Abstract
Vibrio parahaemolyticus is indigenous to coastal environments and a frequent cause of seafood-borne gastroenteritis in the United States, primarily due to raw-oyster consumption. Previous seasonal-cycle studies of V. parahaemolyticus have identified water temperature as the strongest environmental predictor. Salinity has also been identified, although it is evident that its effect on annual variation is not as pronounced. The effects of other environmental factors, both with respect to the seasonal cycle and intraseasonal variation, are uncertain. This study investigated intraseasonal variations of densities of total and pathogenic V. parahaemolyticus organisms in oysters and overlying waters during the summer of 2004 at two sites in the northern Gulf of Mexico. Regression analyses indicated significant associations (P < 0.001) between total V. parahaemolyticus densities and salinity, as well as turbidity in water and in oysters at the Mississippi site but not at the Alabama site. Pathogenic V. parahaemolyticus organisms in Mississippi oyster and water samples were detected in 56% (9 out of 16) and 78% (43 out of 55) of samples, respectively. In contrast, 44% (7 out of 16) of oyster samples and 30% (14 out of 47) of water samples from Alabama were positive. At both sites, there was greater sample-to-sample variability in pathogenic V. parahaemolyticus densities than in total V. parahaemolyticus densities. These data suggest that, although total V. parahaemolyticus densities may be very informative, there is greater uncertainty when total V. parahaemolyticus densities are used to predict the risk of infection by pathogenic V. parahaemolyticus than previously recognized.
Vibrio parahaemolyticus is the leading cause of Vibrio-associated gastroenteritis in the United States (15, 19, 20, 32) and has been isolated from oysters, blue crabs, finfish, and planktonic copepods (6, 7, 18, 22). Vibrio infections are most common in individuals living in states bordering the Gulf of Mexico (23) and are usually associated with the consumption of raw shellfish, primarily oysters (9, 20). Recent outbreaks (4, 5, 10, 14) raised the awareness of public health officials concerning shellfish throughout coastal states and prompted the Interstate Shellfish Sanitation Conference to develop an Interim Control Plan for regulating shellfish harvest areas based on V. parahaemolyticus densities in shellfish (31). The Interim Control Plan employs a colony lift technique using DNA probes that target the species-specific thermolabile hemolysin (tlh) gene and the thermostable direct hemolysin (tdh) gene associated with pathogenic V. parahaemolyticus strains (32). A similar DNA probe colony hybridization method has been developed to target the tdh-related hemolysin gene (trh), which is also associated with pathogenic strains of V. parahaemolyticus (27). While these colony lift techniques make it possible to investigate the distribution of pathogenic V. parahaemolyticus strains directly, they have a relatively high limit of detection (LOD). Thus, the colony lift method is typically sensitive enough to quantify the more abundant tlh-positive V. parahaemolyticus strains from water and oysters but is less effective for the rarer tdh- or trh-positive strains.
V. parahaemolyticus density in oysters has been shown to be positively correlated with water temperature, and higher densities of V. parahaemolyticus are normally detected during warmer months (8, 15). However, V. parahaemolyticus densities vary considerably even at optimal temperatures, and possible links between this variability and other environmental factors remain unclear (29, 35). The U.S. Food and Drug Administration (FDA) released a V. parahaemolyticus risk assessment in which densities of total V. parahaemolyticus organisms in oysters at harvest were predicted based on water temperature measurements obtained from National Oceanographic and Atmospheric Administration (NOAA) buoys (2). Water temperature was estimated to be associated with approximately 50% of the annual variation in total V. parahaemolyticus densities in oysters. In the risk assessment, the effects of additional environmental factors impacting V. parahaemolyticus densities were not incorporated due to insufficient data. While predicted V. parahaemolyticus densities based on water temperature generally agree with average densities determined by microbiological examination and provide an appropriate basis for aggregate seasonal and regional predictions, there remains considerable unexplained intraseasonal and intraregional variability (2).
To address these data gaps, we investigated potential intraseasonal associations between selected non-temperature-based environmental parameters (levels of salinity, chlorophyll, and turbidity) and the densities of total and pathogenic V. parahaemolyticus organisms in oysters and in overlying waters. Turbidity and chlorophyll levels have been previously hypothesized to correlate with V. parahaemolyticus densities (21, 22, 25, 35). Temperature effects were minimized by conducting the study during those months that provide near-optimal temperatures for V. parahaemolyticus growth (May through August). If strong and predictive environmental signatures can be identified based on water quality parameters, then remote sensing of these parameters may prove useful as a means of effectively monitoring estuarine environments and limiting human exposure to dangerous V. parahaemolyticus densities.
Since the densities of pathogenic (tdh- or trh-positive) V. parahaemolyticus organisms in the environment are usually below the LOD (10 CFU g−1) for the DNA probe colony hybridization method, previous studies have not adequately characterized the distribution of pathogenic V. parahaemolyticus densities (15); it appears that a high number of samples contain nondetectable V. parahaemolyticus. To address this inaccuracy, a real-time PCR assay was applied for the simultaneous detection of tlh, tdh, and trh genes in overnight enrichments of oysters in alkaline peptone water (APW) (28).
MATERIALS AND METHODS
Sample collection and preparation.
Surface water samples (n = 102) and oyster samples (n = 32) were collected at two sites from May through August 2004. One site was a pier at the FDA Gulf Coast Seafood Laboratory (GCSL) in Dauphin Island, AL (30°15′52″N, 88°06′47″W), and the other was a pier at the University of Southern Mississippi Gulf Coast Research Laboratory (GCRL) in Ocean Springs, MS (30°15′53″N, 89°06′48″W). Water samples (four 1-liter samples from the Alabama site and one 1-liter sample from the Mississippi site) were collected on average three times per week in sterile 1-liter wide-mouth bottles (Nalgene, Rochester, NY) as recommended previously (1). Oyster samples (∼1-m depth) were collected once per week using tongs at the Alabama site; at the Mississippi site, oysters were collected by hand from tethered holding baskets resting on the bottom. Six (Mississippi) and 12 (Alabama) oysters were analyzed within 1 hour of collection. Surface and bottom water temperature and salinity were measured using a YSI model 85 salinometer (Yellow Springs, OH) or by using a calibrated thermometer and handheld refractometer. A water sample was also taken for chlorophyll and turbidity analyses using a benchtop fluorometer (model TD-700; Turner) and turbidimeter (model 2020; LaMotte).
