Abstract
Helicobacter pylori is an important human pathogen. However, the study of this organism is often limited by a relative shortage of genetic tools. In an effort to expand the methods available for genetic study, an endogenous H. pylori plasmid was modified for use as a transcriptional reporter and as a complementation vector. This was accomplished by addition of an Escherichia coli origin of replication, a kanamycin resistance cassette, a promoterless gfpmut3 gene, and a functional multiple cloning site to form pTM117. The promoters of amiE and pfr, two well-characterized Fur-regulated promoters, were fused to the promoterless gfpmut3, and green fluorescent protein (GFP) expression of the fusions in wild-type and Δfur strains was analyzed by flow cytometry under iron-replete and iron-depleted conditions. GFP expression was altered as expected based on current knowledge of Fur regulation of these promoters. RNase protection assays were used to determine the ability of this plasmid to serve as a complementation vector by analyzing amiE, pfr, and fur expression in wild-type and Δfur strains carrying a wild-type copy of fur on the plasmid. Proper regulation of these genes was restored in the Δfur background under high- and low-iron conditions, signifying complementation of both iron-bound and apo Fur regulation. These studies show the potential of pTM117 as a molecular tool for genetic analysis of H. pylori.
Helicobacter pylori is a microaerophilic, gram-negative bacterium that causes diseases such as gastritis, peptic ulcer disease, mucosa-associated lymphoid tissue lymphoma, and gastric adenocarcinoma. While infection is chronic and often asymptomatic, this bacterium infects over 50% of the world's population (16). The sheer number of infected individuals leads to a significant number of H. pylori-associated disease cases. Moreover, since colonization usually occurs early in childhood and remains throughout the person's life unless he or she is treated (6), the chronicity of the infection increases the likelihood that disease will occur.
H. pylori's success as a pathogen is due in part to the fact that it is well adapted for life in the gastric environment and produces a number of factors that facilitate its survival (16, 41). One of these factors is the ferric uptake regulator (Fur) (3), which has been shown to be important for colonization in the murine and gerbil models of H. pylori infection (7, 20). Fur, which acts as a transcriptional regulator, is crucial for iron homeostasis. While iron is essential for virtually all life forms, too much iron can lead to DNA and cellular damage by interacting with free oxygen to form hydroxyl radicals. Because of this, the regulation of iron uptake and storage is crucial for the bacterium. Thus, critical components in the host-pathogen interaction are the host's attempt to sequester iron from the bacteria and the bacteria's attempt to acquire iron from the host.
Fur functions in H. pylori in much the same way that it does in other bacteria; it binds to specific regions of iron-regulated promoters called “Fur boxes” and represses gene expression when it is bound by iron (Fe2+), an indication of sufficient levels of cellular iron. Not surprisingly, genes regulated in this manner are often involved in iron acquisition. Repression of their expression prevents the deleterious effects of acquiring too much iron. In addition, Fur in H. pylori is known to down regulate another set of genes when it is in its apo form (i.e., Fur is not bound to its iron cofactor). This occurs under iron-depleted conditions and often involves the down regulation of iron storage genes (14). apo Fur regulation has not been identified in any other bacterial species, making the study of Fur in H. pylori of particular interest. Also, Fur has been implicated in the regulation of genes whose functions have no clear link to iron homeostasis and play a role in acid resistance (5, 7, 17, 20, 42), which further emphasizes Fur's global role in stress adaptation and its contribution to the success of H. pylori as a pathogen.
To date, the study of H. pylori has been somewhat limited by the relative lack of genetic tools for the study of this bacterium. For instance, while it is possible to obtain mutations in many strains of H. pylori by allelic replacement (2, 8) and transposon mutagenesis (18, 22, 34), it is often difficult to complement these mutations due to a lack of vectors that efficiently replicate and are stably maintained in the bacterium. One advance in this area occurred when pHP489, a cryptic H. pylori plasmid, was modified for use as an H. pylori-Escherichia coli shuttle vector for genetic analysis of H. pylori (30). In that work, the authors described modification of pHP489 for this purpose and its use as a stable complementation vector (30). This plasmid was the first reported shuttle vector for use in the study of H. pylori and was shown to be useful as a complementation vector (30), although to our knowledge there have been no other reports of its utilization.
