Abstract
The cells of the marine bacterium strain C-21, which is phylogenetically closely related to Arenibacter troitsensis, accumulate iodine in the presence of glucose and iodide (I−). In this study, the detailed mechanism of iodine uptake by C-21 was determined using a radioactive iodide tracer, 125I−. In addition to glucose, oxygen and calcium ions were also required for the uptake of iodine. The uptake was not inhibited or was only partially inhibited by various metabolic inhibitors, whereas reducing agents and catalase strongly inhibited the uptake. When exogenous glucose oxidase was added to the cell suspension, enhanced uptake of iodine was observed. The uptake occurred even in the absence of glucose and oxygen if hydrogen peroxide was added to the cell suspension. Significant activity of glucose oxidase was found in the crude extracts of C-21, and it was located mainly in the membrane fraction. These findings indicate that hydrogen peroxide produced by glucose oxidase plays a key role in the uptake of iodine. Furthermore, enzymatic oxidation of iodide strongly stimulated iodine uptake in the absence of glucose. Based on these results, the mechanism was considered to consist of oxidation of iodide to hypoiodous acid by hydrogen peroxide, followed by passive translocation of this uncharged iodine species across the cell membrane. Interestingly, such a mechanism of iodine uptake is similar to that observed in iodine-accumulating marine algae.
Iodine is one of the essential trace elements for humans and animals because of its important role as a constituent of the thyroid hormones, thyroxine and triiodothyronine. Insufficient iodine in the diet leads to iodine deficiency disorders, such as endemic goiter and cretinism, which are still the most common causes worldwide of mental retardation and brain damage (12, 13). In the thyroid gland in mammals, iodine is taken up as an iodide ion (I−) by a sodium/iodide symporter (9, 11, 23). This transmembrane protein cotransports sodium ions with iodide into the thyroid against the concentration gradient. The driving force for the process is the electrochemical gradient of sodium ions across the cell membrane. As a result of this active transport system, the normal thyroid concentrates iodide by a factor of 20 to 40 (23). The thyroidal uptake of iodide is competitively inhibited by perchlorate, thiocyanate, and nitrate (9).
Other well-known iodine-accumulating organisms are marine algae, such as Laminaria spp. (17), although the physiological functions of iodine in the algal cells are still uncertain. The average concentration of total dissolved iodine in seawater is 0.45 μM, and the predominant chemical forms are iodide (I−; oxidation state, −1) and iodate (IO3−; oxidation state, +5) ions (28). Laminaria spp. take up iodine from seawater and accumulate it by a concentration factor of 1.5 × 105 (17). A number of studies have shown that the uptake of iodine by Laminaria is dependent on oxidative power (17, 18, 19, 22). Kylin (18) and Shaw (22) postulated the involvement of iodide oxidation prior to uptake of iodine. The latter author also suggested that hypoiodous acid (HIO) (oxidation state, +1) is the species finally taken up by Laminaria (22). Tong and Chaikoff (25) suggested the involvement of hydrogen peroxide (H2O2) in the oxidation of iodide in the alga Nereocystis luetkeana. Recently, Küpper et al. (17) found that addition of haloperoxidase, which was partially purified from Ascophyllum nodosum or Laminaria digitata, enhanced the uptake of iodine by Laminaria. Since haloperoxidases had actually been found in the cell walls of Laminaria (8, 15), Küpper et al. (17) proposed that iodide is oxidized to HIO or molecular iodine (I2; oxidation state, 0) by cell wall haloperoxidases and that the oxidized iodine species then freely penetrate algal cells by means of facilitated diffusion.
Until now, detailed mechanisms of iodine uptake by living organisms have been characterized only for the thyroid gland of mammals and for marine algae. Therefore, it is of interest to understand the mechanisms of iodine uptake in other organisms and to compare them with those of mammals and algae. In a previous study, we isolated an iodine-accumulating bacterium, designated strain C-21, from surface marine sediment (4). This strain was phylogenetically closely related to a marine aerobic bacterium, Arenibacter troitsensis, a member of the family Flavobacteriaceae. When C-21 was cultured in a liquid medium containing 0.1 μM iodide, the strain removed 80% of iodide from the medium within 24 h and accumulated iodine in the cells with a maximum concentration factor of 5.5 × 103 (4). The initial uptake rates of iodine by washed cells of C-21 showed substrate saturation kinetics with an apparent affinity constant for transport of 0.073 μM. Interestingly, the cells took up iodine only in the presence of glucose (4). In this study, iodine transport by a washed cell suspension of C-21 was assayed for further understanding of the mechanism of iodine uptake by C-21.
MATERIALS AND METHODS
Bacterial strain.
Strain C-21 has been isolated from surface marine sediment collected from Sagami Bay, Kanagawa, Japan (4). This strain has been deposited in the NBRC culture collection (Biological Resource Center, National Institute of Technology and Evaluation, Chiba, Japan) as NBRC103722.
Culture conditions.