Oysters were cleaned and shucked as recommended previously (1). Oysters (200 to 250 g) were blended with an equal weight of phosphate-buffered saline (PBS; 7.65 g NaCl, 0.724 g Na2HPO4 [anhydrous], 0.21 g KH2PO4 per liter of distilled water, pH 7.4). A 1:10 dilution was made in PBS by adding 20 g of the 1:1 homogenate to 80 ml of PBS.
Direct plating/colony hybridization.
Analysis of the water and oyster samples for V. parahaemolyticus adhered to the FDA protocol (32). Specifically, 1 ml of water was spread plated on triplicate T1N3 agar plates (1% tryptone, 3% NaCl, 2% agar, pH 7.2). Aliquots of oyster homogenate equal to 0.1 g (0.2 g of the 1:1 homogenate) and 0.01 g (100 μl of the 1:10 dilution) were spread plated on triplicate T1N3 agar plates.
After overnight incubation at 35°C, bacterial colonies were lifted from the T1N3 agar plates using Whatman no. 541 filters (8.5-cm diameter; Whatman Inc., Florham Park, NJ), and filters were analyzed by DNA hybridization using alkaline phosphatase-labeled gene probes (DNA Technology, Aarhus, Denmark) for the detection of the tlh, tdh, and trh genes, as previously described (12, 27, 32).
Enrichment and real-time PCR-most probable number (MPN) analysis.
Volumes of 1 liter, 100 ml, and 10 ml of Alabama waters were enriched with 110 ml, 11 ml, and 1.1 ml, respectively, of 10× APW (10% peptone, pH 8.5 ± 0.2). These large sample sizes were used because enrichment followed by real-time PCR detection improved sensitivity, especially for pathogenic V. parahaemolyticus, which is usually below the LOD for colony hybridization, in which only 1 ml is examined. Many of the Mississippi water samples had a salinity of <10 ppt, and 10× APW supplemented with 10% NaCl was used in these samples for optimal growth for V. parahaemolyticus. All water sample enrichments were performed in triplicate.
Four sterile 100-ml bottles containing 80 ml of APW were inoculated with 20 g of the 1:1 homogenate described above. Three were used as 10-g oyster enrichment samples, and the fourth bottle was used to inoculate 10 ml of homogenate into triplicate tubes for the 1-g samples.
After incubation, 100 μl from each enrichment tube or bottle were placed into 0.5-ml Microfuge tubes, which were heated to 100°C for 10 min in a model 200 Peltier thermal cycler (MJ Research, Inc., Watertown, MA) and immediately frozen at −20°C until analysis. Enrichments were analyzed by real-time PCR-MPN, as described by Nordstrom et al. (28), using a multiplex assay for the detection of the tlh, tdh, and trh genes of V. parahaemolyticus. This assay includes an internal amplification control (IAC) for the detection of possible matrix inhibition. A positive control (tlh+ tdh+ trh+ V. parahaemolyticus strain) and a negative control (distilled water) were included for each PCR master mix.
Statistical analyses.
Generalized linear mixed-model (GLMM) regressions were used to estimate the distributions and correlations between total (tlh) and pathogenic (tdh and trh) V. parahaemolyticus densities in oyster and water samples, as well as their relationship to environmental parameters. The significance of contemporaneous associations with environmental factors as predictors of V. parahaemolyticus densities was evaluated using a stepwise backward selection procedure. In univariate and multivariate analyses, the distribution of V. parahaemolyticus densities was assumed to be lognormal, with mean log10 densities being either constant or linearly related to environmental parameters. GLMM regression parameters were estimated by considering the results of colony hybridization or real-time PCR-MPN assays from multiple dilutions obtained from the same sample as repeated measures, marginally distributed as either Poisson or binomial outcomes, respectively, based on the volume of sample examined. The approach of using the GLMM (11, 33) with discrete mixed-type response variables and latent (underlying) lognormal distribution was considered an appropriate method for combining the plate count and real-time PCR-MPN data in a manner that appropriately weighted the outcomes of the different methods in an inverse proportion to their inherent measurement errors. The resulting estimates of the variation in log10 V. parahaemolyticus densities were thereby corrected for the most prominent factor determining measurement error (i.e., assay or dilution volume). For univariate GLMM analyses, a log transformation of the scale parameter was used to improve numerical stability and asymptotic properties of statistical estimates and derived confidence intervals. Similarly, for multivariate GLMM analyses, a spherical parameterization of the variance-covariance matrix was used (30). Because measurements of pathogenic V. parahaemolyticus (tdh and trh) densities were frequently below the LOD, the proportion of the variation explained by environmental parameters was evaluated using Nagelkerke's pseudo-R2 statistic (26) to estimate partial coefficients of determination, as described by Lipsitz et al. (24). The fit of all GLMMs to the data were evaluated using the deviance statistic as a goodness-of-fit measure, and all fits were found to be adequate.
