Abstract
Photorhabdus luminescens is a gram-negative insect pathogen that enters the hemocoel of infected hosts and produces a number of secreted proteins that promote colonization and subsequent death of the insect. In initial studies to determine the exact role of individual secreted proteins in insect pathogenesis, concentrated culture supernatants from various P. luminescens strains were injected into the tobacco hornworm Manduca sexta. Culture supernatants from P. luminescens TT01, the genome-sequenced strain, stimulated a rapid melanization reaction in M. sexta. Comparison of the profiles of secreted proteins from the various Photorhabdus strains revealed a single protein of approximately 37 kDa that was significantly overrepresented in the TT01 culture supernatant. This protein was purified by DEAE ion-exchange and Superdex 75 gel filtration chromatography and identified by matrix-assisted laser desorption ionization-time of flight analysis as the product of the TT01 gene plu1382 (NCBI accession number NC_005126); we refer to it here as PrtS. PrtS is a member of the M4 metalloprotease family. Injection of PrtS into larvae of M. sexta and Galleria mellonella and into adult Drosophila melanogaster and D. melanogaster melanization mutants (Bc) confirmed that the purified protein induced the melanization reaction. The prtS gene was transcribed by P. luminescens injected into M. sexta before death of the insect, suggesting that the protein was produced during infection. The exact function of this protease during infection is not clear. The bacteria might survive inside the insect despite the melanization process, or it might be that the bacterium is specifically activating melanization in an attempt to circumvent this innate immune response.
Photorhabdus luminescens is a gram-negative organism of the family Enterobacteriaceae that lives as a symbiont in the intestine of entomopathogenic nematodes (8). These nematodes, of the family Heterorhabdititae, live in the soil where they seek out and enter insect hosts. P. luminescens once expelled from the nematode enters the hemocoel of the insect, where it produces several toxins and proteins that damage the host. Inside the insect both the bacteria and the nematode replicate, and at the end of the infection cycle nematodes emerge from the insect colonized with P. luminescens.
In a laboratory setting, injection of as few as 10 P. luminescens organisms will result in rapid death of the insect (8). Additionally, bacterial culture supernatants, introduced either by an oral route or injected into the hemocoel, are toxic (1). Many potential bacterial products are implicated in this killing, including a toxin termed “makes caterpillars floppy,” hemolysins, RTX (repeats in toxin) family members, and large toxin complexes (Tc) (1-3, 5, 7). Photorhabdus also secretes numerous proteases and lipases and is able to inhibit competitor bacteria by synthesizing various antibiotics and bacteriocins (7-9, 13).
As with all pathogens, Photorhabdus must avoid the infected host's immune response. Similar to the innate immune response of higher organisms, the insect innate immune response has both a cellular and a humoral component (11). The cellular response includes phagocytic cells called hemocytes, which either take up the invading bacteria or form cellular aggregates called nodules that trap them. The humoral response includes the production of numerous antimicrobial peptides and proteins. In contrast to vertebrates, the humoral arm of the insect innate immune system also has an antimicrobial function termed melanization. Melanization is the deposition of melanin onto invading organisms, which can confine the infection and kill the pathogen. The melanization response is controlled by a cascade of serine proteases that results in the activation of the enzyme prophenoloxidase (PPO). PPO is present in the hemolymph at all times and when activated catalyzes the synthesis of melanin. Insects produce serine protease inhibitor proteins, called serpins, which regulate the melanization cascade through the specific inhibition of the terminal protease. Regulation of this pathway is to prevent excessive melanization (6).
In this report we demonstrate that a Photorhabdus protein induces the melanization reaction in the insects Manduca sexta, Drosophila melanogaster, and Galleria mellonella. This protein was isolated and identified as the product of the P. luminescens gene plu1382. Plu1382 is a predicted M4 metalloprotease family member that has previously been named PrtS (4).
MATERIALS AND METHODS
Chemicals.
All chemicals and supplies were purchased from Sigma (St. Louis, MO) or Fisher Scientific (Pittsburgh, PA) unless otherwise noted.
Bacterial strains and growth conditions.
P. luminescens Hb, Photorhabdus asymbiotica, and Photorhabdus temperata C1 were purchased from the American Type Culture Collection (ATCC 29999, ATCC 43950, and ATCC 29304, respectively). P. luminescens Hm and TT01 were generous gifts from E. Peter Greenberg (University of Washington) and Alain Givaudan (University of Montpellier), respectively. All Photorhabdus strains were maintained on Luria-Bertani (LB) agar plates. For protein purification, bacteria were grown with aeration at 30°C for 72 h in LB broth. At 72 h of growth, cultures of TT01 contain 5 × 108 viable cells per ml, while cultures of all other Photorhabdus strains contain 5 × 109 viable cells per ml.
For expression and purification of recombinant proteins, Escherichia coli TOP10 and BL21* cells were used with the expression plasmid pET101 as suggested by the manufacturer (Invitrogen, Carlsbad, CA). E. coli was routinely grown at 37°C on LB agar or in LB broth supplemented with 100 μg ml−1 of carbenicillin when appropriate (Research Products International, Mount Prospect, IL).