All cultivations and incubations were carried out at 30°C throughout this study. Strain C-21 was routinely cultured in Marine broth 2216 (Difco) with shaking at 180 rpm. In growth experiments, the strain was cultured in a minimal medium, which contained 420 mM NaCl, 9 mM KCl, 9 mM CaCl2·2H2O, 25 mM MgSO4·7H2O, 23 mM MgCl2·6H2O, 2 mM NaHCO3, 2 mM NaNO3, 4 mM NH4Cl, 2 mM KH2PO4, 0.05 g liter−1 yeast extract, and 4 g liter−1 carbon source (glucose, glycerol, or succinate). C-21 did not grow in the medium if the carbon source was omitted.
Iodine transport assays.
Cells were cultured for 2 days in Marine broth 2216 and then harvested by centrifugation (7,000 × g at 4°C for 10 min). The cell pellet was washed twice with 10 mM potassium phosphate buffer (pH 7.0) supplemented with 330 mM NaCl, 30 mM MgCl2·6H2O, and 2 mM CaCl2·2H2O. After washing, cells were resuspended in the same buffer to achieve optical density at 600 nm of 1.0 (equivalent to 0.5 mg (dry weight)] ml−1).
The transport assay was carried out essentially as described previously (4). Briefly, the cell suspension was incubated aerobically with 0.1 μM potassium iodide and 74 kBq ml−1 radioactive iodine tracer (Na125I; Amersham Bioscience). The transport experiment was initiated by the addition of 25 mM glucose (time zero). Aliquots of the cell suspension were periodically removed and centrifuged through silicone oil (35:65 mixture of SH556 and SH550; Toray Dow Corning Silicone). The activity of 125I in the cells was measured using an Aloka ARC-370 M scintillation counter. The initial uptake rates were determined from the initial slopes of transport kinetic curves and expressed as nmoles of iodine per minute per gram dry weight of the cells. The radioactivity at time zero was subtracted from activities at subsequent times to calculate the net uptake by the cells. When the cells were incubated anaerobically, the suspension (20 ml) was dispensed into a 60-ml serum bottle. After the headspace was flushed with N2 gas (99.5% purity) for 5 min, the bottle was sealed with a thick butyl rubber stopper and an aluminum cap.
Potential competitive inhibitors, metabolic inhibitors, and reducing agents were added 30 min before the addition of glucose. Potential metabolic inhibitors tested were valinomycin, nigericin, carbonylcyanide-m-chlorophenylhydrazone, 2,4-dinitrophenol (DNP), gramicidin D, monensin, N,N′-dicyclohexylcarbodiimide (DCCD), potassium cyanide (KCN), sodium azide (NaN3), and orthovanadate. Water-insoluble metabolic inhibitors were dissolved in ethanol and added to the cell suspension to give a final ethanol concentration of 1% (vol/vol). Preliminary experiments showed that 1% ethanol does not affect the initial uptake of iodine significantly. In the case of orthovanadate, potassium phosphate in the buffer was replaced with 10 mM Tris-HCl (pH 8.0).
In some experiments, one of the following exogenous enzymes was added to the cell suspension: glucose oxidase (from Aspergillus niger; Sigma Chemical, St. Louis, MO), alcohol oxidase (from Pichia pastoris; MP Biomedicals, Aurora, OH), catalase (from bovine liver; Sigma Chemical), and iodide oxidase. Iodide oxidase was partially purified from culture supernatant of an iodide-oxidizing Alphaproteobacteria strain, Q-1, which is phylogenetically closely related to Rhodothalassium salexigens (5). Strain Q-1 was isolated from an iodide-enriched natural gas brine water in Japan (5). Its extracellular enzyme (iodide oxidase) catalyzed the oxidation of iodide to molecular iodine with oxygen as an electron acceptor (5). A culture supernatant of strain Q-1 was concentrated by ultrafiltration and was applied to a DEAE-cellulose (DE-52; Whatman, United Kingdom) column preequilibrated with 20 mM sodium acetate buffer (pH 5.5). The column was eluted with a linear gradient of 0.1 to 0.6 M NaCl, and the iodide oxidase-containing fractions were pooled and concentrated by ultrafiltration. The specific activity of the partially purified enzyme was 2.1 U mg−1.
Radiotracer experiments on abiotic and enzymatic oxidation of iodide.
To determine whether abiotic or enzymatic oxidation of iodide occurs under our experimental conditions, iodide (0.1 μM) and Na125I (74 kBq ml−1) were incubated in the sealed serum bottle either with H2O2 (1 mM) or with iodide oxidase (0.1 U ml−1). The volume of reaction mixtures was 10 ml with a headspace of 50 ml. After incubation for 10 to 60 min, the bottle was heated and volatile radioiodine (125I2) was introduced into a silver wool trap by sweeping nitrogen gas as described elsewhere (2, 3). The trap was transferred to counting vials, and its 125I activity was measured using a scintillation counter. The detection limit of this method was approximately 0.01% of volatilization, which corresponds to 5.0 × 10−6 μM of I2 in the reaction mixtures.
Enzyme assays.
