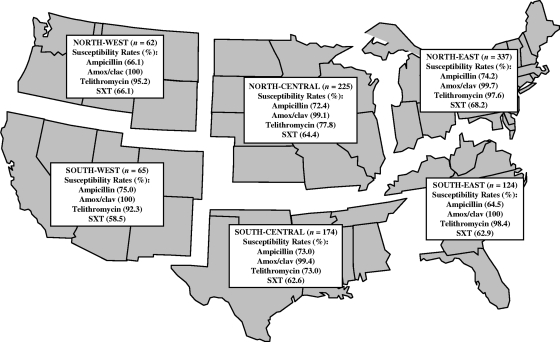
All H. influenzae isolates were tested for their abilities to produce β-lactamase; 270 (27.4%) were β-lactamase positive, and 717 (72.6%) were β-lactamase negative. Faropenem exhibited equivalent activity levels against both β-lactamase-positive and β-lactamase-negative isolates, with an MIC90 of 1 μg/ml against both phenotypes (Table 1). Although amoxicillin-clavulanate was active against 99.6% of H. influenzae isolates, the MIC90s for β-lactamase-positive and β-lactamase-negative isolates were 2 and 1 μg/ml, respectively. The MIC90 for ampicillin was >16 μg/ml, with 27.6% of the isolates being resistant. There were three β-lactamase-negative, ampicillin-resistant isolates of H. influenzae identified in the study, resulting in a prevalence value of 0.4%. Faropenem was active against the β-lactamase-negative ampicillin-resistant strains, with MICs ranging from 2 to 4 μg/ml. Although telithromycin was active against 96.4% of H. influenzae isolates, there were 36 isolates that were nonsusceptible, with MICs that ranged from 8 to 32 μg/ml. Levofloxacin was the most active agent, with 99.9% of the isolates being susceptible, and SXT was the least active agent, with only 64.9% of the isolates being susceptible (Table 1). Regional antimicrobial susceptibility results for H. influenzae are shown in Fig. 2. The prevalence values of ampicillin-susceptible H. influenzae ranged from 64.5% in the Southeast to 75% in the Southwest. There were little or no regional differences in the susceptibility of H. influenzae to amoxicillin-clavulanate, with ≥99.9% of isolates being susceptible in all regions. In contrast, there were regional differences in the susceptibility of H. influenzae to telithromycin, with percent susceptible rates ranging from 73% in the South Central region to 98.4% in the Southeast. The least effective agent was SXT, with percent susceptible rates ranging from 58.5% in the Southwest to 68.2% in the Northeast.
FIG. 2.
Antimicrobial susceptibility results for H. influenzae isolated from subjects in six different U.S. geographic regions during the 2005-2006 respiratory season. Amox/clav, amoxicillin-clavulanate.
Among the 486 M. catarrhalis isolates, 445 (91.6%) were β-lactamase positive and 32 (6.6%) were β-lactamase negative. Meropenem was the most active β-lactam agent against M. catarrhalis, with MICs that ranged from ≤0.004 to 0.12 μg/ml and an MIC90 of 0.008 μg/milliliter. Meropenem was followed by amoxicillin-clavulanate as the next most active agent, with an MIC90 of 0.25 μg/ml. Faropenem was also active against M. catarrhalis, with MICs that ranged from 0.03 to 1 μg/ml and an MIC90 of 0.5 μg/ml. Ampicillin was the least active agent (MIC90, 16 μg/ml), as activity was compromised by the ability of the majority of the isolates to produce β-lactamase.
DISCUSSION
The results of this study provide an update on resistance trends in U.S. respiratory pathogens during the 2005-2006 respiratory season. For example, the continued prevalence of penicillin-nonsusceptible S. pneumoniae was confirmed and found to be comparable with results of other recent surveillance studies (1, 16, 23, 28). In this study, 92.1% of S. pneumoniae isolates remained susceptible to amoxicillin-clavulanate, showing that there has been little change when these results are compared with those of a previous surveillance study conducted in the 1999-2000 respiratory season (4) that reported 95.1% of isolates as susceptible. However, the MIC90 for amoxicillin-clavulanate and S. pneumoniae increased twofold from 1 to 2 μg/ml during the 6 years from the 1999-2000 respiratory season to the 2005-2006 respiratory season. A similar twofold increase in MIC90 was also observed for amoxicillin-clavulanate and penicillin-resistant S. pneumoniae, increasing from 4 μg/ml in 1999 to 8 μg/ml in this study. Faropenem was previously evaluated in a U.S. surveillance study against respiratory pathogens collected in 1999. Since no interpretive criteria are available to monitor changes in the susceptibility of S. pneumoniae to faropenem, it was possible to compare MIC90s to show a twofold increase in MIC90, from 0.25 μg/ml in 1999 to 0.5 μg/ml in the 2005-2006 respiratory season. In both studies, no isolates of S. pneumoniae exhibited faropenem MICs that were >2 μg/ml. The twofold increase in MIC90s for most β-lactams and S. pneumoniae may be due to the increase in the prevalence of penicillin-resistant strains from 10.4% in 1999 to 16% in this study.