Purification of native PrtS.
P. luminescens TT01 was inoculated from a single colony into 3 ml LB and grown overnight at 30°C with aeration. The culture was diluted 1:250 into LB and grown for 72 h at 30°C with aeration. The resulting dark-brown-pigmented culture was centrifuged, and the supernatant was filtered through a 0.22-μm filter (Corning, Acton, MA) to remove cells and stored at 4°C until purification. The supernatant was diluted 1:5 into 25 mM Tris (pH 8.0) to adjust the pH, and a 100-ml volume of diluted sample was run over a HiTrap DEAE column (GE Healthcare, Piscataway, NJ) using an Akta Purifier fast protein liquid chromatograph (GE Healthcare). Fractions were eluted in steps of increasing NaCl concentration. Eluates were examined by A280 and sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). PrtS eluted with 25 mM Tris, 0.1 M NaCl, pH 8.0. PrtS-containing fractions were concentrated using a Vivaspin 10-kDa concentrator (ISC BioExpress, Kaysville, UT) and further purified on either a Superdex 75 or a Superdex 200 sizing column. A sample (100 μl) of concentrated DEAE-purified protein was loaded onto an Akta Purifier fast protein liquid chromatography sizing column and run with 25 mM Tris, 0.1 M NaCl, pH 8.0. Fractions containing PrtS were further concentrated using a 10-kDa Vivaspin concentrator. The bicinchoninic acid assay (Pierce, Rockford, IL) was used to determine total protein concentration when possible (at times pigments in supernatants interfered with colorimetric assays).
Purified PrtS was identified by matrix-assisted laser desorption ionization-time of flight (MALDI-TOF) analysis performed at the University of Washington Mass Spectrometry Facility.
Construction of recombinant PrtS (rPrtS) and rPrtS-E168A expression plasmids.
DNA encoding PrtS (plu1382) was amplified by PCR from strain TT01 genomic DNA utilizing PfuUltra DNA polymerase (Stratagene, La Jolla, CA), the forward primer CACCATGCAAATACAAAACAATAACTAC, and the reverse primer CTCTTCAGTTTTATCTTTATTTTTG (Operon, Huntsville, AL). The PCR product was inserted into pET101 according to the manufacturer's suggestions, to form pKH7. The DNA sequence of the plasmid insert was confirmed by automated DNA sequencing. Expression from this plasmid results in full-length PrtS with a carboxy-terminal histidine tag to facilitate purification.
A construct encoding PrtS with a mutation in the predicted catalytic glutamate at position 168 (E168) was also generated. The codon for E168 was mutated to code for alanine using the QuikChange site-directed mutagenesis kit (Stratagene). The primer CGATGTGATCGGCCATGCATTATCACATGGTG (underlined is the site of the A-to-C base change) and its reverse complement were used with pKH7 as the DNA template. The DNA sequences of the resulting mutated plasmids were determined, and a plasmid containing the correct change was isolated and designated pKH8.
Recombinant protein expression and purification.
rPrtS and rPrtS-E168A were expressed and purified using the pET101 constructs pKH7 and pKH8, according to the manufacturer's suggestions with some exceptions. Briefly, 100 ml of E. coli BL21* freshly transformed with pKH7 or pKH8 was grown in LB-carbenicillin (100 μg ml−1) at 37°C with aeration. When the cell culture reached an optical density at 600 nm of 0.8, isopropyl-β-d-1-thiogalactopyranoside (IPTG) was added to a final concentration of 0.8 mM. Induction was allowed to proceed at 30°C with aeration for 2 h. Bacterial cells were collected by centrifugation, and the pellets were lysed using a French pressure cell. The lysate was clarified by centrifugation, followed by filtration through a 0.45-μm filter. The E. coli lysate containing rPrtS-E168A was loaded onto a His-bind (Novagen, La Jolla, CA) column, and fractions were eluted with steps of 0.1, 0.5, and 1 M imidazole. The eluted proteins were equilibrated with 0.1 M Tris (pH 8.0) using Spectra 10,000-molecular-weight-cutoff dialysis tubing and stored at 4°C. rPrtS was purified by the same method as described for rPrtS-E168A above except that, from the time of induction with IPTG, all solutions contained 1× Complete protease inhibitor (Roche, Indianapolis, IN), and the protein was eluted with 0.1 M imidazole.
SDS-PAGE and Western immunoanalysis.
Protein samples were mixed with equal volumes of 2× sample buffer containing 10% β-mercaptoethanol; heated at 60°C for 1 h (16); and loaded onto a 12%, 15%, or 4 to 20% gradient polyacrylamide gel for SDS-PAGE. Protein standards were purchased from Bio-Rad (Hercules, CA). Western analyses were performed on a Millipore Immobilon P membrane (Bedford, MA). Histidine tag-labeled proteins were detected using anti-His6-horseradish peroxidase antibody from Abcam (Cambridge, MA) diluted 1:5,000. Autoradiography was performed with enhanced chemiluminescence detection (GE Healthcare) and blue autoradiography film (ISC Bioexpress).