For the preparation of crude extracts, cells cultured as described above were harvested, washed twice, and resuspended in 50 mM Tris-HCl buffer (pH 8.0) to achieve an optical density at 600 nm of 20. They were disrupted by sonication (Ohtake ultrasonic disintegrator 5202) at 100 W and 20 kHz for 3 min, followed by centrifugation (10,000 × g, 10 min, 4°C) to remove cell debris. The soluble fraction was separated from the membrane fraction by ultracentrifugation (100,000 × g, 1 h, 4°C).
Since only low levels of glucose oxidase activity were found in the crude extracts (approximately 1 mU mg protein−1), it was difficult to determine the activity by using an oxygen consumption rate measured with an oxygen electrode. Thus, the activity was determined colorimetrically by an oxidative coupling reaction of N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline sodium salt with 4-aminoantipyrine in the presence of H2O2 and peroxidase (24). The resulting blue dye has a relatively low molar extinction coefficient (ɛ) of 17.5 mM−1 cm−1 at 593 nm. The reaction mixture (1.5 ml) contained 67 mM Tris-HCl (pH 9.0), 10 mM glucose, 10 mM CaCl2·2H2O, 0.62 mM 4-aminoantipyrine, 0.42 mM N-ethyl-N-(2-hydroxy-3-sulfopropyl)-3,5-dimethoxyaniline sodium salt (MP Biomedicals), and 0.025 mg horseradish peroxidase (Sigma Chemical). In a parallel experiment, glucose was omitted from the reaction mixture as a control, and corresponding A593 values of the controls were subtracted to calculate the glucose-dependent H2O2 production. In the assay, cumulative production of H2O2 proceeded linearly, and the amount of H2O2 produced was proportional to the amount of enzyme preparations added to the reaction mixture. Boiled enzyme was completely inactive. One unit of glucose oxidase activity was defined as the amount of crude enzyme catalyzing the production of 1 μmol of H2O2 per min. The protein concentration was determined by the Bradford method (7) with bovine serum albumin as a standard protein.
The reaction mixture for the assay of peroxidase contained 50 mM potassium phosphate (pH 6.0), 48 mM o-dianisidine, and 7.2 mM H2O2. The oxidized product of o-dianisidine was determined by measuring the increase in absorbance at 460 nm using a ɛ value of 11.3 mM−1 cm−1 (20). The reaction mixture for the assay of bromoperoxidase contained 50 mM potassium phosphate (pH 6.0), 10 mM bromide, 44 μM monochlorodimedon (MCD), and 7.2 mM H2O2. The decrease in absorbance at 290 nm due to the bromination of MCD (ɛ = 19.9 mM−1 cm−1) was measured (20). The reaction mixture for the assay of iodoperoxidase (triiodide-forming activity) contained 50 mM sodium acetate (pH 5.5), 10 mM iodide, and 1 mM H2O2. Formation of triiodide (I3−) was determined by measuring the increase in absorbance at 353 nm using a ɛ value of 25.5 mM−1 cm−1 (1).
RESULTS
Iodine uptake under various incubation conditions.
The washed cell suspension of C-21 took up iodine in the presence of glucose with an initial uptake rate of 0.663 nmol iodine min−1 gram dry cells−1 (Table 1). Only a limited uptake rate of 0.111 nmol iodine min−1 gram dry cells−1 was observed in the absence of glucose. The cells also showed limited uptake rates when glucose was replaced with glycerol or succinate. Even in the presence of glucose, no significant uptake was observed if the cells were incubated under anaerobic conditions or if calcium ions (Ca2+) were omitted from the cell suspension. The initial uptake rates decreased with decreasing pH, and no significant uptake was observed at pH 5.0 (Table 1). In minimal medium, C-21 was able to grow on glucose, glycerol, or succinate as the sole source of energy and carbon (data not shown).
TABLE 1.
Initial uptake rates of iodine by washed cells of C-21 under various incubation conditions
| Incubation condition | Initial uptake rate of iodine (nmol min−1 g dry cells−1)c | |||
|---|---|---|---|---|
| pH value | Carbon source | Oxygena | Calcium ionb | |
| 7.0 | Glucose | + | + | 0.663 ± 0.008 |
| —d | + | + | 0.111 ± 0.015 | |
| Glycerol | + | + | 0.135 ± 0.010 | |
| Succinate | + | + | 0.091 ± 0.006 | |
| Glucose | − | + | 0 | |
| Glucose | + | − | 0.009 ± 0.000 | |
| 6.0 | Glucose | + | + | 0.404 ± 0.007 |
| 5.5 | Glucose | + | + | 0.195 ± 0.012 |
| 5.0 | Glucose | + | + | 0 |
+, aerobic condition; −, anaerobic condition.
+, the incubation buffer contained 2 mM CaCl2; −, CaCl2 was omitted from the buffer.
All values are the means from duplicate analyses, and the errors indicate the ranges of the means.
—, no carbon source was added.
To evaluate the specificity of the iodine uptake system, the washed cells were incubated with potential competitive inhibitors (Table 2). Nitrate, nitrite, sulfate, chlorate, perchlorate, and bromide did not affect the initial uptake rates of iodine, while thiocyanate slightly inhibited it. Effects of various metabolic inhibitors, including ionophores and ATPase inhibitors, were also tested (Table 2). Some of these compounds (nigericin, valinomycin plus nigericin, DNP, monensin, DCCD, and KCN) inhibited the uptake significantly, but none of the metabolic inhibitors showed more than 50% inhibition. On the other hand, the initial uptake rates were strongly inhibited by various reducing agents and catalase (Table 2). Inhibitions by 5 mM of l-cysteine, l-ascorbate, dithiothreitol, and glutathione were 100, 91, 81, and 91%, respectively. Similarly, 1,000 U ml−1 of catalase inhibited 84% of the uptake.
