Abstract
Secretion of the cytokine interleukin-2 (IL-2) was investigated in Lactococcus lactis using the secretory machinery of the bacteriocin lactococcin A. Surprisingly, the lcnCD transport genes were not essential for mouse IL-2 secretion. Furthermore, expression of a mature mouse IL-2 gene resulted in interleukin secretion without the requirement for a leader sequence.
In most cases secretion of heterologous proteins and peptides in Lactococcus lactis has involved fusion to a signal peptide recognized by the general secretory (Sec) pathway (6, 8, 21). Also, the dedicated secretion systems (10) associated with bacteriocins produced by lactic acid bacteria have been exploited (19). In this case leader peptides serve as recognition signals for the corresponding ABC transporters and are cleaved during export at a specific processing site adjacent to two conserved glycine residues located at positions −1 and −2 (11). The flexibility of ABC transporters for secretion and processing of class II bacteriocins has been reported previously (1, 14, 15, 16, 22). In previous studies we exploited the lactococcin A secretion pathway for lactococcal production of pediocin PA-1 (14, 15) and the Escherichia coli bacteriocin colicin V (16). Here we describe lactococcal expression and secretion of the eukaryotic cytokine interleukin-2 (IL-2) using the lactococcin A secretion system under control of the nisin promoter PnisA (17) and the unexpected observation that mouse IL-2 (mIL-2) can be secreted without the requirement for a signal peptide.
Construction of pFI2398, a chimeric gene (L-mIL-2) consisting of an in-frame fusion of sequences encoding the lactococin A leader and mature mIL-2.
Primers pAD1 (5′-CCTGAATAATATAGAGATAGGTT-3′) and pIL2a (5′-CTTGAAGTGGGTGCTCCTCCGTTAGCTTC-3′) were used to amplify a fragment containing PnisA and the part of lcnA gene encoding the lactococcin A leader. Plasmid pFI2396 (7) was used as the template. Primers pIL2c (5′-GAAGCTAACGGAGGAGCACCCACTTCAAG-3′) and pIL2f (5′-GGATCCTTATTGAGGGCTTGTTGAG-3′) were used to amplify a fragment comprising part of the mIL-2 structural gene. Plasmid pT1-IL2 (20) was used as the template.
Plasmids pFI2398 and pFI2148 were introduced into L. lactis strains FI5876 (4, 13), FI7847 (5), and UKLc10 (23) (Table 1). The growth conditions were the growth conditions used previously (4, 5, 13, 23). Plasmid pFI2148 contains the lactococcin A-dedicated translocatory function genes (lcnCD). Strains FI7847 and UKLc10 were induced with nisin (at the optimal concentration, 50 ng ml−1; added at an optical density at 600 nm of 0.5), whereas FI5876 was autoinduced due to the presence of a complete nisin biosynthesis gene cluster.
TABLE 1.
Lactococcal strains used in this study
| Strain | Host | Plasmid | Presence of gene(s)a
|
mIL-2 concn (pg/ml)b | Reference or source | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| L-mIL-2 | mIL-2 | usp-mIL-2 | lcnCD | nis | Δnis | nisRK | |||||
| FI5876 | + | 0 | 6 | ||||||||
| FI7847 | + | 0 | 8 | ||||||||
| UKLc10 | + | 0 | 29 | ||||||||
| FI9955 | FI5876 | + | |||||||||
| pFI2398 | + | 160 | This study | ||||||||
| FI9957 | FI5876 | + | |||||||||
| pFI2398 | + | ||||||||||
| pFI2148 | + | 215 | This study | ||||||||
| FI9947 | FI7847 | + | |||||||||
| pFI2398 | + | 150 | This study | ||||||||
| FI9949 | FI7847 | + | |||||||||
| pFI2398 | + | ||||||||||
| pFI2148 | + | 180 | This study | ||||||||
| FI10537 | UKLc10 | + | |||||||||
| pFI2398 | + | 195 | This study | ||||||||
| FI10538 | UKLc10 | + | |||||||||
| pFI2398 | + | ||||||||||
| pFI2148 | + | 355 | This study | ||||||||
| FI10585 | FI5876 | + | |||||||||
| pFI2584 | + | 175 | This study | ||||||||
| FI10584 | FI7847 | + | |||||||||
| pFI2584 | + | 140 | This study | ||||||||
| FI10586 | UKLc10 | + | |||||||||
| pFI2584 | + | 285 | This study | ||||||||
| FI10608 | FI5876 | + | |||||||||
| pFI2593 | + | 490 | This study | ||||||||
L-mIL-2 contains the lcnA leader and mIL-2 genes preceded by the nisin promoter; mIL-2 contains the mIL-2 gene preceded by the nisin promoter; usp-mIL-2 contains the usp45 leader and mIL-2 genes preceded by the nisin promoter; nis is the nisin gene cluster; Δnis is the nisin gene cluster with a frameshift mutation in codon 16 of the nisin structural gene impeding nisin biosynthesis (the sequences of the downstream nisin cluster genes are not affected); nisRK is the nisRK genes integrated into the fivefold peptidase-deficient mutant IM16, a derivative of L. lactis MG1363.