For graphical presentation of the data, a generalized linear model approach was used to obtain maximum likelihood estimates of the densities of tlh, tdh, and trh organisms corresponding to each sample based on the combined outcomes of both enumeration methods. For samples enumerated by real-time PCR alone, this corresponds to the usual estimates for three-tube MPN format data. For samples enumerated by colony hybridization and real-time PCR, the resulting estimate is a weighted combination of the estimates that would be obtained by considering the outcome of each method alone. This weighting is based on the maximum likelihood principle. When densities were below the LOD by both methods, half the LOD of the most sensitive method (generally real-time PCR) was used for graphing purposes. Improbable MPNs in the real-time PCR data were corrected prior to the GLMM analysis by excluding the questionable outcome (i.e., based on the inhibition of an internal amplification control) in lower dilutions. The significance of site differences in the distribution of salinity, turbidity, and chlorophyll was evaluated by the Mann-Whitney-Wilcoxon test. All statistical analyses were conducted using the SAS statistical software (SAS Institute, Cary, NC); significance was defined as a P of <0.05, and relations with P values in the range of 0.05 to 0.10 were considered marginally significant.
RESULTS
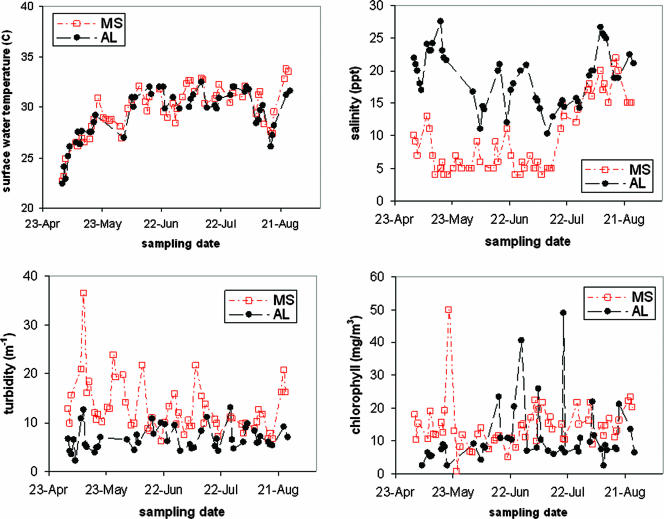
Water temperatures were similar at the Mississippi and Alabama sites (ranging from 22.4 to 33.8°C), but there were statistically significant (P < 0.001) site differences in the distributions of salinity, turbidity, and chlorophyll (Fig. 1). The salinity range was higher at the Alabama site (10 to 28 ppt) than at the Mississippi site (4 to 13 ppt) from May through June, but the ranges were similar during July and August. Turbidity and chlorophyll exhibited day-to-day variability at both sites, with higher levels being observed at the Mississippi site; median turbidity and chlorophyll levels were 10.9 m−1 and 13.5 mg/m3 at the Mississippi site, compared to 6.1 m−1 and 7.9 mg/m3 at the Alabama site. At both sampling sites, V. parahaemolyticus densities were generally higher (∼2 logs) and more consistent in oysters than in water (Fig. 2).
FIG. 1.
Environmental parameters (temperature, salinity, turbidity, and chlorophyll) measured at the Alabama and Mississippi sampling sites.
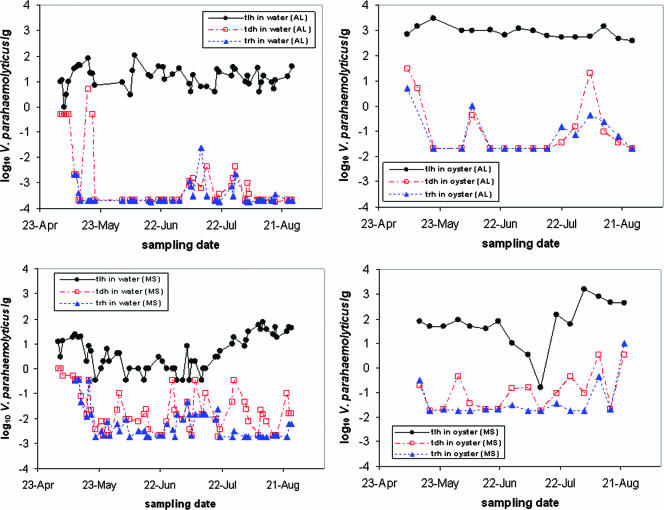
FIG. 2.
Vibrio parahaemolyticus (tlh, tdh, and trh) levels in water and oysters at the Alabama and Mississippi sampling sites.
A summary and inferential statistics of V. parahaemolyticus measurements in water and oysters are shown in Table 1. With the exception of one water sample collected at the Alabama site and examined only by DNA probe colony hybridization (LOD = 1 CFU ml−1), all samples contained detectable levels of total V. parahaemolyticus (tlh) organisms. Because of the infrequency of tdh+ and trh+ V. parahaemolyticus strains in environmental samples, most of the information on total V. parahaemolyticus (tlh) densities was obtained using colony hybridization, and most of the information on pathogenic V. parahaemolyticus (tdh and trh) densities was obtained from MPN-PCR analyses.
TABLE 1.