Pneumococcal resistance to azithromycin and SXT continues to remain prevalent, with resistance rates of 30.4% and 23.5%, respectively, which were consistent with data from other studies (4, 16). Telithromycin was active against 99.4% of the S. pneumoniae isolates, suggesting little change in susceptibility compared with the results from the PROTEKT US studies conducted between 2000 and 2003 (14). Levofloxacin remained active against 99.2% of S. pneumoniae isolates, and although resistance rates were low, there was a small but noticeable stepwise increase in resistance, ranging from 0.2% among penicillin-susceptible isolates to 1.6% among penicillin-resistant strains, which was similar to data previously reported in a study conducted in the 2000-2001 respiratory season (20). Not surprisingly, S. pneumoniae isolates from children younger than 2 years were more resistant to the oral β-lactams, azithromycin, and SXT than were isolates from subjects older than 65 years, since these agents have a history of widespread use in children to treat respiratory infections such as acute otitis media and sinusitis (6, 21). Comparison of the MIC90s of the oral β-lactams showed that faropenem was the most active agent against isolates from children younger than 2 years, with an MIC90 of 1 μg/ml, compared with amoxicillin-clavulanate and cefdinir, with MIC90s of 8 and >8 μg/ml, respectively. The more potent activity of faropenem compared with those of other β-lactams against pediatric isolates of S. pneumoniae was also confirmed in another study that evaluated middle ear fluid isolates from children with acute otitis media in Costa Rica and Israel, showing that it was typically two- to fourfold more active than amoxicillin-clavulanate (27). In contrast, no levofloxacin-resistant S. pneumoniae was detected in isolates from children 14 years old or younger. Since resistance among the β-lactams and macrolides is driven by extensive pediatric use, the low usage level of fluoroquinolones such as levofloxacin in children may be a factor that is keeping resistance rates relatively low in the adult population (11).
Monitoring the prevalence of MDR phenotypes of S. pneumoniae is important and a growing concern that may ultimately limit therapeutic options in the future (30). In the 1997-1998 respiratory season, 5.9% of S. pneumoniae isolates were MDR, increasing to 11% in the 1998-1999 respiratory season (23). In this study, conducted in the 2005-2006 respiratory season, the MDR S. pneumoniae rates ranged from 8.8% in the Northwest to 24.9% in the Southeast, showing that MDR rates have continued to increase.
Among H. influenzae isolates the incidence of β-lactamase production was 27.4% in the 2005-2006 respiratory season, confirming the recently observed stabilization of prevalence rates in the United States (10, 17). There was a noticeable decrease in the susceptibility of H. influenzae to SXT when the results of this study, where only 64.9% of the isolates were susceptible to SXT, were compared with those from a study conducted in 1999, where 86.5% of isolates were susceptible to SXT (4). Although azithromycin and telithromycin exhibited potent in vitro activity levels against H. influenzae, with susceptibility rates of 98.7% and 96.4%, respectively, there were isolates with MICs that were >16 μg/ml for both agents that are unlikely to be eradicated in the clinical setting. Among the M. catarrhalis isolates collected, 91.6% were β-lactamase positive, suggesting that there has been no change in prevalence in recent years.
The present study has shown that penicillin-resistant and MDR S. pneumoniae as well as β-lactamase-producing H. influenzae and M. catarrhalis remain prevalent in the United States. Faropenem has demonstrated in vivo efficacy against MDR S. pneumoniae, with faropenem MICs as high as 2 μg/ml in a murine neutropenic thigh infection model (3a). These in vivo pharmacodynamic studies also showed that the T>MIC for free faropenem (89.1% protein bound) was minimally affected by resistance and that the goal for effective therapy should be to maintain free serum levels above the MIC for 13.9% of the dosing interval. Faropenem has also been shown to be effective against respiratory infections caused by MDR S. pneumoniae in phase III clinical studies (31), and future studies will continue to monitor efficacy against infections caused by resistant organisms.
These data highlight the need for new oral agents that are active against contemporary resistant phenotypes of respiratory pathogens. Faropenem is an oral penem being developed for the treatment of community-acquired respiratory tract infections. This study shows that faropenem retains activity against recent clinical isolates of respiratory pathogens collected in the United States during the 2005-2006 respiratory season. The results of this study will provide a longitudinal component to future studies in the United States to monitor changes in the susceptibility of respiratory pathogens to faropenem and other comparator agents.
Footnotes
Published ahead of print on 1 October 2007.