Another cryptic plasmid, pHel1, was also modified for use as a genetic tool in this organism (25, 26). This plasmid was modified to create pHel2 and pHel3 (26). In the study of Heuermann and Haas, these plasmids were shown to replicate autonomously in H. pylori. Moreover, the pHel derivatives were presented as useful complementation vectors, although the authors did note that not all attempts at complementation had been successful with the pHel vector system (26). Perhaps in keeping with this, these plasmids are currently not widely used as complementation vectors in the study of H. pylori; instead, many investigators currently achieve complementation by expression of the gene of interest from a nonnative locus within the chromosome. One such popular system described for Helicobacter involves allelic complementation in the rdxA locus, which, when disrupted, confers metronidazole (Mtz) resistance to the bacteria (11, 36). Since rdxA is responsible for the conversion of the nontoxic compound Mtz to the toxic compound hydroxylamine, rdxA mutation results in an inability to convert the nontoxic drug into the toxic by-product (21, 44). In addition to the rdxA system, another system for chromosome-based complementation has recently been described by Langford et al. (28). In this system complementation is achieved by inserting the gene of interest into the intergenic region between the HP0203 and HP0204 genes by use of a suicide plasmid vector containing the intergenic flanking regions, a multiple cloning site (MCS), and a chloramphenicol resistance cassette (28). Despite the definite advances that these tools have provided in the study of H. pylori, the number of complementation strategies available for this organism still lags behind the number of complementation strategies available for many other model organisms.
In addition to the relative lack of systems for complementation, currently there are no convenient systems that allow creation of transcriptional fusions in H. pylori. While lacZ transcriptional fusions have been shown to function in H. pylori (4, 15, 23), the studies required plasmid integration into the bacterial chromosome, thus creating the concern that the integration may have polar effects. In addition to lacZ, green fluorescent protein (GFP) has also been used to monitor gene expression in H. pylori (27). In their study, Josenhans et al. described transcriptional promoter fusions to GFP that were integrated into the bacterial chromosome, requiring complicated strategies for cloning and integration and once again creating the possibility of polar effects. Additionally, these authors described expression of GFP from the pHel2 (27) vector to create fluorescent “marker strains.”
In light of the limited number of genetic tools that are available for the study of H. pylori compared to the number of tools available for other bacterial pathogens, there is a definite need to develop more vectors and methods for investigation of this important pathogen. Therefore, we describe here modification of another endogenous H. pylori plasmid, pHP666 (45), for use both as a GFP reporter plasmid and as a complementation vector. As proof of principle, we show that the new system can be used to complement an H. pylori fur mutation and to study iron regulation by flow cytometry.
MATERIALS AND METHODS
Bacterial strains and growth.
All strains and plasmids used in this study are listed in Table 1, and primer sequences are shown in Table 2. H. pylori strains were maintained as frozen stocks in brain heart infusion medium supplemented with 20% glycerol and 10% fetal bovine serum (Gibco) at −80°C. Bacterial strains were grown on horse blood agar (HBA) plates containing 4% Columbia agar base (EMD Chemicals, Inc.), 5% defibrinated horse blood (HemoStat Laboratories, Dixon, CA), 0.2% β-cyclodextrin (Sigma), 10 μg/ml vancomycin (Amresco), 5 μg/ml cefsulodin (Sigma), 2.5 U/ml polymyxin B (Sigma), 5 μg/ml trimethoprim (Sigma), and 8 μg/ml amphotericin B (Amresco). H. pylori liquid cultures were grown with shaking at 100 rpm in brucella broth supplemented with 10% fetal bovine serum and 10 μg/ml vancomycin at 37°C. Where noted (Table 1), plates and cultures were supplemented with 25 μg/ml kanamycin (Kan) (Gibco) and/or 25 or 8 μg/ml chloramphenicol (Cm) (EMD Chemicals, Inc.). Both plate and liquid cultures were grown in gas evacuation jars under microaerophilic conditions (5% O2, 10% CO2, 85% N2) generated with an Anoxomat gas evacuation and replacement system (Spiral Biotech).