Activity assays.
The proteolytic activities of PrtS, rPrtS, and PrtS-E168A were examined by caseinolytic macroassay with a few modifications (4). Azocasein and proteins were in 0.1 M Tris (pH 8), and the assays were performed at 50°C. The metalloprotease inhibitor 1,10-phenanthroline was included in these assays at 8 mM.
The autocatalytic activity of rPrtS was examined by a size shift from approximately 42 kDa to approximately 37 kDa. rPrtS (0.6 μg or 1.2 μg) and rPrtS-E168A (100 μg) were incubated together at room temperature in the presence and absence of 8 mM 1,10-phenanthroline for 0, 2, and 18 h. Portions of the protein mixtures were prepared for SDS-PAGE as described above.
Insect toxicity assays.
Manduca sexta fifth-instar larvae were the generous gift of Lynn Riddiford, Department of Biology, University of Washington. Larvae were rested on ice for 5 min, injected with 20-μl volumes using BD Ultra-Fine II insulin syringes, and kept at room temperature for the remainder of the experiment. Larvae were observed for 120 h when concentrated supernatant was injected, 96 h when purified PrtS was injected, and 72 h when recombinant protein was used. Injections contained 2.2 μg or 4.4 μg PrtS purified from supernatants of TT01 or greater than 5 μg rPrtS-E168A. Supernatants from strains grown at 30°C for 72 h were concentrated 2.5-fold in an Amicon 10,000-molecular-weight-cutoff concentrator (Millipore) and injected as 20-μl volumes. At least three larvae were injected for each protein, and each experiment was performed at least twice. Larvae were scored as dead when they did not respond to repeated painful stimulation.
Galleria mellonella insects were purchased from Petco, chilled on ice for 2 min, and injected with 20 μl containing approximately 5.6 μg of supernatant purified PrtS, again done in triplicate.
Adult Drosophila melanogaster wild type (Oregon R) and D. melanogaster Black cells (Bc[1] fj[1] wt[1]; Bloomington stock number 1036), which contain a mutation in the humoral melanization pathway, were used. Proteins were introduced into the flies by pricking the thorax with a 25-gauge needle that had been dipped into solutions of either buffer alone (33% glycerol, 0.07 M Tris, pH 8.00), rPrtS (2.6 μg μl−1 in 33% glycerol, 0.07 M Tris, pH 8.00), or rPrtS-E168A (2.6 μg μl−1 in 33% glycerol, 0.07 M Tris, pH 8.00). Insects were scored for mortality at 4, 24, and 48 h postinjection. Student's t test analyses of results were performed using the program at http://www.physics.csbsju.edu/stats/t-test.html.
N-terminal sequence of PrtS.
To determine the N-terminal sequence of purified rPrtS, the protein was run on a 4 to 20% gradient polyacrylamide gel (Ready Gel; Bio-Rad), transferred to polyvinylidene difluoride (SequiBlot; Bio-Rad), and stained with Coomassie brilliant blue R-250. The stained membrane was sent to Midwest Analytical (St. Louis, MO) for protein sequence analysis.
Reverse transcription-PCR (RT-PCR) analysis of prtS transcription.
Fifth-instar M. sexta insects were chilled on ice for 5 min and then injected with a 20-μl volume of phosphate-buffered saline (PBS) containing approximately 10,000 CFU of E. coli VCS257 (Stratagene) or 10 CFU of Photorhabdus sp. strains. At 2-, 4-, 12-, 24-, and 48-h time points postinjection, the Manduca insects were chilled on ice for 5 min and surface sterilized with ethanol and the hemolymph was collected by bleeding from the horn. The hemolymph was immediately frozen at −20°C for RNA collection, or dilutions were made in PBS and plated on LB agar. CFU were counted after 48 h of growth at 30°C.
Total RNA was isolated from 100-μl aliquots of hemolymph using the Qiagen RNeasy mini-RNA isolation system, by the manufacturer's protocol, with the addition of the on-column DNase treatment (Qiagen). Postcleanup, the RNA was again DNase treated by the manufacturer's protocol (Invitrogen) and suspended in a 20-μl volume.
RT-PCR was performed in a two-step reaction (ImProm II reverse transcription system; Promega), with 10 μl of purified total RNA for the initial cDNA amplification with random primers. PCR primers obtained from Operon were designed to amplify an approximately 200-nucleotide unique internal region of Photorhabdus prtS. Primers amplifying the Photorhabdus species 16S rRNA served as a PCR control. Amplification products were visualized on a 0.8% agarose gel.
RESULTS
Photorhabdus culture supernatants induced melanization in Manduca sexta.