REFERENCES
- 1.Brown, S. D., and M. J. Rybak. 2004. Antimicrobial susceptibility of Streptococcus pneumoniae, Streptococcus pyogenes and Haemophilus influenzae collected from patients across the USA, in 2001-2002, as part of the PROTEKT US study. J. Antimicrob. Chemother. 54(Suppl. 1):i7-i15. [DOI] [PubMed] [Google Scholar]
- 2.Clinical and Laboratory Standards Institute. 2006. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 7th ed. Approved standard M7-A7. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3.Clinical Laboratory and Standards Institute/NCCLS. 2006. Performance standards for antimicrobial susceptibility testing; 16th informational supplement. Clinical and Laboratory Standards Institute, Wayne, PA.
- 3a.Craig, W. A., and D. R. Andes. 2001. Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-2094.
- 4.Critchley, I. A., J. A. Karlowsky, D. C. Draghi, M. E. Jones, C. Thornsberry, K. Murfitt, and D. F. Sahm. 2002. Activities of faropenem, an oral beta-lactam, against recent U.S. isolates of Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis. Antimicrob. Agents Chemother. 46:550-555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalhoff, A., N. Janjic, and R. Echols. 2006. Redefining penems. Biochem. Pharmacol. 71:1085-1095. [DOI] [PubMed] [Google Scholar]
- 6.Doern, G. V., K. P. Heilmann, H. K. Huynh, P. R. Rhomberg, S. L. Coffman, and A. B. Brueggemann. 2001. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in the United States during 1999-2000, including a comparison of resistance rates since 1994-1995. Antimicrob. Agents Chemother. 45:1721-1729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Draghi, D. C., M. E. Jones, D. F. Sahm, and G. S. Tillotson. 2006. Geographically-based evaluation of multidrug resistance trends among Streptococcus pneumoniae in the USA: findings of the FAST surveillance initiative (2003-2004). Int. J. Antimicrob. Agents 28:525-531. [DOI] [PubMed] [Google Scholar]
- 8.Felmingham, D., and J. Washington. 1999. Trends in the antimicrobial susceptibility of bacterial respiratory tract pathogens—findings of the Alexander Project 1992-1996. J. Chemother. 11(Suppl. 1):5-21. [DOI] [PubMed] [Google Scholar]
- 9.Hadley, J. A., G. S. Tillotson, R. Tosiello, and R. M. Echols. 2006. Faropenem medoxomil: a treatment option in acute bacterial rhinosinusitis. Expert Rev. Anti-Infect. Ther. 4:923-937. [DOI] [PubMed] [Google Scholar]
- 10.Heilmann, K. P., C. L. Rice, A. L. Miller, N. J. Miller, S. E. Beekmann, M. A. Pfaller, S. S. Richter, and G. V. Doern. 2005. Decreasing prevalence of beta-lactamase production among respiratory tract isolates of Haemophilus influenzae in the United States. Antimicrob. Agents Chemother. 49:2561-2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hooper, D. C. 1998. Expanding uses of fluoroquinolones: opportunities and challenges. Ann. Intern. Med. 129:908-910. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs, M. R. 1997. Respiratory tract infection: epidemiology and surveillance. J. Chemother. 9(Suppl. 3):10-17. [PubMed] [Google Scholar]
- 13.Jacobs, M. R. 1999. Emergence of antibiotic resistance in upper and lower respiratory tract infections. Am. J. Manag. Care 5:S651-S661. [PubMed] [Google Scholar]
- 14.Jenkins, S. G., D. J. Farrell, M. Patel, and B. S. Lavin. 2005. Trends in anti-bacterial resistance among Streptococcus pneumoniae isolated in the USA, 2000-2003: PROTEKT US years 1-3. J. Infect. 51:355-363. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, D. M., M. G. Stilwell, T. R. Fritsche, and R. N. Jones. 2006. Emergence of multidrug-resistant Streptococcus pneumoniae: report from the SENTRY Antimicrobial Surveillance Program (1999-2003). Diagn. Microbiol. Infect. Dis. 56:69-74. [DOI] [PubMed] [Google Scholar]
- 16.Jones, M. E., J. A. Karlowsky, R. Blosser-Middleton, I. A. Critchley, E. Karginova, C. Thornsberry, and D. F. Sahm. 2002. Longitudinal assessment of antipneumococcal susceptibility in the United States. Antimicrob. Agents Chemother. 46:2651-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones, M. E., J. A. Karlowsky, R. Blosser-Middleton, I. A. Critchley, C. Thornsberry, and D. F. Sahm. 2002. Apparent plateau in β-lactamase production among clinical isolates of Haemophilus influenzae and Moraxella catarrhalis in the United States: results from the LIBRA surveillance initiative. Int. J. Antimicrob. Agents 19:119-123. [DOI] [PubMed] [Google Scholar]