Observed and estimated distributions of total (tlh) and pathogenic (tdh and trh) V. parahaemolyticus organisms in oysters and water collected from Mississippi and Alabama sites
| Site and sample type | Gene detected/unit of measure | No. of samples | No. of positive samples | % of samples that were positive | Rangea | Meanb | SDb |
|---|---|---|---|---|---|---|---|
| MS water | tlh/ml | 55 | 55 | 100 | 0.11-76 ml | 0.65 (0.45, 0.86) | 0.68 (0.53, 1.15) |
| tdh/ml | 55 | 43 | 78 | ND-1 ml | −1.90 (−2.12, −1.69) | 0.71 (0.52, 0.98) | |
| trh/ml | 51 | 28 | 55 | ND-1 ml | −2.57 (−2.87, −2.26) | 0.79 (0.54, 1.16) | |
| MS oyster | tlh/g | 16 | 16 | 100 | 0.16-1,600 g | 1.90 (1.50, 2.29) | 0.70 (0.43, 1.10) |
| tdh/g | 16 | 9 | 56 | ND-0.46 g | −1.34 (−2.03, −0.65) | 1.03 (0.55, 1.91) | |
| trh/g | 16 | 5 | 31 | ND-10 g | −2.87 (−4.76, −0.97) | 2.00 (0.68, 5.86) | |
| AL water | tlh/ml | 47 | 46 | 98 | ND-104 ml | 1.18 (1.06, 1.29) | 0.37 (0.29, 0.47) |
| tdh/ml | 47 | 12 | 26 | ND-5 ml | −4.13 (−4.68, −3.59) | 0.88 (0.54, 1.44) | |
| trh/ml | 41 | 10 | 24 | ND-3 ml | −4.52 (−5.26, −3.79) | 1.04 (0.62, 1.76) | |
| AL oyster | tlh/g | 16 | 16 | 100 | 380-3,000 g | 2.91 (2.79, 3.03) | 0.21 (0.14, 0.32) |
| tdh/g | 16 | 7 | 44 | ND-30 g | −2.12 (−3.66, −0.58) | 1.90 (0.91, 3.98) | |
| trh/g | 15 | 6 | 40 | ND-1.1 g | −1.74 (−2.57, −0.92) | 0.97 (0.45, 2.11) |
ND, not detected.
Estimated values are followed by parenthetical 95% confidence intervals for log10 V. parahaemolyticus (tlh, tdh, trh) densities based on GLMM regression analyses.
The previously described IAC was used to differentiate PCR inhibition from negative signals (28). Complete inhibition was observed only with a single MPN tube inoculated with a 10-g portion of oyster homogenate from Mississippi, and partial inhibition (threshold cycle, 21 to 30) was generally limited to oysters collected from Mississippi. No adjustments were made to data from MPN tubes in which the IAC was partially inhibited because often one or more of the V. parahaemolyticus targets (10/15 for tlh, 3/15 for tdh, and 1/15 trh) were detected in these samples.
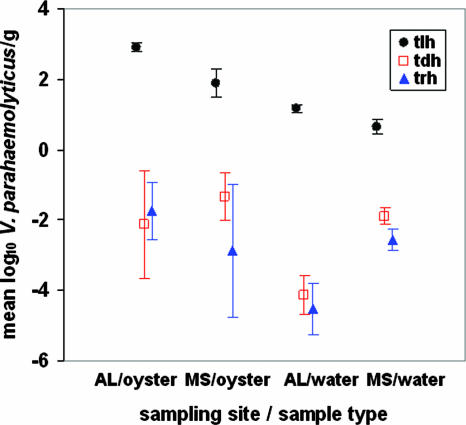
Pathogenic V. parahaemolyticus (tdh+ and/or trh+) organisms were detected in 56% and 78% of Mississippi oyster and water samples and in 44% and 30% of Alabama oyster and water samples, respectively. Mean densities of total V. parahaemolyticus (tlh) were higher in oysters than in water at both sampling sites (Fig. 3). Mean densities of pathogenic V. parahaemolyticus (tdh+ and trh+) organisms were similar in oyster samples from Mississippi and Alabama and lower in water samples from Alabama. Estimated standard deviations indicate greater variability among tdh+ and trh+ V. parahaemolyticus densities than among tlh+ V. parahaemolyticus densities in both water and oyster samples at both sites (Table 1).
FIG. 3.
Maximum likelihood estimates and 95% confidence intervals for mean log10 Vibrio parahaemolyticus (tlh, tdh, trh) densities in Alabama versus in Mississippi oysters and water.
Based on multivariate GLMM analysis, total V. parahaemolyticus (tlh) densities in oysters and water were significantly correlated at the Mississippi site (r = 0.89; P < 0.001) but not at the Alabama site. Similar analyses comparing tdh and trh organism densities indicated positive correlations for each sample type and site. The estimated correlations were statistically significant (P < 0.001) when tdh and trh organism densities in water were compared but only marginally significant (P < 0.10) when tdh and trh organism densities in oysters were compared. These positive associations between tdh and trh organism densities in water and oysters are clearly evident in Fig. 2.
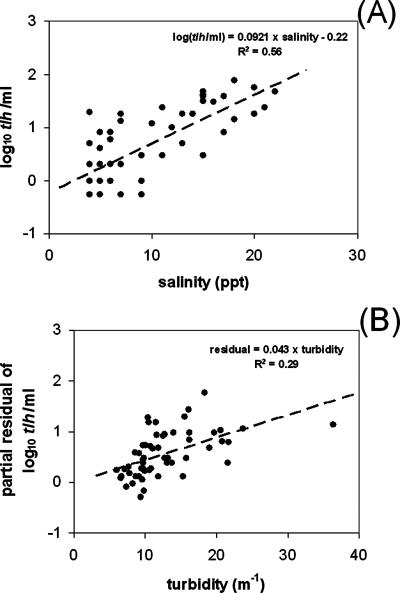
The association of water temperature, salinity, turbidity, and chlorophyll with V. parahaemolyticus densities differed at the two sampling sites. Water temperature varied from 22.4 to 33.8°C and was not significantly correlated with either total or pathogenic V. parahaemolyticus densities at either site. No significant associations between V. parahaemolyticus and these environmental parameters were evident at the Alabama site. In contrast, at the Mississippi site, salinity and turbidity were positively associated with V. parahaemolyticus densities in water and oysters. The results of the GLMM regression analyses for the Mississippi data are shown in Table 2. As indicated by estimated regression coefficients, the associations between total V. parahaemolyticus (tlh) densities and these two parameters were consistent across the sample types (water versus oyster). The associations were highly significant (P < 0.001), except the association between turbidity and V. parahaemolyticus (tlh) densities in oysters, which was only marginally significant (P = 0.081). Based on the regression coefficients, point estimates of the effects that salinity (in parts per thousand) had on total numbers of V. parahaemolyticus (tlh) organisms were 0.10 log10 ml−1 and 0.12 log10 g−1 for water and oysters, respectively. That is, a 10-ppt change in salinity was estimated to correspond to a 1.0- and a 1.2-log10 change in total numbers of V. parahaemolyticus (tlh) organisms in water and oysters, respectively. For turbidity, the regression coefficient estimates differed by smaller amounts: 0.043 log10 ml−1 and log10 g−1 m−1 for water and oysters, respectively. Consistent with the differences in effect sizes, salinity corresponded to more of the variation in V. parahaemolyticus (tlh) densities than did turbidity. Partial coefficients of determination (i.e., pseudo-R2's) summarizing the amount of variation explained by each factor are shown in Table 2. Based on these statistics, salinity was estimated to be associated with ca. 64% and 76% of the variation of V. parahaemolyticus (tlh) densities in water and oysters, respectively. Similarly, turbidity was estimated to be associated with ca. 26% and 18% of the variation. A graphical summary of the correlations of turbidity with V. parahaemolyticus (tlh) densities in water is shown in Fig. 4. Although similar patterns of statistical significance and magnitude of effect sizes were evident for salinity and turbidity versus pathogenic V. parahaemolyticus (tdh and trh) densities at the Mississippi site, the results were inconsistent between sample types. The proportion of variation in pathogenic V. parahaemolyticus densities associated with salinity and turbidity was generally much smaller than that estimated for total V. parahaemolyticus (tlh) densities (Table 2).