As an initial step to determine the profile of toxins produced by various Photorhabdus strains, culture supernatants from 72-h cultures were isolated, concentrated 2.5-fold, and injected into the hemocoel of fifth-instar M. sexta larvae. Culture supernatants were collected from the entomophagous strains P. luminescens TT01, Hb, and Hm; the closely related P. temperata C1; and the human pathogen P. asymbiotica. Supernatants were injected into M. sexta, and the mean time to death of the larvae was determined (Table 1). The P. luminescens TT01 supernatant appeared the most toxic, killing larvae as early as 24 h, and with a mean time to death of 56 h. Larvae injected with either the P. luminescens Hb or Hm supernatants or the P. asymbiotica supernatants had a mean time to death of 96 h. Larvae injected with either P. temperata C1 supernatant or boiled P. luminescens TT01 supernatant remained alive at 120 h, and the experiment was terminated at that time.
TABLE 1.
Mortality and melanization of Manduca sexta injected with supernatants from different Photorhabdus strainsa
| Strain supernatant | Mean time to death (h postinjection) | % Melanized |
|---|---|---|
| P. luminescens | ||
| TT01 | 56 | 67 |
| TT01 (boiled) | >120b | 0 |
| Hb | 96 | 0 |
| Hm | 96 | 0 |
| P. temperata C1 | >120b | 0 |
| P. asymbiotica | 96 | 0 |
Three larvae were injected with 20 μl each of 2.5-fold-concentrated supernatants from 72-h growth at 30°C for each strain.
Larvae injected with either boiled TT01 supernatant or C1 supernatant were alive at 120 h and sacrificed at the end of the experiment.
The phenotypes of the injected M. sexta insects differed depending on the supernatant used. The supernatant from strain TT01 induced a melanization response in the injected larvae within 1 h. This rapid melanization response was not observed when the other supernatants were injected. The increased melanization with the P. luminescens TT01 supernatant might result from the increased concentration of a specific component found in all supernatants or from a component produced by strain TT01 not produced by the other strains. Analysis of the culture supernatants by SDS-PAGE demonstrated that an approximately 37-kDa protein was abundant in the P. luminescens TT01 culture supernatant but was apparently absent (P. asymbiotica and P. luminescens Hb) or significantly decreased (P. temperata C1 and P. luminescens Hm) in the other examined strains (Fig. 1). We hypothesized that this protein was responsible for the observed TT01-specific melanization response.
FIG. 1.

PrtS is found in abundance in Photorhabdus luminescens strain TT01 culture supernatant. Photorhabdus cultures were grown for 72 h at 30°C, and supernatant proteins were separated by 12% SDS-PAGE and stained with Coomassie blue. Lanes: A, P. asymbiotica (ATCC 43950); B, P. luminescens TT01; C, P. luminescens Hb; D, P. temperata C1; E, P. luminescens Hm. The asterisk indicates the position of PrtS.
Identification of PrtS.
The 37-kDa protein was purified from supernatants of strain TT01 by ion-exchange and gel filtration chromatography, and either 2.2 or 4.4 μg was injected into six M. sexta larvae for each concentration (Fig. 2). As a control similar amounts of bovine serum albumin (BSA) were injected. Within 15 min of injection of 4.4 μg of purified 37-kDa protein, four of the six larvae exhibited obvious melanization reactions. All insects injected with the 37-kDa protein melanized within 2 h. At 96 h, all larvae injected with 4.4 μg purified protein lost weight (mean, −0.17 g), and larvae injected with 2.2 μg gained less weight (mean, 0.14 g) than those injected with 2.2 or 4.4 μg BSA (5.1 and 5.6 g, respectively). Over the course of the experiment (96 h), three larvae injected with the 37-kDa protein died. Larvae injected with BSA did not melanize, and all survived the course of the experiment.
FIG. 2.
PrtS stimulates melanization in M. sexta. (A) Coomassie blue-stained 12% SDS-polyacrylamide gel of purified PrtS (lane 1) and BSA (lane 2). (B) Photograph of M. sexta fifth-instar larvae at 71 h post-injection of 4.4 μg of PrtS (panel 1) and BSA (panel 2). The black pigment on the larva in panel 1 is an indication of melanization response.
To test if the induction of melanization was insect specific, we injected purified 37-kDa protein into six Galleria mellonella larvae. All three Galleria larvae injected with 5.6 μg and two of three injected with 2.8 μg of the protein visibly blackened or melanized in less than 1 h. One of the larvae injected with 2.8 μg and all three injected with 5.6 μg died during the 7-day course of the experiment. None of the Galleria larvae injected with buffer alone melanized, and all three survived the course of the experiment.
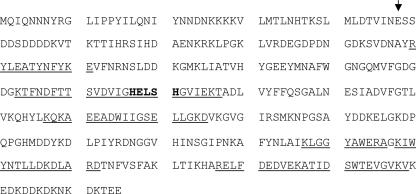
The identity of the 37-kDa protein was determined by MALDI-TOF analysis (Fig. 3). This approach revealed the molecular mass of nine 37-kDa-protein-specific peptides; comparison of these peptides to the TT01 published sequence (NCBI accession number NC_005126) identified the unknown protein as a predicted M4 metalloprotease encoded by plu1382, previously named PrtS (4).