- 18.Jones, R. N. 1999. The impact of antimicrobial resistance: changing epidemiology of community-acquired respiratory-tract infections. Am. J. Health Syst. Pharm. 56:S4-S11. [DOI] [PubMed] [Google Scholar]
- 19.Karchmer, A. W. 2004. Increased antibiotic resistance in respiratory tract pathogens: PROTEKT US—an update. Clin. Infect. Dis. 39(Suppl. 3):S142-S150. [DOI] [PubMed] [Google Scholar]
- 20.Karlowsky, J. A., D. C. Draghi, C. Thornsberry, M. E. Jones, I. A. Critchley, and D. F. Sahm. 2002. Antimicrobial susceptibilities of Streptococcus pneumoniae, Haemophilus influenzae and Moraxella catarrhalis isolated in two successive respiratory seasons in the US. Int. J. Antimicrob. Agents 20:76-85. [DOI] [PubMed] [Google Scholar]
- 21.Karlowsky, J. A., C. Thornsberry, I. A. Critchley, M. E. Jones, A. T. Evangelista, G. J. Noel, and D. F. Sahm. 2003. Susceptibilities to levofloxacin in Streptococcus pneumoniae, Haemophilus influenzae, and Moraxella catarrhalis clinical isolates from children: results from 2000-2001 and 2001-2002 TRUST studies in the United States. Antimicrob. Agents Chemother. 47:1790-1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mera, R. M., L. A. Miller, J. J. Daniels, J. G. Weil, and A. R. White. 2005. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States over a 10-year period: Alexander Project. Diagn. Microbiol. Infect. Dis. 51:195-200. [DOI] [PubMed] [Google Scholar]
- 23.Sahm, D. F., J. A. Karlowsky, L. J. Kelly, I. A. Critchley, M. E. Jones, C. Thornsberry, Y. Mauriz, and J. Kahn. 2001. Need for annual surveillance of antimicrobial resistance in Streptococcus pneumoniae in the United States: 2-year longitudinal analysis. Antimicrob. Agents Chemother. 45:1037-1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schrag, S. J., L. McGee, C. G. Whitney, B. Beall, A. S. Craig, M. E. Choate, J. H. Jorgensen, R. R. Facklam, and K. P. Klugman. 2004. Emergence of Streptococcus pneumoniae with very-high-level resistance to penicillin. Antimicrob. Agents Chemother. 48:3016-3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schurek, K. N., R. Wiebe, J. A. Karlowsky, E. Rubinstein, D. J. Hoban, and G. G. Zhanel. 2007. Faropenem: review of a new oral penem. Expert Rev. Anti-Infect. Ther. 5:185-198. [DOI] [PubMed] [Google Scholar]
- 26.Siegert, R., O. Berg, P. Gehanno, A. Leiberman, J. L. Martinkenas, P. Nikolaidis, P. Arvis, M. Alefelder, and P. Reimnitz. 2003. Comparison of the efficacy and safety of faropenem daloxate and cefuroxime axetil for the treatment of acute bacterial maxillary sinusitis in adults. Eur. Arch. Otorhinolaryngol. 260:186-194. [DOI] [PubMed] [Google Scholar]
- 27.Stone, K. C., R. Dagan, A. Arguedas, E. Leibovitz, E. Wang, R. M. Echols, N. Janjic, and I. A. Critchley. 2007. Activity of faropenem against middle ear fluid pathogens from children with acute otitis media in Costa Rica and Israel. Antimicrob. Agents Chemother. 51:2230-2235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stratton, C. W., and S. D. Brown. 2004. Comparative in vitro activity of telithromycin and beta-lactam antimicrobials against community-acquired bacterial respiratory tract pathogens in the United States: findings from the PROTEKT US study, 2000-2001. Clin. Ther. 26:522-530. [DOI] [PubMed] [Google Scholar]
- 29.Upchurch, J., M. Rosemore, R. Tosiello, S. Kowalsky, and R. Echols. 2006. Randomized double-blind study comparing 7- and 10-day regimens of faropenem medoxomil with a 10-day cefuroxime axetil regimen for treatment of acute bacterial sinusitis. Otolaryngol. Head Neck Surg. 135:511-517. [DOI] [PubMed] [Google Scholar]
- 30.Whitney, C. G., M. M. Farley, J. Hadler, L. H. Harrison, C. Lexau, A. Reingold, L. Lefkowitz, P. R. Cieslak, M. Cetron, E. R. Zell, J. H. Jorgensen, and A. Schuchat. 2000. Increasing prevalence of multidrug-resistant Streptococcus pneumoniae in the United States. N. Engl. J. Med. 343:1917-1924. [DOI] [PubMed] [Google Scholar]
- 31.Young, C. L., I. A. Critchley, and N. Janjic. 2005. Abstr. 45th Intersci. Conf. Antimicrob. Agents Chemother., abstr. E-0224.