TABLE 1.
Plasmids and strains used in this study
| Plasmid or strain | Descriptiona | Reference |
|---|---|---|
| Plasmids | ||
| pHP666 | Endogenous H. pylori plasmid isolated from CCUG 17874 | 45 |
| pHel2 | E. coli-H. pylori shuttle vector | 26 |
| pTM117 | pHP666 modified to include E. coli origin of replication, aphA-3 cassette (Kanr), MCS, and promoterless gfpmut3 | This study |
| pDSM221 | pTM117 amiE promoter::gfpmut3 fusion | This study |
| pDSM368 | pTM117 pfr promoter::gfpmut3 fusion | This study |
| pDSM227 | fur::pHel2 complementation vector | This study |
| pDSM340 | fur::pTM117 complementation vector | This study |
| H. pylori strains | ||
| G27 | WT H. pylori | 10 |
| DSM145 | G27 Δfur::aphA-3, Kanr (25 μg/ml) | 20 |
| DSM300 | G27 Δfur::cat, Cmr (25 or 8 μg/ml) | This study |
| DSM215 | G27(pTM117), Kanr (25 μg/ml) | This study |
| DSM235 | G27(pDSM221), Kanr (25 μg/ml) | This study |
| DSM305 | DSM300(pDSM221), Kanr Cmr (25 and 25 μg/ml) | This study |
| DSM369 | G27(pDSM368), Kanr (25 μg/ml) | This study |
| DSM370 | DSM300(pDSM368), Kanr Cmr (25 and 25 μg/ml) | This study |
| DSM279 | G27(pDSM227), Cmr (8 μg/ml) | This study |
| DSM281 | DSM145(pDSM227), Kanr Cmr (25 and 8 μg/ml) | This study |
| DSM341 | G27(pDSM340), Kanr (25 μg/ml) | This study |
| DSM343 | DSM300(pDSM340), Kanr Cmr (25 and 8 μg/ml) | This study |
Appropriate antibiotic concentrations are indicated in parentheses.
TABLE 2.
Primers used in this study
| Primerb | Sequence (5′-3′)a | Reference |
|---|---|---|
| pTM117 construction primers | ||
| F666NotI | ATAAGAATGCGGCCGCAGAGATTGAAACAGACTATTTAGAAAA | This study |
| R666NotI | ATAAGAATGCGGCCGCTCATTATATTTTCTAACTCACTCTCTC | This study |
| BR322-F (PstI, HpaI) | AAAACTGCAGTTAACTCCCGCCGCATCCATACCGCC | This study |
| BR322-R (NotI) | ATAAGAATGCGGCCGCCACTGAGCGTCAGACCCCGTA | This study |
| AphA-F (XbaI, BamHI, SmaI, KpnI, SacII) | GATTTCTAGAGGATCCCCGGGTACCGCGGTCGATACTATGTTATACGCC | This study |
| AphA-R (NotI) | ATAAGAATGCGGCCGCAGACATCTAAATCTAGGTACTA | This study |
| GF1 (XbaI) | GATTTCTAGATTTAAGAAGGAGATATACATATGAGTAAAGGAGAAG | 9 |
| GFP-R (PstI) | AAAACTGCAGGTCTGGACATTTATTTGTATAG | This study |
| Sequencing primers | ||
| gfp-1 | AAGTCGTGCTGCTTCATGTG | This study |
| gfp-F1 | CCTGTTCCATGGCCAACACTTG | This study |
| gfp-F1a | CCAGACCTGCAGTTAACTC | This study |
| gfp-F2 | CCACGCTGATGAGCTTTAC | This study |
| gfp-F3 | GGTAAGACACGACTTATCG | This study |
| gfp-F4 | GCTTTCAAGCAAGTAATTC | This study |
| apha3 | GAAAGAGCCTGATGCACTCC | This study |
| apha3-2 | CGGTGATATTCTCATTTTAGCC | This study |
| apha3-R1 | GGCTGGAGCAATCTGCTCATG | This study |