FIG. 3.
Amino acid sequence of PrtS from Photorhabdus luminescens strain TT01 plu1382. The protein shown in Fig. 2 was sent for MALDI-TOF analysis. Peptides obtained from that analysis are underlined. Residues in bold are the characteristic HEXXH motif of M4 metalloproteases. The arrow denotes the last residue of the cleaved leader sequence.
Generation of rPrtS and rPrtS-E168A.
M4 metalloproteases all share a HEXXH motif where E is the catalytic residue (10). To test the relevance of the metalloprotease activity in our studies, two recombinant proteins were generated: recombinant full-length PrtS and full-length PrtS with a mutation in the predicted catalytic site, PrtS-E168A (see Materials and Methods). Both recombinant proteins contained C-terminal histidine tags to facilitate purification.
We easily purified the mutant rPrtS-E168A from E. coli by standard protocols but were unable to purify wild-type rPrtS from E. coli unless Roche Complete protease inhibitor was added to the culture medium. rPrtS-E168A and rPrtS purified in the presence of Complete protease inhibitor had the same apparent molecular mass of approximately 42 kDa, which is the predicted molecular mass of the entire protein encoded by plu1382 (41.4 kDa) plus the C-terminal histidine tag. When the protease inhibitor was removed from the rPrtS preparation by dialysis, the apparent molecular mass of rPrtS shifted to 37 kDa as determined by SDS-PAGE. This molecular mass agrees with the predicted molecular mass of PrtS isolated from the culture supernatants. The change in size of rPrtS in the absence of inhibitor suggested that an autocatalytic event was occurring. The size of rPrtS-E168A did not change when the protease inhibitor was removed, indicating that the predicted autocatalytic event was not occurring. Additional experiments demonstrated that rPrtS-E168A did not degrade azocasein (not shown), and this protein appears to be catalytically inactive.
rPrtS was able to cleave rPrtS-E168A to 37 kDa (Fig. 4). rPrtS-E168A was incubated at room temperature with 220- to 110-fold less rPrtS; aliquots were removed at 0, 2, and 18 h; and the proteins were separated by SDS-PAGE. After 2 h of incubation, approximately 50% of the rPrtS-E168A ran as the smaller protein, and at 18 h all of the rPrtS-E168A had been converted to the lower molecular mass. This cleavage was not observed if the metalloprotease inhibitor 1,10-phenanthroline was present. There was no decrease in the size of rPrtS-E168A incubated at room temperature for 18 h, again suggesting that this protein is catalytically inactive.
FIG. 4.
rPrtS is active and can cleave rPrtS-E168A. rPrtS and rPrtS-E168A were incubated at room temperature in the presence (+) and absence (−) of the metalloprotease inhibitor 1,10-phenanthroline (8 mM) for 0 h (lanes A to E), 2 h (lanes F to J), and 18 h (lanes K to M). Lanes A, F, and K are 2.2 μg rPrtS-E168A; lanes B, D, G, and I are 2.2 μg rPrtS-E168A with 0.01 μg rPrtS; lanes C, E, H, J, L, and M are 2.2 μg rPrtS-E168A with 0.02 μg rPrtS; and lane N is rPrtS used as a size control.
N-terminal sequence of PrtS.
Many metalloproteases are activated by cleavage of their N termini. We therefore analyzed the N-terminal amino acid sequence of the 37-kDa rPrtS. Initial characterization of recently purified rPrtS revealed two N-terminal sequences: 64% of the protein sample appeared to have the N-terminal sequence VINES (amino acids 45 to 48, Fig. 3) and 36% of the proteins in the sample appeared to have the N-terminal sequence SSDDS (amino acids 49 to 53, Fig. 3). To determine if the two N-terminal sequences resulted from incompletely processed protein, the same sample was stored for several weeks at 4°C and resubmitted for N-terminal sequence analysis. The sequence obtained from the older sample was 32% VINES and 68% SSDDS, suggesting that SSDDS is most likely the N-terminal sequence of the activated protein.
Melanization is associated with the active form of PrtS.
To confirm that the black deposit that we were observing did in fact result from a melanization reaction, we obtained wild-type D. melanogaster and D. melanogaster flies that contained the Black cell mutation (Bc) and are deficient for humoral melanization (15).
Wild-type Drosophila flies injected with rPrtS developed a visible blackening at the injection site and at times a discolored body, which likely was a function of the amount of protein internalized (Fig. 5B). Wild-type Drosophila flies injected with rPrtS were immediately moribund. In contrast, wild-type flies did not melanize when injected with rPrtS-E168A at amounts equal to those of rPrtS. To illustrate the degree of melanization, we compared the color of injected flies to noninjected ebony mutant flies, which naturally have darker pigmentation (Fig. 5C). The melanization site is much darker. Finally, Bc flies injected with rPrtS did not produce a melanization reaction, as predicted since these flies lack phenoloxidase (not shown).