TABLE 2.
Measures of association between numbers of total (tlh) and pathogenic (tdh and trh) V. parahaemolyticus organisms and environmental parameters
| Site and sample type | Gene detected/unit of measure | Environmental parameter | Regression coefficient (effect size)a | P value | Partial coefficient of determination (%)b |
|---|---|---|---|---|---|
| MS water | tlh/ml | Salinity | 0.10 (0.079, 0.12) | <0.001 | 64 |
| tlh/ml | Turbidity | 0.0433 (0.023, 0.064) | <0.001 | 26 | |
| MS oyster | tlh/g | Salinity | 0.12 (0.082, 0.15) | <0.001 | 76 |
| tlh/g | Turbidity | 0.0431 (−0.006, 0.092) | 0.081 | 18 | |
| MS water | tdh/ml | Salinity | |||
| tdh/ml | Turbidity | 0.063 (0.023, 0.10) | 0.003 | 4 | |
| MS oyster | tdh/g | Salinity | 0.11 (0.003, 0.22) | 0.044 | 8 |
| tdh/g | Turbidity | 0.18 (0.03, 0.33) | 0.023 | 10 | |
| MS water | trh/ml | Salinity | −0.076 (−0.13, −0.02) | 0.009 | 4 |
| trh/ml | Turbidity | 0.060 (0.017, 0.10) | 0.008 | 4 | |
| MS oyster | trh/g | Salinity | 0.26 (0.07, 0.45) | 0.01 | 21 |
| trh/g | Turbidity | 0.38 (0.10, 0.66) | 0.01 | 23 |
Maximum likelihood estimates and parenthetical 95% confidence intervals of regression coefficients corresponding to environmental parameters identified to be significant (P < 0.05) or marginally significant (P < 0.10) by backward stepwise identification in GLMM analyses.
Based on Nagelkerke's pseudo-R2 statistic.
FIG. 4.
Relationship of total numbers of V. parahaemolyticus (tlh) organisms in water to salinity and turbidity at the Mississippi site. (A) Correlation plot of log10 numbers of tlh organisms/ml versus salinity; (B) partial residual plot of tlh numbers/ml, adjusted for salinity, versus turbidity.
DISCUSSION
The present study examined intraseasonal relationships between selected environmental parameters (chlorophyll, turbidity, and salinity) and the densities of total and pathogenic V. parahaemolyticus organisms in the northern Gulf of Mexico over a sampling period during which the effects of water temperature were minimal. The determination of predictive intraseasonal associations may help identify the environmental conditions under which V. parahaemolyticus is most likely to persist at high densities. If strong environmental signatures exist and can be identified to be predictive of high and persistent V. parahaemolyticus abundance, then monitoring of water quality via remote sensing may prove to be an effective tool for controlling or mitigating human exposure.
The present study identified marked and persistent differences between geographic sites and between ratios of total organisms to pathogenic V. parahaemolyticus organisms. Pathogenic V. parahaemolyticus densities in both Alabama and Mississippi oysters and in overlying waters fluctuated more than did total V. parahaemolyticus densities. Additionally, higher total V. parahaemolyticus densities were observed in Alabama samples, but higher pathogenic V. parahaemolyticus densities were observed in Mississippi samples. Quantitative data on pathogenic V. parahaemolyticus densities have been limited in previous studies and lacked the precision needed to address the issue of spatial variation (e.g., site-to-site differences). Furthermore, pathogenic V. parahaemolyticus estimates have been based on the ratios of pathogenic to total V. parahaemolyticus isolates grown on thiosulfate citrate bile sucrose agar (TCBS) after overnight APW enrichment (2). These ratios could have been affected by differing growth rates in APW or differing plating efficiencies on TCBS between pathogenic and nonpathogenic V. parahaemolyticus strains. The use of the real-time PCR-MPN format in the present study enhances sensitivity by allowing for the inoculation of large sample portions (>3 liters of water and >30 g of oysters) and the examination of many V. parahaemolyticus cells (log10 4) from the APW enrichment by real-time PCR without the tedious and resource-intensive approach of colony isolation, identification, and characterization on TCBS or other growth media (2).