TABLE 2.
Effects of competitive inhibitors, metabolic inhibitors, reducing agents, and catalase on initial uptake rates of iodine
| Category | Additivea | Concn (μM) | Uptake activity (% of control)b |
|---|---|---|---|
| Competitive inhibitors | Nitrate | 10 | 100 ± 4 |
| Nitrite | 10 | 95 ± 2 | |
| Sulfate | 10 | 100 ± 1 | |
| Chlorate | 10 | 100 ± 3 | |
| Perchlorate | 10 | 95 ± 1 | |
| Thiocyanate | 10 | 63 ± 5 | |
| Bromide | 10 | 100 ± 4 | |
| Metabolic inhibitorsc | Valinomycin | 100 | 89 ± 3 |
| Nigericin | 10 | 51 ± 3 | |
| Val + Nig | 10 | 61 ± 7 | |
| CCCP | 100 | 98 ± 1 | |
| DNP | 500 | 59 ± 5 | |
| Gramicidin D | 10 | 82 ± 8 | |
| Monensin | 100 | 64 ± 0 | |
| DCCD | 100 | 68 ± 3 | |
| KCN | 1,000 | 73 ± 1 | |
| NaN3 | 1,000 | 96 ± 2 | |
| Orthovanadate | 5,000 | 84 ± 0 | |
| Reducing agents | Cysteine | 100 | 54 ± 1 |
| 1,000 | 24 ± 1 | ||
| 5,000 | 0 | ||
| Ascorbate | 100 | 38 ± 2 | |
| 1,000 | 21 ± 1 | ||
| 5,000 | 9 ± 0 | ||
| DTT | 100 | 36 ± 0 | |
| 1,000 | 26 ± 1 | ||
| 5,000 | 19 ± 1 | ||
| Glutathione | 100 | 51 ± 1 | |
| 1,000 | 33 ± 1 | ||
| 5,000 | 9 ± 0 | ||
| Miscellaneous | Catalase | 100d | 61 ± 2 |
| 1,000d | 16 ± 1 |
CCCP, carbonylcyanide-m-chlorophenylhydrazone; DTT, dithiothreitol.
The initial uptake rates in controls (no addition) ranged from 0.441 to 0.979 nmol iodine min−1 g dry cells−1 depending on the experiment, and the mean value ± standard deviation was 0.599 ± 0.144 nmol iodine min−1 g dry cells−1 (n = 18). All values are the means from duplicate analyses of the initial uptake rates, and errors indicate the ranges for the means.
Metabolic inhibitors were added to the cell suspension at various concentrations, and results at a maximum concentration are shown.
Value is expressed in U ml−1.
H2O2-dependent uptake of iodine.
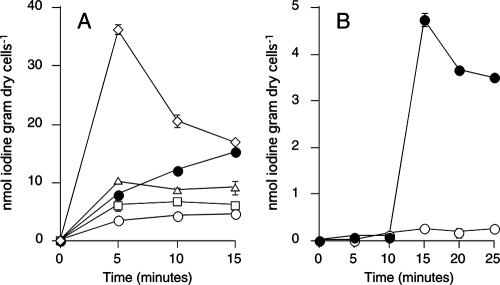
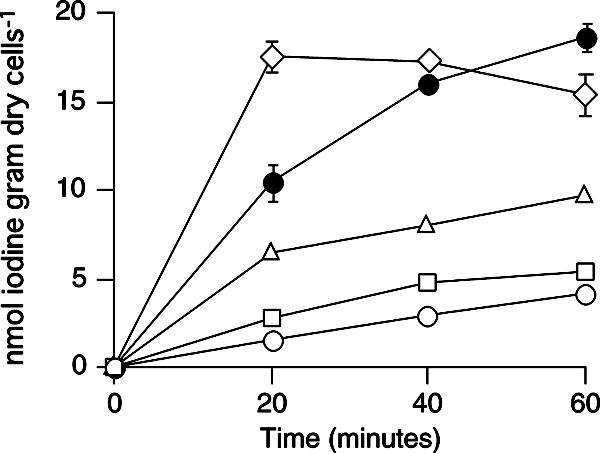
The uptake of iodine by the washed cells of C-21 was enhanced by an addition of exogenous glucose oxidase (Fig. 1). In the presence of 0.1 and 1 U ml−1 of glucose oxidase, the initial uptake rates increased, respectively, to 2.3 and 3.4 times higher than that observed in the absence of the enzyme. No significant iodine uptake occurred in the absence of glucose when glucose oxidase was present, indicating that the enhanced uptake was not due to glucose oxidase itself but to H2O2 produced by the enzyme. The cells took up iodine in the absence of glucose when 10 to 1,000 μM of H2O2 was directly added to the cell suspension (Fig. 2A). Glucose-independent uptake was also observed when methanol (0.1%) plus alcohol oxidase (0.01 to 1 U ml−1) were added to the cell suspension (data not shown). Furthermore, significant uptake of iodine occurred even in the absence of oxygen when H2O2 (10 μM) was added to the anaerobic cell suspension (Fig. 2B). To check whether H2O2 oxidizes iodide spontaneously, iodide (0.1 μM) and Na125I were incubated with 1,000 μM H2O2 for 10 min in acetate buffer (pH 5.0). However, volatile iodine (I2) production was below the detection limit (less than 0.01% of volatilization), indicating that abiotic oxidation of iodide by H2O2 did not occur under our experimental conditions.