FIG. 5.
rPrtS but not rPrtS-E168A induces melanization in Drosophila. (A) Wild-type fly pricked with 2.6 μg μl−1 rPrtS-E168A. (B) Wild-type fly pricked with 2.6 μg μl−1 rPrtS; the arrow points to the site of injection. (C) Untreated ebony fly as color control.
We assayed mortality rates for the wild-type and Bc flies after injection with either rPrtS or rPrtS-E168A. Only wild-type flies injected with rPrtS melanized, and mortality was directly related to active PrtS. The percentages of wild-type flies that died upon injection of either rPrtS-E168A or buffer were essentially the same (Table 2). The Drosophila Bc mutation decreases fitness and size of the flies in general, so these flies were less likely to survive the injection procedure; thus, increased mortality was observed with all injections. None of the Bc flies melanized, regardless of injection material. The mortality observed with rPrtS-injected Bc flies (52%) was compared to the mortality observed with rPrtS-E168A-injected Bc flies (28%). An unpaired Student t test indicated that there was a significant difference in the observed mortalities (P = 0.086). This suggests that PrtS-induced death can occur in the absence of melanization and that the injected protease was damaging the fly.
TABLE 2.
Mortality of wild-type and Bc Drosophila after injection with rPrtS and rPrtS-E168Aa
| Fly type/injection | % Mortality |
|---|---|
| Wild type/buffer | 4 |
| Wild type/rPrtS | 42 |
| Wild type/rPrtS-E168A | 2 |
| Bc/buffer | 20 |
| Bc/rPrtS | 52 |
| Bc/rPrtS-E168A | 28 |
Fifty wild-type Drosophila and 25 Bc Drosophila flies were injected with 2.6 μg μl−1 protein or buffer alone. Percent mortality was scored at 48 h postinjection.
The prtS gene is transcribed during infection.
As an initial experiment to determine if PrtS is produced during infection of the insect host, M. sexta insects were injected with either P. luminescens TT01 or E. coli VCS257 and the infected hemolymph was collected and analyzed by RT-PCR for the prtS transcript. Fifth-instar M. sexta insects were injected in the hemocoel with either 10,000 CFU of E. coli or 10 CFU of Photorhabdus. At 2-, 4-, 12-, 24-, and 48-h time points postinjection, the hemolymph was collected by bleeding from the horn and immediately frozen at −20°C for RNA collection or dilutions were made in PBS and plated on LB agar.
As shown in Fig. 6A, no viable E. coli bacteria were detected in the hemolymph 2 h after injection of 104 CFU. In contrast, P. luminescens grew at an exponential rate to the density of 106 CFU per 100 μl hemolymph at 48 h. At 48 h the Photorhabdus-infected M. sexta died. RT-PCR analysis indicated that at all time points postinjection the prtS transcript was present in the Photorhabdus-infected insect (Fig. 6B). Thus, PrtS is most likely produced during the active infection, when it could induce melanization in the host.
FIG. 6.
The prtS gene is transcribed during infection of M. sexta. (A) Growth of P. luminescens TT01 in M. sexta. M. sexta hemocoel was injected with either 10 CFU P. luminescens TT01 or 10,000 CFU E. coli VCS257. At indicated time points postinjection, 100 μl of hemolymph was collected from the horn of the insect and dilutions were made in PBS and plated to obtain colony counts. At 48 h postinjection with P. luminescens, the M. sexta died. (B) Detection of prtS transcript using RT-PCR. Hemolymph samples from M. sexta were collected as described above, total RNA was isolated, and RT-PCR was performed as described in Materials and Methods. Samples from three M. sexta insects per time point were examined. Hours postinjection are indicated above the horizontal lines. “0” represents hemolymph sample immediately after injection; − represents samples from uninfected controls.
DISCUSSION
Photorhabdus luminescens subsp. laumondii strain TT01 was isolated from a Heterorhabditis bacteriophora insect isolated on Trinidad and Tobago (7). The genome sequence of this strain has been reported, and this complete sequence contributes significantly to the understanding of the numerous toxic proteins produced by this pathogen. One of these proteins is a protease product of plu1382. This protein has been previously identified in the culture supernatants of Photorhabdus strains and was named PrtS (4) and Php-C (13). We use the PrtS designation here. The levels of PrtS produced by Photorhabdus strains vary (13), and for undetermined reasons this protein is made in abundance by TT01.
The predicted amino acid sequence of PrtS indicated that it is a member of the M4 metalloprotease family. The M4 family of metalloproteases consists of endopeptidases that share a HEXXH active site motif, where the histidines coordinate a zinc ion and the glutamate is the catalytic residue (10). Thermolysin from Bacillus thermoproteolyticus is the prototype of this family, and there are family members produced by many other bacterial species including Pseudomonas aeruginosa, Listeria monocytogenes, Legionella pneumophila, and other bacterial pathogens. Here we demonstrated that PrtS is a protease that can cleave a catalytically inactive mutant (Fig. 4) and azocasein (data not shown). We have not yet demonstrated an association with a metal ion, which is needed for a definitive assignment of this protease as an M4 family member.