The concentration of mIL-2 (pg ml−1) in culture supernatants of lactococcal strains was determined by a sandwich ELISA. The values are the means from quadruplicate samples (standard deviation, ≤8%).
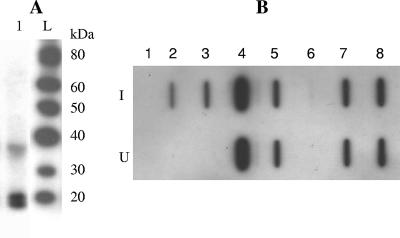
Western blotting of culture supernatants from all three constructs revealed two intense bands consistent with unprocessed L-mIL-2 (19.6 kDa) and processed mIL-2 (17.2 kDa) (Fig. 1A) (L-mIL-2 is a chimeric protein containing the lactococcin A leader and mIL-2). Culture supernatants were assayed for the presence of mIL-2 by slot blot analysis (Fig. 1B), and the concentration of the cytokine was measured by a sandwich enzyme-linked immunosorbent assay (ELISA) (Table 1). For each strain, the intensities of the slot blot bands correlated well with the values obtained by the sandwich ELISA. Interestingly, extracellular production of mIL-2 was also detected in the supernatants from the transformants carrying only pFI2398 (L-mIL-2), suggesting that extracellular production was independent of the lactococcin A lcnCD genes. However, in this case only unprocessed L-mIL-2 was observed, and the presence of pFI2148 was associated with a higher concentration of mIL-2 in the culture supernatants than in the equivalent strains lacking this plasmid (Table 1 and Fig. 1B).
FIG. 1.
(A) Detection of mIL-2 in culture supernatant of L. lactis FI9957 by Western blot analysis. Lane 1, FI9957; lane L, MagicMark Western protein standard. (B) Slot blot detection of mIL-2 in the culture supernatants of L. lactis strains. Row I, nisin induced (50 ng ml−1); row U, not induced with exogenous nisin. Lane 1, FI7847; lane 2, FI9947; lane 3, FI9949; lane 4, murine IL-2 (2 ng ml−1); lane 5, murine IL-2 (0.2 ng ml−1); lane 6, FI5876; lane 7, FI9955; lane 8, FI9957.
Construction of pFI2584, a plasmid containing the mature mIL-2 sequence under control of PnisA.
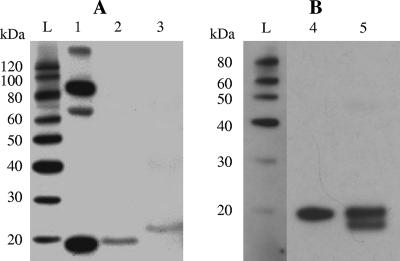
Cytokine secretion was evaluated in the absence of a leader peptide. Primers pAD1 and pIL2nl3′ (5′-AGCTTGAAGTGGGTGCCATTTTGAGTGCCTCC-3′) were used to amplify a fragment encoding PnisA and a part of mature mIL-2. Plasmid pFI2398 was used as the template. Primers pIL2nl5′ (5′-GGAGGCACTCAAAATGGCACCCACTTCAAGC-3′) and pIL2f were used to amplify a fragment comprising a part of the sequence encoding mIL-2. Western blot analysis of culture supernatants and intracellular content samples from FI5876, FI7847, and UKLc10 derivatives transformed with pFI2584 revealed that the anti-mIL-2 polyclonal antibodies specifically recognized a 17.2-kDa band, which is the expected size for mIL-2 (Fig. 2A). The ELISA confirmed that there was extracellular production of mIL-2 (Table 1).