FIG. 1.
Iodine uptake is stimulated by exogenous glucose oxidase. Washed cells were incubated aerobically with glucose in the presence of 0 (solid circles), 0.1 (solid squares), or 1 U ml−1 (solid triangles) of glucose oxidase. In some experiments, cells were incubated without glucose in the presence (crosses) or absence (open circles) of glucose oxidase (10 U ml−1). All values represent the means of duplicate analyses, and the error bars indicate the range for the mean. The absence of bars indicates that the error is smaller than the symbol.
FIG. 2.
(A) Glucose-independent uptake of iodine in the presence of H2O2. Cells were incubated aerobically with 0 (open circles), 10 (open squares), 100 (open triangles), or 1,000 μM (open diamonds) of H2O2 in the absence of glucose. Iodine uptake by the cells incubated with glucose (but without H2O2) is also shown as a control (solid circles). (B) Anaerobic uptake of iodine in the presence of H2O2. The cells (20 ml with a headspace of 40 ml) were incubated under anaerobic conditions. At 10 min, H2O2 (solid symbols) or deionized water (open symbols) was added to the cell suspension. The final concentration of H2O2 was 10 μM. All values represent the means from duplicate analyses, and the error bars indicate the ranges for the means. The absence of bars indicates that the error is smaller than the symbol.
Effect of iodide oxidation on iodine uptake.
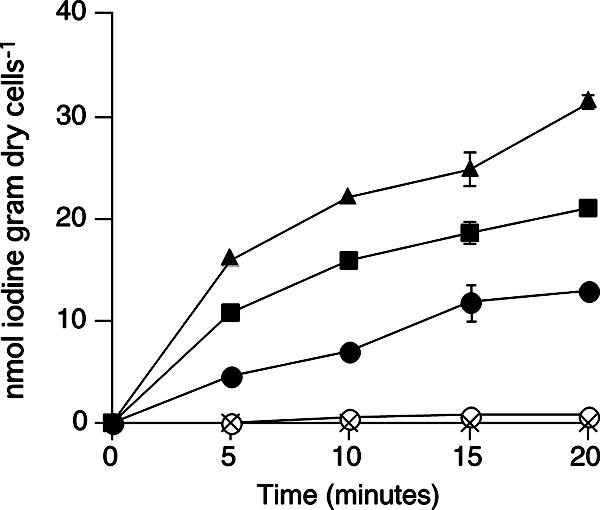
When iodide oxidase (0.1 U ml−1) was incubated with iodide and Na125I for 60 min in acetate buffer (pH 5.0), 19.7% of the total iodine was volatilized. This indicated that the enzyme actually oxidized iodide under our experimental conditions. To evaluate the effect of iodide oxidation on the iodine uptake, the cells of C-21 were then incubated with iodide oxidase in the absence of glucose. As shown in Fig. 3, the addition of 0.01 to 1 U ml−1 iodide oxidase stimulated and enhanced the glucose-independent uptake of iodine in a dose-dependent manner.
FIG. 3.
Effect of iodide oxidation on iodine uptake in the absence of glucose. Cells were incubated aerobically with 0 (open circles), 0.01 (open squares), 0.1 (open triangles), or 1 U ml−1 (open diamonds) of iodide oxidase in the absence of glucose. Iodine uptake by cells incubated with glucose is also shown as a control (solid circles). All values represent the means of duplicate analyses, and the error bars indicate the ranges for the means. The absence of bars indicates that the error is smaller than the symbol.
Possible enzymes involved in iodine uptake.
A significant glucose oxidase activity (0.95 mU mg−1) was found in the crude extracts of C-21, and 83% of the total activity was located in the membrane fraction (Table 3). When Ca2+ was omitted from the reaction mixture, only 45% of the maximum activity was observed. This suggested that the activity was, at least in part, Ca2+ dependent. The extracts also showed peroxidase activity (o-dianisidine-oxidizing activity) with specific activities of 15.9 and 9.57 U mg−1 in the soluble and membrane fractions, respectively. However, neither bromoperoxidase (MCD-brominating activity) nor iodoperoxidase (I3−-forming activity) activity was detected in the extracts.
TABLE 3.