M4 metalloproteases are synthesized as precursors that then cleave their N termini for activation (12). The removed N-terminal propeptide may assist in folding and secretion of the protein (14, 17). To purify active rPrtS, a protease inhibitor mix was added to the supernatants during the purification process. This approach allowed purification of a full-length protein. When the protease inhibitor was removed, the protein underwent an apparent autocatalytic event, clipping off the N-terminal 48 amino acids. We determined the N-terminal sequence of the active rPrtS to be SSDDS, which corresponds to the N-terminal sequence determined for this protein by Marokhazi et al. (13). The apparent molecular weight of the cleaved rPrtS correlated with that of the native PrtS found in culture supernatants; we are confident that this result reflects the actual N-terminal sequence of the active protein. The primary amino acid sequence of PrtS does not contain a gram-negative bacterial secretion signal peptide associated with Sec-dependent secretion systems, and yet this protein is found in Photorhabdus culture supernatants. Therefore, the mechanism used to secrete or release this protein from the bacteria remains undefined.
Injection of rPrtS into the three insect species examined here resulted in rapid accumulation of black pigment. We conclude that this discoloration indeed represents melanization, as injection into the Drosophila melanization mutant Black cells did not result in the development of the dark pigment. Melanization in the insect results from a complex enzyme cascade that results in the activation of PPO, and it is tempting to speculate that PrtS is activating one of the critical enzymes in the pathway. Work by others showed that injection of thermolysin into Galleria resulted in melanization, and the same study indicated that in vitro the Galleria PPO pathway could be activated by thermolysin (19). Therefore, it is possible that rPrtS directly activated this reaction in the insects examined here. However, death from rPrtS injection was not strictly a function of the activation of melanization, as there was significant lethality after injection of Bc flies with rPrtS. We speculate that death can occur due to the nonspecific proteolytic activity of the protein.
Many insect pathogens, including bacteria and fungi, produce proteases, and as such, insects have developed defense mechanisms to combat them. Injection of thermolysin into Galleria induces the expression of an inhibitor of metalloproteases from insects (IMPI) (19). IMPI is a component of the innate humoral immune response of this insect and is induced in Galleria in response to microbial invasion. This protein is inhibitory not only to thermolysin but to other metalloproteases as well. In addition, Photorhabdus itself secretes a factor, named Inh, that is inhibitory to metalloproteases (18). This suggests that expression and inhibition of protease activity are important factors in the insect-pathogen interaction.
P. luminescens is found only in association with either the insect or the nematode host, and most likely PrtS is produced specifically to aid the bacterium-host interaction. We demonstrate here that the prtS transcript is present when P. luminescens is growing in an insect, and it is present before the insect expires. PrtS could have a number of virulence-associated roles in the infected insect. It is possible that Photorhabdus produces PrtS simply to damage the insect host and increase colonization by both the bacterium and the nematode. PrtS cleaves insect antibacterial peptides, and this activity might be the primary function (4). Photorhabdus might produce PrtS to generate small peptides as a source of amino acids. Lastly, the rapid induction of the melanization reaction suggests that one function of PrtS might be to overinduce and confuse the insect's innate immune response as a mechanism to evade this response. A recent report indicates that P. luminescens produces a small-molecule antibiotic that inhibits phenoloxidase, a critical enzyme in the melanization pathway (7a). It appears that P. luminescens attempts to manipulate this important insect innate immune response to promote its own growth and survival. The exact sequence of events involved in this manipulation is still to be determined.
Acknowledgments
We thank L. Riddiford and B. Nguyen for the generous gift of the Manduca sexta insects used in these experiments. We thank M. Scotcher and J. Hoff for critical reading of the manuscript and I. Gendlina for helpful suggestions.
This work was supported by start-up funds from the University of Washington to C.M.C.
Footnotes
Published ahead of print on 12 October 2007.