FIG. 2.
Western blot detection of mIL-2 in culture supernatants. Lane 1, mIL-2; lane 2, L. lactis FI10586; lane 3, L. lactis FI10537; lane 4, intracellular contents of L. lactis FI10608; lane 5, culture supernatant of L. lactis FI10608; lane L, MagicMark Western protein standard.
Previously, Steidler et al. (20) reported mIL-2 secretion using the usp45 signal peptide, but they observed the presence of a single 20.1-kDa band. A pTG262 derivative plasmid (pFI2593) carrying the lactococcal usp45 signal peptide fused with the sequence encoding mature mIL-2 under the control of the nisin promoter was engineered and introduced into L. lactis FI5876 (strain FI10608). Western blot analysis of concentrated culture supernatants of L. lactis FI10608 revealed two distinct bands corresponding to processed and unprocessed mIL-2 (Fig. 2B), as observed when the lcnA leader was used (Fig. 1A).
The CTLL-2 assay described by Gillis et al. (9) and modified by Hopkins and Failla (12) was used to evaluate mIL-2 activity. The values obtained after addition of supernatants from L. lactis derivative strains to the CTLL-2 cells agreed with the results obtained with the sandwich ELISA. In addition, we demonstrated that L. lactis strains with complete nisin biosynthesis gene clusters (FI9955, FI9957, and FI10585) coproduced nisin A (6.5 to 7.9 μg ml−1) and mIL-2.
To our knowledge, this is the first report of secretion of a eukaryotic peptide in L. lactis assisted by the secretory machinery of a bacteriocin. The supernatants of the transformed strains contained a mixture of both processed and unprocessed mIL-2, which has not occurred previously using this system (7, 16). The lactococcin secretory machinery encoded by the lcnCD genes was not necessary for secretion of unprocessed mIL-2 when this peptide was fused to the lactococcin A leader. As a result, transformation of the three lactococcal hosts with a plasmid (pFI2584) containing only the sequence of mature mIL-2 without any signal peptide under the control of PnisA led to some extracellular production of mIL-2. A number of proteins secreted by bacteria without leader sequences have been reported previously (2), but to date such secretion has involved only noneukaryotic proteins. Proteins using this nonclassical secretion pathway can be predicted using methods such as the method described at the SecretomeP 2.0 website (http://www.cbs.dtu.dk/services/SecretomeP). This method gives FASTA protein sequences scores between 0 and 1, and a score greater than 0.5 is considered indicative of nonclassical secretion. Using this system to test the mIL-2 protein sequence resulted in a score of 0.875 when it was tested in the mammalian category, which suggests that mIL-2 might be secreted in eukaryotic cells without the requirement for a signal peptide.
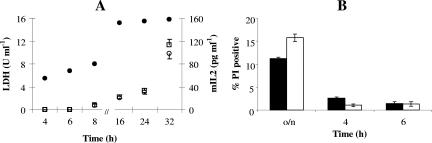
There is evidence that several proteins secreted by nonclassical systems are also present at high intracellular concentrations (3). This observation suggests that when some proteins are abundant in the cytoplasm, they may actually leak out of the cell. In this study, significant mIL-2 levels were detected intracellularly as well as extracellularly. To evaluate cell leakage, the release of cytoplasmic lactate dehydrogenase (LDH) into culture supernatant was investigated using a modified version of the method of Wittenberger and Angelo (24). The results for LDH activity measured in culture supernatants from L. lactis FI10585 did not differ from the results for the parental strain carrying the empty vector (Fig. 3A). The cytokine mIL-2 is smaller than LDH, and the possibility that mIL-2 is released from the cell in a passive way cannot be dismissed.
FIG. 3.
(A) LDH activity in culture supernatants of L. lactis FI10585 (○) and L. lactis FI5876(pTG262) (□) and mIL-2 concentrations in the supernatant of L. lactis FI10585 (•). (B) Flow cytometric analysis of PI-stained bacteria during different phases of growth. PI permeability is expressed as the percentage of PI-positive events compared to the total number. The error bars indicate standard deviations of three biological replicates. Filled bars, L. lactis FI10585; open bars, L. lactis FI5876(pTG262).