Localization of glucose oxidase in cell fractions of strain C-21
| Cell fraction | Total activitya (mU) | % of activity | Sp acta (mU mg−1) |
|---|---|---|---|
| Crude extracts | 49.2 ± 8.80 | 100 | 0.95 ± 0.11 |
| Soluble fraction | 8.13 ± 1.24 | 16.5 | 0.26 ± 0.04 |
| Membrane fraction | 40.9 ± 5.26 | 83.1 | 2.61 ± 0.08 |
All values represent means from triplicate analyses ± standard deviations.
DISCUSSION
In a previous study, we found that iodine uptake by washed cells of C-21 occurred only in the presence of glucose (4). Since C-21 can utilize glucose as a sole source of energy and carbon, we assumed that iodine uptake is an active transport process (4). However, in this study, only limited uptake was observed in the presence of glycerol and succinate (Table 1), both of which could also support the growth of C-21. In addition, the uptake was not inhibited or was only partially inhibited by various metabolic inhibitors, whereas reducing agents and catalase strongly inhibited the uptake (Table 2). From these results, we inferred that the uptake is not energy dependent and that certain oxidants, such as H2O2, are involved in the process.
The cells of C-21 took up iodine even in the absence of glucose and oxygen if H2O2 was added to the cell suspension (Fig. 2). This indicates that H2O2 is primarily necessary for the uptake of iodine while glucose and oxygen play a secondary role by providing cells with H2O2 (see below). Furthermore, efficient uptake of iodine occurred when the cells were incubated with iodide oxidase instead of glucose (Fig. 3). These results suggest that the cells do not take up iodine in the form of iodide ion but oxidize it with H2O2 before the translocation across the cell membrane. There are several candidates for the oxidized iodine species translocated into the cells, viz., molecular iodine (I2), HIO, hypoiodite (IO−), and iodate (IO3−). In aqueous solution, molecular iodine is hydrolyzed spontaneously to form HIO and iodide with an equilibrium constant of 4.3 × 10−13 (10), as follows: I2 + H2O ⇔ HIO + I− + H+.
Assuming that 0.1 μM of iodide is oxidized completely to 0.05 μM molecular iodine and that molecular iodine is then hydrolyzed to form HIO and iodide according to the above equation at pH 7.0, the concentration ratio between HIO and molecular iodine ([HIO]/[I2]) at the steady state should be 600. This ratio increases if the concentration of molecular iodine is much lower than 0.05 μM. Thus, molecular iodine is not stable and is readily hydrolyzed to HIO under our experimental conditions. Since the dissociation constant of HIO is 10.6, its undissociated form (hypoiodite) should be ignored at pH 7.0. HIO also disproportionates spontaneously to form iodate (6, 21): 3HIO ⇔ IO3− + 2I− + 3H+.
However, the rate of this reaction at pH 7.0 is very slow, with the half-life of HIO ranging from a week to several years (6). In addition, we previously found that the cells of C-21 did not take up iodate (4). Therefore, we consider the oxidized form of iodine, which is translocated into the cells, to be HIO. The pH dependence of the uptake (Table 1) may reflect the decreased hydrolysis rate of molecular iodine under acidic conditions.
Strain C-21 possessed glucose oxidase in the membrane fraction (Table 3). This result, together with the fact that glucose/oxygen or H2O2 is required for the uptake of iodine, strongly suggests that glucose oxidase plays an important role in the process by providing the cells with H2O2. The calcium-dependent uptake of iodine by the cells (Table 1) might be due in part to the Ca2+ dependence of this enzyme.
A detailed mechanism of iodide oxidation by H2O2 is still unclear. Our radiotracer experiment showed that abiotic oxidation of iodide by H2O2 was not detectable under our experimental conditions. Thus, it is reasonable to consider that enzymatic oxidation of iodide takes place before iodine is translocated into the cells. Bromoperoxidase and iodoperoxidase are well-known enzymes that oxidize iodide with H2O2 as an electron acceptor (19, 20). Although we detected neither activity in the crude extracts, Leblanc et al. (19) pointed out the difficulty in determining whether these enzymes are absent because of relatively high detection limits of the haloperoxidase assays. Under the optimum conditions, the cells of C-21 took up iodine at rates of approximately 0.7 nmol iodine min−1 g dry cells−1 (Table 1). Assuming that cellular proteins comprise one-half of the dry weight of the bacterial cells (14), it is enough for the cells to possess at least 1.4 μU mg−1 of these enzyme activities. Thus, it cannot be excluded that a very low level of either haloperoxidase catalyzes the oxidation of iodide.
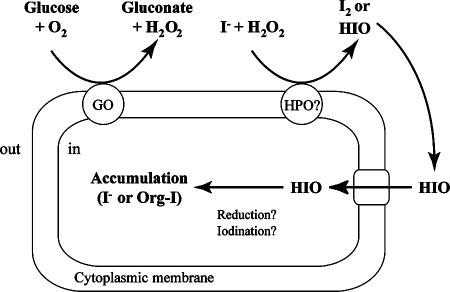
In Fig. 4, we illustrate a proposed mechanism of iodine uptake by C-21. First, glucose is oxidized by the membrane-bound glucose oxidase, and H2O2 is produced. Second, iodide is oxidized to molecular iodine or HIO, probably by a yet-unidentified haloperoxidase. Even if molecular iodine is produced at this stage, it is readily hydrolyzed spontaneously to form HIO. Finally, HIO is translocated freely across the cell membrane. Since the initial uptake rates of iodine by C-21 showed substrate saturation kinetics (4), HIO is probably transported via carrier-mediated facilitated diffusion. In the cells, HIO should be reduced to iodide again or be associated with certain organic compounds to avoid a release of iodine from the cells. At present, however, the chemical form of iodine in the cells is still unclear.