Differences in salinity, turbidity, and chlorophyll levels between the two sampling sites during the study period may account for some of the discrepancies in V. parahaemolyticus densities. A significant correlation between salinity and total (tlh) V. parahaemolyticus densities in water and oysters was identified at the Mississippi site. The lack of significance for salinity at the Alabama site is likely a consequence of a higher and narrower range of salinity levels at that site than at the Mississippi site. Salinities at the Mississippi site were generally <10 ppt during the first half of the study, which is well below the reported optimum salinity of 23 ppt for V. parahaemolyticus growth (2). When salinity increased at the Mississippi site in mid-July, densities of total V. parahaemolyticus organisms increased ca. 10-fold to levels typical of the Alabama site. Previous studies indicated that V. parahaemolyticus densities decrease as salinity increases (2, 14, 15). In the present study, the densities of pathogenic V. parahaemolyticus organisms were generally positively associated with increasing salinity. The GLMM regression analyses indicated no significant association between V. parahaemolyticus densities and water temperature at either site. This finding is consistent with the relatively narrow range of temperature variations during the study time frame.
Turbidity was generally higher in Mississippi than in Alabama, and regression analysis indicated a positive association between turbidity and V. parahaemolyticus densities at the Mississippi site. Lower levels and less variability in turbidity may have obscured the effects of turbidity at the Alabama site. The association between turbidity and V. parahaemolyticus densities at the Mississippi site was generally consistent and statistically significant (P < 0.05) except with total V. parahaemolyticus in oysters, which association was only marginally significant (P = 0.081). However, the effect sizes for turbidity on total V. parahaemolyticus organisms were nearly identical for water and oysters, and the lack of statistical significance per se is likely a consequence of the smaller number of oyster samples analyzed. Due to the discrete nature of the data for pathogenic V. parahaemolyticus, a pseudo-R2 measure of association was used to evaluate the proportion of variation explained. Based on this statistic, turbidity was associated with up to 26% of the variation in total V. parahaemolyticus densities and up to 23% of the variation in pathogenic V. parahaemolyticus densities. Although statistically significant associations between turbidity and pathogenic V. parahaemolyticus densities were identified, the estimates of effect sizes were not as robust as with total V. parahaemolyticus densities. The adequacy of the fitted regression model was found to be more problematic for pathogenic than for total V. parahaemolyticus strains. Increased turbidity could potentially affect V. parahaemolyticus densities in water and oysters in various ways. The most obvious is by resuspension of sediments, which have higher V. parahaemolyticus densities than water, and subsequent uptake by oyster filtration. Nutrients that were previously sediment bound may also become more available in the water column, resulting in more V. parahaemolyticus growth. On the other hand, high turbidity resulting from the suspension of inorganic particles or other particles that are not digestible by oysters may cause them to shut down filter feeding and allow V. parahaemolyticus to accumulate in their tissues (2).
No significant associations with chlorophyll were observed at either site. This is contradictory to our previous study (29), which indicated a significant association (P < 0.05) between remotely sensed chlorophyll and total V. parahaemolyticus organisms, after correction for the effects of temperature and salinity. However, the previous data were collected year round, and the current data were collected only during warm weather, when chlorophyll levels are typically higher. It is possible that a relationship is evident in a comparison of the effects of seasonal differences in chlorophyll levels that are not similarly apparent in the effects of day-to-day variations within a single season. It is also possible that the effects of day-to-day variations have a stronger temporal (i.e., lagging) relationship than those of other environmental parameters and cannot be appropriately identified based on contemporaneous statistical associations alone. However, the examination of potential temporal associations based on the current data was found to be problematic due to intermittent sampling dates over the course of the study.
Other variables (chemical, biological, geological, and hydrological, etc.) may be responsible for differences in V. parahaemolyticus densities at the two study sites. The Mississippi site is within a highly eutrophic estuary known as the Mississippi Sound. While this site is protected seaward from the Gulf of Mexico by barrier islands, the ca. 1-km fetch between the site and the innermost island allows some wave energy to impact the site, especially during southerly wind flows, which prevailed during the study period. A marine aquaculture facility housed at the GCRL adjacent to the sampling site was considered a potential source of pathogenic V. parahaemolyticus, but tests did not detect V. parahaemolyticus in culture raceways or in the effluent. The Alabama site is located along a bulkhead shoreline in a small (2- to 3-km) bay on the north side of Dauphin Island near the mouth of Mobile Bay. The low-wave energy, especially during the study period, when prevailing winds were from southerly directions, and relative isolation from major freshwater sources (the Mobile River system is approximately 50 km to the north and had relatively low flow during the study period) contributed to the lower turbidity and higher salinity at this site.
Total V. parahaemolyticus densities in oysters paralleled those in the overlying waters at both sites, as previously reported (13). Regression analyses indicated a significant correlation between total V. parahaemolyticus densities in oysters and water collected on the same day at the Mississippi site but not at the Alabama site. The correlation at the Mississippi site was likely induced by the effects of salinity and turbidity, and the lack of correlation at the Alabama site is consistent with the absence of identifiable environmental effects at that site and relatively constant V. parahaemolyticus densities. However, differences in mean densities of pathogenic V. parahaemolyticus in oysters and their overlying waters were much greater in Alabama than in Mississippi. It is possible that Alabama oysters retain pathogenic V. parahaemolyticus, perhaps by colonization, even when their densities in the overlying water are very low. These data suggest that V. parahaemolyticus populations in oysters and their overlying waters are controlled quantitatively and qualitatively by different factors. It is unlikely that selective filtration of pathogenic V. parahaemolyticus alone can account for the magnitude of different concentrations in oysters at the two sites. Previous studies demonstrated the potential for V. parahaemolyticus growth (2), phagocytosis (16), and phage lysis (3) in oysters, and these processes could affect ratios of pathogenic to nonpathogenic V. parahaemolyticus organisms in oyster tissues.