REFERENCES
- 1.Bowen, D., T. Rocheleau, M. Blackburn, O. Andreev, E. Golubeva, R. Bhartia, and R. ffrench-Constant. 1998. Insecticidal toxins from the bacterium Photorhabdus luminescens. Science 280:2129-2132. [DOI] [PubMed] [Google Scholar]
- 2.Bowen, D. J., T. Rocheleau, C. K. Grutzmacher, L. Meslet, M. Valens, D. Marble, A. Dowling, R. ffrench-Constant, and M. A. Blight. 2003. Genetic and biochemical characterization of PrtA, an RTX-like metalloprotease from Photorhabdus. Microbiology 149:1581-1591. [DOI] [PubMed] [Google Scholar]
- 3.Brillard, J., E. Duchaud, N. Boemare, F. Kunst, and A. Givaudan. 2002. The PhlA hemolysin from the entomopathogenic bacterium Photorhabdus luminescens belongs to the two-partner secretion family of hemolysins. J. Bacteriol. 184:3871-3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cabral, C., A. Cherqui, A. Pereira, and N. Simoes. 2004. Purification and characterization of two distinct metalloproteases secreted by the entomopathogenic bacterium Photorhabdus sp. strain Az29. Appl. Environ. Microbiol. 70:3831-3838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Daborn, P., N. Waterfield, C. Silva, C. Au, S. Sharma, and R. ffrench-Constant. 2002. A single Photorhabdus gene, makes caterpillars floppy (mcf), allows Escherichia coli to persist within and kill insects. Proc. Natl. Acad. Sci. USA 99:10742-10747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Gregorio, E., P. Spellman, P. Tzou, G. Rubin, and B. Lemaitre. 2002. The Toll and Imd pathways are the major regulators of the immune response in Drosophila. EMBO J. 21:2568-2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duchaud, E., C. Rusniok, L. Frangeul, C. Buchrieser, A. Givaudan, S. Taourit, S. Bocs, C. Boursaux-Eude, M. Chandler, J. Charles, E. Dassa, R. Derose, S. Derzelle, G. Freyssinet, S. Gaudriault, C. Medigue, A. Lanois, K. Powell, P. Siguier, R. Vincent, V. Wingate, M. Zouine, P. Glaser, N. Boemare, A. Danchin, and F. Kunst. 2003. The genome sequence of the entomopathogenic bacterium Photorhabdus luminescens. Nat. Biotechnol. 21:1307-1313. [DOI] [PubMed] [Google Scholar]
- 7a.Eleftherianos, I., S. Boundy, S. A. Joyce, S. Aslam, J. W. Marshall, R. J. Cox, T. J. Simpson, D. J. Clarke, R. H. ffrench-Constant, and S. E. Reynolds. 2007. An antibiotic produced by an insect-pathogenic bacterium suppresses host defenses through phenoloxidase inhibition. Proc. Natl. Acad. Sci. USA 104:2419-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forst, S., B. Dowds, N. Boemare, and E. Stackebrandt. 1997. Xenorhabdus and Photorhabdus spp.: bugs that kill bugs. Annu. Rev. Microbiol. 51:47-72. [DOI] [PubMed] [Google Scholar]
- 9.Forst, S., and K. Nealson. 1996. Molecular biology of the symbiotic-pathogenic bacteria Xenorhabdus spp. and Photorhabdus spp. Microbiol. Rev. 60:21-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jongeneel, C., J. Bouvier, and A. Bairoch. 1989. A unique signature identifies a family of zinc-dependent metallopeptidases. FEBS Lett. 242:211-214. [DOI] [PubMed] [Google Scholar]
- 11.Kanost, M., H. Jiang, and X. Yu. 2004. Innate immune responses of a lepidopteran insect, Manduca sexta. Immunol. Rev. 198:97-105. [DOI] [PubMed] [Google Scholar]
- 12.Kessler, E., and M. Safrin. 1994. The propeptide of Pseudomonas aeruginosa elastase acts as an elastase inhibitor. J. Biol Chem. 269:22726-22731. [PubMed] [Google Scholar]
- 13.Marokhazi, J., K. Lengyel, S. Pekar, G. Felfoldi, A. Patthy, L. Graf, A. Fodor, and I. Venekei. 2004. Comparison of proteolytic activities produced by entomopathogenic Photorhabdus bacteria: strain- and phase-dependent heterogeneity in composition and activity of four enzymes. Appl. Environ. Microbiol. 70:7311-7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McIver, K., E. Kessler, and D. Ohman. 2004. Identification of residues in the Pseudomonas aeruginosa elastase propeptide required for chaperone and secretion activities. Microbiology 150:3969-3977. [DOI] [PubMed] [Google Scholar]
- 15.Rizki, T. M., R. M. Rizki, and E. H. Grell. 1980. A mutant affecting the crystal cells in Drosophila melanogaster. Roux Arch. Dev. Biol. 188:91-99. [DOI] [PubMed] [Google Scholar]
- 16.Sambrook, J., and D. W. Russell. 2001. Molecular cloning: a laboratory manual, 3rd ed., vol. 3. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- 17.Tang, B., S. Nirasawa, M. Kitaoka, C. Marie-Claire, and K. Hayashi. 2003. General function of N-terminal propeptide on assisting protein folding and inhibiting catalytic activity based on observations with a chimeric thermolysin-like protease. Biochem. Biophys. Res. Commun. 301:1093-1098. [DOI] [PubMed] [Google Scholar]
- 18.Valens, M., A. Broutelle, M. Lefebvre, and M. A. Blight. 2002. A zinc metalloprotease inhibitor, Inh, from the insect pathogen Photorhabdus luminescens. Microbiology 148:2427-2437. [DOI] [PubMed] [Google Scholar]
- 19.Wedde, M., C. Weise, P. Kopacek, P. Franke, and A. Vilcinskas. 1998. Purification and characterization of an inducible metalloprotease inhibitor from the hemolymph of greater wax moth larvae, Galleria mellonella. Eur. J. Biochem. 255:535-543. [DOI] [PubMed] [Google Scholar]