In order to answer this question, flow cytometry was used to evaluate intact, permeabilized, and lysed (dead) cells in bacterial cultures of FI10585 and FI5876(pTG262). Intact, nisin-induced, and cetyltrimethylammonium bromide-treated bacteria were labeled with thiazole orange and/or propidium iodide (PI) and analyzed by flow cytometry to measure the scatter and fluorescence intensity of individual cells. Thiazole orange labeled all cells, facilitating discrimination of bacteria from the background noise, while cells with permeable membranes were easily distinguished by PI labeling. Samples were taken during different growth phases, and the results showed that in overnight cultures the PI-positive cells accounted for 10 to 15% of the cells of both of the strains (Fig. 3B). After inoculation, the percentage of PI-permeable cells dropped gradually in the exponential growth phase to 1 to 3%, which was also observed at 8 h (data not shown).
Therefore, FI10585 and FI5876(pTG262) showed no significant differences in membrane PI permeability, suggesting the possibility that mIL-2 is secreted without the requirement for a signal peptide by an alternative ABC transporter, as has been reported previously for other secreted leaderless bacterial peptides (18).
Acknowledgments
This work was partially supported by grant EX2005-0015 from the Ministerio de Educacion y Ciencia, Madrid, Spain. A.F. holds a fellowship from the Universidad Complutense de Madrid, Spain.
We are grateful to Claire Shearman and especially to Udo Wegmann (IFR, Norwich, United Kingdom) for his interesting and stimulating comments on the work and for providing L. lactis UKLc10.
Footnotes
Published ahead of print on 28 September 2007.
REFERENCES
- 1.Allison, G. E., R. W. Worobo, M. E. Stiles, and T. R. Klaenhammer. 1995. Heterologous expression of the lactacin F peptides by Carnobacterium piscicola LV17. Appl. Environ. Microbiol. 61:1371-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bendtsen, J. D., L. Kiemer, A. Fausboll, and S. Brunak. 2005. Non-classical protein secretion in bacteria. BMC Microbiol. 5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Buttner, K., J. Bernhardt, C. Scharf, R. Schmid, U. Mader, C. Eymann, H. Antelmann, A. Volker, U. Volker, and M. Hecker. 2001. A comprehensive two-dimensional map of cytosolic proteins of Bacillus subtilis. Electrophoresis 22:2908-2935. [DOI] [PubMed] [Google Scholar]
- 4.Dodd, H. M., N. Horn, and M. J. Gasson. 1990. Analysis of the genetic determinant for production of the peptide antibiotic nisin. J. Gen. Microbiol. 136:555-566. [DOI] [PubMed] [Google Scholar]
- 5.Dodd, H. M., N. Horn, W. C. Chan, C. J. Giffard, B. W. Bycroft, G. C. K. Roberts, and M. J. Gasson. 1996. Molecular analysis of the regulation of nisin immunity. Microbiology 142:2385-2392. [DOI] [PubMed] [Google Scholar]
- 6.Enouf, V., P. Langella, J. Commissaire, J. Cohen, and G. Corthier. 2001. Bovine rotavirus non-structural protein 4 produced by Lactococcus lactis is antigenic and immunogenic. Appl. Environ. Microbiol. 67:1423-1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fernández, A., N. Horn, M. J. Gasson, H. M. Dodd, and J. M. Rodríguez. 2004. High-level coproduction of the bacteriocins nisin A and lactococcin A by Lactococcus lactis. J. Dairy Res. 71:216-221. [DOI] [PubMed] [Google Scholar]
- 8.Gaeng, S., S. Scherer, H. Neve, and M. J. Loessner. 2000. Gene cloning and expression and secretion of Listeria monocytogenes bacteriophage-lytic enzymes in Lactococcus lactis. Appl. Environ. Microbiol. 66:2951-2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gillis, S., M. M. Fern, W. Ou, and K. A. Smith. 1978. T cell growth factor: parameters of production and quantitative microassay for activity. J. Immunol. 120:2027-2032. [PubMed] [Google Scholar]