The real-time PCR assay has two advantages over other assays (28, 34): (i) the IAC is included to eliminate reporting of false-negative results caused by sample matrix inhibition and (ii) concentrations of tlh primers are limited, which improves the detection of pathogenic targets (tdh and trh) in the presence of high densities of total V. parahaemolyticus (tlh) organisms. By applying this assay to 10-fold serial dilutions of oysters in APW in an MPN-PCR format, the LOD is lowered ∼300-fold (i.e., a three-tube MPN starting with a sample of 10 g of homogenate has a theoretical LOD of 0.03 MPN g−1) over the 10-CFU g−1 LOD for the colony hybridization method.
The IAC data indicated that matrix inhibition of the PCR in the current study was primarily limited to oysters collected from Mississippi. Partial matrix inhibition would likely affect the detection of tdh and trh more than tlh, since they are usually present at much lower copy numbers. The impact of matrix inhibition on estimates of pathogenic V. parahaemolyticus densities was reduced by the adjustments made with improbable MPN data (excluding data at lower dilutions [10-g portions] when more tubes at higher dilutions [1-g portions] were positive). In most cases, the failure to detect V. parahaemolyticus targets at the lower dilutions when higher dilutions were positive appeared to be unrelated to matrix inhibition of the PCR because the IAC was not inhibited. Failure to detect the V. parahaemolyticus targets was likely due to their copy number in APW enrichments being below the assay LOD of 500 ml−1. The low frequency of partial IAC inhibition in Alabama oysters (ca. 1%) and all water samples (<1%) is similar to that reported for this V. parahaemolyticus assay in a study of Alaskan oysters (28). While the IAC assists with the identification of the few samples with matrix inhibition and prevents reporting of false-negative results, efficient methods to eliminate these inhibitors need to be developed.
In conclusion, these results demonstrate greater temporal and spatial variations in the densities of pathogenic V. parahaemolyticus organisms in Gulf oysters during warm weather than those observed for total-V. parahaemolyticus densities; hence, there may be more uncertainty in the use of densities of total V parahaemolyticus organisms as a surrogate for risk predictions than was previously recognized. While these results underscore a continued poor understanding of the ecology and distribution of pathogenic bacteria, including V. parahaemolyticus in coastal environments (17), other important aspects of V. parahaemolyticus risk assessment, such as seasonality and postharvest growth, are firmly established for risk predictions. The site-to-site variability demonstrated in this study emphasizes the need to use remote-sensing algorithms that are site specific and to take niche-based signatures into account.
Acknowledgments
This study was funded by an Oceans and Human Health Initiative grant (NA-04-OAR4600214) from the NOAA.
We are grateful to the following individuals and agencies for providing laboratory equipment, space, and aid during sample collection/analyses: John Tennyson and Angela Ruple, NOAA, National Marine Fisheries Service, National Seafood Inspection Laboratory; Scott Gordon, Mississippi Department of Marine Resources; Dawn Rebarchik, GCRL; and George Blackstone, GCSL.
Footnotes
Published ahead of print on 5 October 2007.
REFERENCES
- 1.American Public Health Association. 1970. Recommended procedures for the examination of seawater and shellfish, 4th ed. American Public Health Association, Washington, DC.
- 2.Anonymous. 2005. Quantitative risk assessment on the public health impact of pathogenic Vibrio parahaemolyticus in raw oysters. U.S. Food and Drug Administration, Washington, DC.
- 3.Baross, J. A., P. A. Tester, and R. Y. Morita. 1984. Incidence, microscopy and etiology of exoskeleton lesions in the tanner crab Chionoecetes tanneri. J. Fish. Res. Board Can. 35:1141-1149. [Google Scholar]
- 4.Centers for Disease Control and Prevention. 1999. Outbreak of Vibrio parahaemolyticus infection associated with eating raw oysters and clams harvested from Long Island Sound—Connecticut, New Jersey, and New York. Morb. Mortal. Wkly. Rep. 48:48-51. [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention. 1998. Outbreak of Vibrio parahaemolyticus infections associated with eating raw oysters—Pacific Northwest, 1997. Morb. Mortal. Wkly. Rep. 47:457-462. [PubMed] [Google Scholar]
- 6.Colwell, R. R., and D. J. Grimes. 1984. Vibrio diseases of marine fish populations. Helgol. Meeresunters 37:265-287. [Google Scholar]
- 7.Colwell, R. R., and J. Kaper. 1977. Vibrio cholerae, Vibrio parahaemolyticus, and other vibrios: occurrence and distribution in Chesapeake Bay. Science 198:394-396. [PubMed] [Google Scholar]
- 8.Cook, D. W., J. D. Bowers, and A. DePaola. 2002. Density of total and pathogenic (tdh+) Vibrio parahaemolyticus in Atlantic and Gulf Coast molluscan shellfish at harvest. J. Food Prot. 65:1873-1880. [DOI] [PubMed] [Google Scholar]
- 9.Daniels, N. A., L. MacKinnon, R. Bishop, S. Altekruse, B. Ray, R. M. Hammond, S. Thompson, S. Wilson, N. H. Bean, P. M. Griffin, and L. Slutsker. 2000. Vibrio parahaemolyticus infections in the United States, 1973-1998. J. Infect. Dis. 181:1661-1666. [DOI] [PubMed] [Google Scholar]
- 10.Daniels, N. A., B. Ray, A. Easton, N. Marana, E. Kahn, A. L. McShan, L. Del Rosario, T. Baldwin, M. A. Kingsley, N. D. Puhr, J. G. Wells, and F. J. Angulo. 2000. Emergence of a new Vibrio parahaemolyticus serotype in raw oysters. JAMA 284:1541-1545. [DOI] [PubMed] [Google Scholar]
- 11.Davidian, M., and D. M. Giltinan. 1995. Nonlinear models for repeated measurement data. Chapman & Hall, New York, NY.