FIG. 4.
Schematic representation of possible mechanism of iodine uptake and accumulation by strain C-21. GO, glucose oxidase; HPO, haloperoxidase; Org-I, organic iodine compound. For clarity, the periplasmic space and outer membrane are not shown.
It is interesting that such a mechanism of iodine uptake is very similar to that proposed for iodine-accumulating marine algae, Laminaria (17, 19, 22). In the early study, Shaw (22) pointed out the importance of iodide oxidation prior to iodine uptake by L. digitata. More recently, Küpper et al. (17) found that an addition of H2O2 or haloperoxidase to algal tissue discs enhanced the uptake of iodine. They proposed that iodide is oxidized to HIO or molecular iodine by a cell wall haloperoxidase and that these oxidized iodine species freely diffuse through the plasma membrane. However, in Laminaria, it is still uncertain how H2O2 is provided for the cell wall haloperoxidase, although involvements of cell wall oxidases, membrane-bound oxidases, mitochondrial respiration, and photosynthesis were proposed (17).
The physiological reason why C-21 takes up and accumulates iodine remains uncertain. As shown in Table 2, various competitive inhibitors did not strongly affect the uptake of iodine. This suggests that the uptake system is specific for iodine and that C-21 does not take up iodine as an analogue of other anions. Iodine is not known to be an essential trace element for bacteria (26), and C-21 is capable of growing in iodine-free media, i.e., Marine broth 2216 and the minimal medium. Thus, it is also unlikely that the uptake of iodine is linked to the assimilation of this element. It is postulated that marine algae take up and accumulate iodine for biosynthesis of volatile iodinated carbons, such as diiodomethane (CH2I2) and chloroiodomethane (CH2ClI), which may act as a chemical defense against herbivores and surface-attached microorganisms (16, 27). Since haloperoxidase catalyzes the production of these compounds, iodine uptake could also take part in the detoxification of H2O2 generated in algal cells by mitochondrial respiration or photosynthesis (17, 19). However, we have previously observed that C-21 grown with iodide produced few volatile iodinated carbons (S. Amachi and Y. Muramatsu, unpublished result). Considering that H2O2 is generated outside of the cells, probably by the membrane-bound glucose oxidase, C-21 should possess certain extracellular H2O2-scavenging systems. Thus, oxidation of iodide to HIO could function as an extracellular detoxification process for the reactive oxygen species. In this case, however, the role of H2O2 generation by glucose oxidase is still unclear. In addition, it seems unnecessary for the cells to take up the oxidation product of iodide (HIO). Therefore, it might be possible that iodine possesses a yet-unidentified internal physiological function in the cells. Further study is needed for a full understanding of the uptake system and possible physiological function of iodine accumulated by C-21.
Acknowledgments
We thank H. Suzuki (Radioisotope Research Center, Chiba University) and Y. Eda (Chiba University) for technical support.
This work was partly supported by a fund from the Forum on Iodine Utilization (FIU) to S.A.
Footnotes
Published ahead of print on 12 October 2007.
REFERENCES
- 1.Alexander, N. M. 1962. A spectrophotometric assay for iodide oxidation by thyroid peroxidase. Anal. Biochem. 4:341-345. [DOI] [PubMed] [Google Scholar]
- 2.Amachi, S., Y. Kamagata, T. Kanagawa, and Y. Muramatsu. 2001. Bacteria mediate methylation of iodine in marine and terrestrial environments. Appl. Environ. Microbiol. 67:2718-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Amachi, S., M. Kasahara, S. Hanada, Y. Kamagata, H. Shinoyama, T. Fujii, and Y. Muramatsu. 2003. Microbial participation in iodine volatilization from soils. Environ. Sci. Technol. 37:3885-3890. [DOI] [PubMed] [Google Scholar]
- 4.Amachi, S., Y. Mishima, H. Shinoyama, Y. Muramatsu, and T. Fujii. 2005. Active transport and accumulation of iodide by newly isolated marine bacteria. Appl. Environ. Microbiol. 71:741-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Amachi, S., Y. Muramatsu, Y. Akiyama, K. Miyazaki, S. Yoshiki, S. Hanada, Y. Kamagata, T. Ban-nai, H. Shinoyama, and T. Fujii. 2005. Isolation of iodide-oxidizing bacteria from iodide-rich natural gas brines and seawaters. Microb. Ecol. 49:547-557. [DOI] [PubMed] [Google Scholar]