- 10.Havarstain, L. S., H. Holo, and I. F. Nes. 1994. The leader peptide of colicin V shares consensus sequences with leader peptide that are common among peptide bacteriocins produced by Gram-positive bacteria. Microbiology 140:2383-2389. [DOI] [PubMed] [Google Scholar]
- 11.Havarstain, L. S., D. B. Diep, and I. F. Nes. 1995. A family of bacteriocin ABC transporters carry out proteolytic processing of their substrates concomitant with export. Mol. Microbiol. 16:229-240. [DOI] [PubMed] [Google Scholar]
- 12.Hopkins, R. G., and M. L. Failla. 1995. Chronic intake of a marginally low copper diet impairs in vitro activities of lymphocytes and neutrophils from male rats despite minimal impact on conventional indicators of copper status. J. Nutr. 125:2658-2668. [DOI] [PubMed] [Google Scholar]
- 13.Horn, N., S. Swindell, H. M. Dodd, and M. J. Gasson. 1991. Nisin biosynthesis genes are encoded by a novel conjugative transposon. Mol. Gen. Genet. 228:129-135. [DOI] [PubMed] [Google Scholar]
- 14.Horn, N., M. I. Martínez, J. M. Martínez, P. E. Hernández, M. J. Gasson, J. M. Rodríguez, and H. M. Dodd. 1998. Production of pediocin PA-1 by Lactococcus lactis using the lactococcin A secretory apparatus. Appl. Environ. Microbiol. 64:818-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horn, N., M. I. Martínez, J. M. Martínez, P. E. Hernández, M. J. Gasson, J. M. Rodríguez, and H. M. Dodd. 1999. Enhanced production of pediocin PA-1 and coproduction of nisin and pediocin PA-1 by Lactococcus lactis. Appl. Environ. Microbiol. 65:4443-4450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horn, N., A. Fernández, H. M. Dodd, M. J. Gasson, and J. M. Rodríguez. 2004. Nisin-controlled production of pediocin PA-1 and colicin V in nisin- and non-nisin-producing Lactococcus lactis strains. Appl. Environ. Microbiol. 70:5030-5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuipers, O. P., P. G. G. A. de Ruyter, M. Kleerebezem, and W. M. de Vos. 1998. Quorum sensing-controlled gene expression in lactic acid bacteria. J. Biotechnol. 64:15-21. [Google Scholar]
- 18.Recchi, C., J. Rauzier, B. Gicquel, and J. M. Reyrat. 2002. Signal-sequence-independent secretion of the staphylococcal nuclease in Mycobacterium smegmatis. Microbiology 148:529-536. [DOI] [PubMed] [Google Scholar]
- 19.Rodríguez, J. M., M. I. Martínez, and J. Kok. 2002. Pediocin PA-1, a wide-spectrum bacteriocin from lactic acid bacteria. Crit. Rev. Food Sci. Nutr. 42:91-121. [DOI] [PubMed] [Google Scholar]
- 20.Steidler, L., J. M. Wells, A. Raeymaekers, J. Vandekerckhove, W. Fiers, and E. Remaut. 1995. Secretion of biologically active murine interleukin-2 by Lactococcus lactis subsp. lactis. Appl. Environ. Microbiol. 61:1627-1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Steidler, L., K. Robinson, L. Chamberlain, K. M. Schofield, E. Remaut, R. W. F. Le Page, and J. M. Wells. 1998. Mucosal delivery of murine interleukin-2 (IL-2) and IL-6 by recombinant strains of Lactococcus lactis coexpressing antigen and cytokine. Infect. Immun. 66:3183-3189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Belkum, M. J., R. W. Worobo, and M. E. Stiles. 1997. Double-glycine-type leader peptides direct secretion of bacteriocins by ABC transporters: colicin V secretion in Lactococcus lactis. Mol. Microbiol. 23:1293-1301. [DOI] [PubMed] [Google Scholar]
- 23.Wegmann, U., J. R. Klein, Drumm, O. P. Kuipers, and B. Henrich. 1999. Introduction of peptidase genes from Lactobacillus delbrueckii subsp. lactis into Lactococcus lactis and controlled expression. Appl. Environ. Microbiol. 65:4729-4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wittenberger, C. L., and N. Angelo. 1970. Purification and properties of a fructose-1,6-diphosphate-activated lactate dehydrogenase from Streptococcus faecalis. J. Bacteriol. 101:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]