- 12.Deepanjali, A., H. S. Kumar, I. Karunasagar, and I. Karunasagar. 2005. Seasonal variation in abundance of total and pathogenic Vibrio parahaemolyticus bacteria in oysters along the southwest coast of India. Appl. Environ. Microbiol. 71:3575-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DePaola, A., L. H. Hopkins, J. T. Peeler, B. Wentz, and R. M. McPhearson. 1990. Incidence of Vibrio parahaemolyticus in U.S. coastal waters and oysters. Appl. Environ. Microbiol. 56:2299-2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.DePaola, A., C. A. Kaysner, J. Bowers, and D. W. Cook. 2000. Environmental investigations of Vibrio parahaemolyticus in oysters after outbreaks in Washington, Texas, and New York (1997 and 1998). Appl. Environ. Microbiol. 66:4649-4654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DePaola, A., J. L. Nordstrom, J. C. Bowers, J. G. Wells, and D. W. Cook. 2003. Seasonal abundance of total and pathogenic Vibrio parahaemolyticus in Alabama oysters. Appl. Environ. Microbiol. 69:1521-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Genthner, F. J., A. K. Volety, L. M. Oliver, and W. S. Fisher. 1999. Factors influencing in vitro killing of bacteria by hemocytes of the eastern oyster (Crassostrea virginica). Appl. Environ. Microbiol. 65:3015-3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grimes, D. J. 1991. Ecology of estuarine bacteria capable of causing human disease: a review. Estuaries 14:345-360. [Google Scholar]
- 18.Grimes, D. J., P. Brayton, R. R. Colwell, and S. H. Gruber. 1985. Vibrios as autochthonous flora of neritic sharks. Syst. Appl. Microbiol. 6:221-226. [Google Scholar]
- 19.Hervio-Heath, D., R. R. Colwell, A. Derrien, A. Robert-Pillot, J. M. Fournier, and M. Pommepuy. 2002. Occurrence of pathogenic vibrios in the coastal areas of France. J. Appl. Microbiol. 92:1123-1135. [DOI] [PubMed] [Google Scholar]
- 20.Hlady, G. A., and K. C. Klontz. 1996. The epidemiology of vibrio infections in Florida, 1981-1993. J. Infect. Dis. 173:1176-1183. [DOI] [PubMed] [Google Scholar]
- 21.Huq, A., and R. R. Colwell. 1996. Vibrios in the marine and estuarine environment: tracking Vibrio cholerae. Ecosyst. Health 2:198-214. [Google Scholar]
- 22.Huq, A., and R. R. Colwell. 1995. Vibrios in the marine and estuarine environments. J. Mar. Biotechnol. 3:60-63. [Google Scholar]
- 23.Levine, W. C., and P. M. Griffin. 1993. Vibrio infections on the Gulf Coast: results of first year of regional surveillance. J. Infect. Dis. 167:479-483. [DOI] [PubMed] [Google Scholar]
- 24.Lipsitz, S. R., T. Leong, J. Ibrahim, and S. Lipschultz. 2001. A partial correlation coefficient and coefficient of determination for multivariate normal repeated measures data. Statistician 50:87-95. [Google Scholar]
- 25.Lobitz, B., L. Beck, A. Huq, B. Wood, G. Fuchs, A. S. G. Faruque, and R. R. Colwell. 2000. Climate and infectious disease: use of remote sensing for detection of Vibrio cholerae by indirect measurement. Proc. Natl. Acad. Sci. USA 97:1438-1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nagelkerke, N. J. D. 1991. A note on a general definition of the coefficient of determination. Biometrika 78:691-692. [Google Scholar]
- 27.Nordstrom, J. L., R. Rangdale, M. C. L. Vickery, A. M. B. Phillips, S. L. Murray, and A. DePaola. 2006. Evaluation of an alkaline phosphatase-labeled oligonucleotide probe for the detection and enumeration of the thermostable-related hemolysin (trh) gene of Vibrio parahaemolyticus. J. Food Prot. 69:2770-2772. [DOI] [PubMed] [Google Scholar]
- 28.Nordstrom, J. L., M. C. L. Vickery, G. M. Blackstone, S. L. Murray, and A. DePaola. 2007. Development of a multiplex real-time PCR assay with an internal amplification control for the detection of total and pathogenic Vibrio parahaemolyticus bacteria in oysters. Appl. Environ. Microbiol. 73:5840-5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phillips, A. M. B., A. DePaola, J. Bowers, S. Ladner, and D. J. Grimes. 2007. An evaluation of the use of remotely sensed parameters for prediction of incidence and risk associated with Vibrio parahaemolyticus in Gulf Coast oysters (Crassostrea virginica). J. Food Prot. 70:879-884. [DOI] [PubMed] [Google Scholar]
- 30.Pinheiro, J. C., and D. M. Bates. 1996. Unconstrained parametrizations for variance-covariance matrices. Stat. Comput. 6:289-296. [Google Scholar]
- 31.U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition. 1986. Sanitation of the harvesting and processing of shellfish. National Shellfish Sanitation Program manual of operations, part II. U.S. Food and Drug Administration, Washington, DC.
- 32.U.S. Food and Drug Administration Center for Food Safety and Applied Nutrition. 2004. Vibrio cholerae, V. parahaemolyticus, V. vulnificus, and other Vibrio spp. Bacteriological analytical manual online, chapter 9. http://www.cfsan.fda.gov/∼ebam/bam-9.html.
- 33.Vonesh, E. F., and V. M. Chinchilli. 1997. Linear and nonlinear models for the analysis of repeated measurements. Marcel Dekker, Inc., New York, NY.
- 34.Ward, L. N., and A. K. Bej. 2006. Detection of Vibrio parahaemolyticus in shellfish by use of multiplexed real-time PCR with TaqMan fluorescent probes. Appl. Environ. Microbiol. 72:2031-2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Watkins, W. D., and V. J. Cabelli. 1985. Effect of fecal pollution on Vibrio parahaemolyticus densities in an estuarine environment. Appl. Environ. Microbiol. 49:1307-1313. [DOI] [PMC free article] [PubMed] [Google Scholar]