- 6.Bichsel, Y., and U. von Gunten. 2000. Hypoiodous acid: kinetics of the buffer-catalyzed disproportionation. Water Res. 34:3197-3203. [Google Scholar]
- 7.Bradford, M. M. 1976. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Chem. 72:248-254. [DOI] [PubMed] [Google Scholar]
- 8.De Boer, E., M. G. M. Tromp, H. Plat, G. E. Krenn, and R. Wever. 1986. Vanadium (V) as an essential element for haloperoxidase activity in marine brown algae: purification and characterization of a vanadium (V)-containing bromoperoxidase from Laminaria saccharina. Biochim. Biophys. Acta 872:104-115. [Google Scholar]
- 9.De La Vieja, A., O. Dohan, O. Levy, and N. Carrasco. 2000. Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol. Rev. 80:1083-1105. [DOI] [PubMed] [Google Scholar]
- 10.Eigen, M., and K. Kustin. 1962. The kinetics of halogen hydrolysis. J. Am. Chem. Soc. 84:1355-1361. [Google Scholar]
- 11.Eskandari, S., D. D. F. Loo, G. Dai, O. Levy, E. M. Wright, and N. Carrasco. 1997. Thyroid Na+/I− symporter: mechanism, stoichiometry, and specificity. J. Biol. Chem. 272:27230-27238. [DOI] [PubMed] [Google Scholar]
- 12.Hetzel, B. S. 1983. Iodine deficiency disorders (IDD) and their eradication. Lancet ii:1126-1129. [DOI] [PubMed] [Google Scholar]
- 13.Hetzel, B. S., and M. T. Mano. 1989. A review of experimental studies of iodine deficiency during fetal development. J. Nutr. 119:145-151. [DOI] [PubMed] [Google Scholar]
- 14.Ingraham, J. L., O. Maaløe, and F. C. Neidhardt. 1983. Growth of the bacterial cell. Sinauer Associates, Sunderland, MA.
- 15.Jordan, P., and H. Vilter. 1991. Extraction of proteins from material rich in anionic mucilages: partition and fractionation of vanadate-dependent bromoperoxidases from the brown algae Laminaria digitata and L. saccharina in aqueous polymer two-phase systems. Biochim. Biophys. Acta 1073:98-106. [DOI] [PubMed] [Google Scholar]
- 16.Küpper, F. C., B. Kloareg, J. Guern, and P. Potin. 2001. Oligoguluronates elicit an oxidative burst in the brown algal kelp Laminaria digitata. Plant Physiol. 125:278-291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Küpper, F. C., N. Schweigert, E. Ar Gall, J.-M. Legendre, H. Vilter, and B. Kloareg. 1998. Iodine uptake in Laminariales involves extracellular, haloperoxidase-mediated oxidation of iodide. Planta 207:163-171. [Google Scholar]
- 18.Kylin, H. 1929. Über das Vorkommen von Jodiden, Bromiden und Jodidoxydasen bei Meeresalgen. Hoppe-Seyler's Z. Physiol. Chem. 186:50-84. [Google Scholar]
- 19.Leblanc, C., C. Colin, A. Cosse, L. Delage, S. La Barre, P. Marin, B. Fiévet, C. Voiseux, Y. Ambroise, E. Verhaeghe, D. Amouroux, O. Donard, E. Tessier, and P. Potin. 2006. Iodine transfers in the coastal marine environment: the key role of brown algae and of their vanadium-dependent haloperoxidases. Biochimie 88:1773-1785. [DOI] [PubMed] [Google Scholar]
- 20.Murphy, C. D., R. M. Moore, and R. L. White. 2000. Peroxidase from marine microalgae. J. Appl. Phycol. 12:507-513. [Google Scholar]
- 21.Rahn, R. O. 1991. Determination of iodide formed from inorganic iodine in aqueous solution. Anal. Chim. Acta 248:595-602. [Google Scholar]
- 22.Shaw, T. I. 1959. The mechanism of iodine accumulation by the brown sea weed Laminaria digitata. The uptake of 131I. Proc. R. Soc. Lond. Ser. B 150:356-371. [DOI] [PubMed] [Google Scholar]
- 23.Smyth, P. P. A., and R. M. Dwyer. 2002. The sodium iodide symporter and thyroid disease. Clin. Endocrinol. 56:427-429. [DOI] [PubMed] [Google Scholar]
- 24.Tamaoku, K., Y. Murao, K. Akiura, and Y. Ohkura. 1982. New water-soluble hydrogen donors for the enzymatic spectrophotometric determination of hydrogen peroxide. Anal. Chim. Acta 136:121-127. [Google Scholar]
- 25.Tong, W., and I. L. Chaikoff. 1955. Metabolism of I131 by the marine alga, Nereocystis luetkeana. J. Biol. Chem. 215:473-484. [PubMed] [Google Scholar]
- 26.Wackett, L. P., A. G. Dodge, and L. B. M. Ellis. 2004. Microbial genomics and the periodic table. Appl. Environ. Microbiol. 70:647-655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wever, R., M. G. M. Tromp, B. E. Krenn, A. Marjani, and M. van Tol. 1991. Brominating activity of the seaweed Ascophyllum nodosum: impact on the biosphere. Environ. Sci. Technol. 25:446-449. [Google Scholar]
- 28.Wong, G. T. F. 1991. The marine geochemistry of iodine. Rev. Aquat. Sci. 4:45-73. [Google Scholar]