| apha3-R2 | GGAAGAACAGTATGTCGAG | This study |
| apha3-R2a | CGTGTTCTTGCATAAAGGTTG | This study |
| apha3-R3 | GCTCTCTTTCAAGTTCAAGG | This study |
| BB-F1 | GCGGTGTTGTCAATCTGC | This study |
| BB-F2 | GCTAGGTTTGTTCAATTC | This study |
| BB-F3 | CGGTATAATAGTGCAAGTC | This study |
| BB-F4 | GGTTTGCGCTATCTTGTTTG | This study |
| BB-F5 | CCGTATAATGCTTAAAGAC | This study |
| BB-R1 | GGAGCTGTTGATATTTCTC | This study |
| BB-R2 | GGATAAGCACCCTACACATG | This study |
| BB-R3 | CCGTTCTTTGTAGCTACAC | This study |
| BB-R4 | GGTTGCTTAGGTTAGGTAG | This study |
| BB-R5 | CCACTCAGGAAATTTGAG | This study |
| Promoter::gfp fusion primers | ||
| amiE-F (SacII) | CCGCGGGCATGCACCTTTGAAATTGC | This study |
| amiE-R (BamHI) | GGATCCGCTGCTGCTACTAATATCTCCATG | This study |
| HP0653_Promoter_F (SacII) | CCGCGGTGGTTAAATTGCCCTTTCGT | This study |
| HP0653_Promoter_R (BamHI) | GGATCCGATAACATAGTATCTCCTTTGTGTTGG | This study |
| Complementation primers | ||
| FurCF (XbaI) | TCTAGAAAGGCTCACTCTACCCTATT | This study |
| FurCR (SalI) | GTCGACAAGACTTTCACCTGGAAACGC | This study |
| RPA primers | ||
| amiE-RPA-F | GGTTTGCCTGGGTTGGAT | 20 |
| amiE-RPA-R | GATTTTGCGGTATTTTG | 20 |
| pfr-RPA-F | GCGGCTGAAGAATACGAG | This study |
| pfr-RPA-R | CTGATCAGCCAAATACAA | This study |
| fur-RPA-F | GAGCGCTTGAGGATGTCTATC | This study |
| fur-RPA-R | GTGATCATGGTGTTCTTTAGC | This study |
Restriction endonuclease sites are underlined, and linker bases are in bold type.
Important restriction sites are indicated in parentheses.
All H. pylori strains in this work are derivatives of G27 (10). Two isogenic fur (HP1027) mutants, strains DSM145 (Δfur1) and DSM300 (Δfur2), were utilized in this study. DSM145 was described previously and contains a deletion insertion of the fur coding sequence with the aphA-3 gene (conferring Kan resistance) from Campylobacter coli (20). To create DSM300, the aphA-3 gene was removed from the original pΔHP1027-K7 suicide vector and was replaced by the chloramphenicol acetyltransferase (cat) gene from C. coli. This was accomplished by digestion of pΔHP1027-K7 with ClaI (Invitrogen) to remove the aphA-3 gene, followed by replacement with the NarI (New England Biolabs)-digested cat gene. The cat gene had been amplified from the pGPS-cat vector (34) with primers catF-nar and catR-nar, which contain NarI restriction sites. NarI and ClaI have compatible sticky ends, and thus their digestion products can be ligated. The ΔHP1027::cat construct was then naturally transformed into wild-type (WT) strain G27 to generate DSM300.
pTM117 construction.
pHP666, an endogenous H. pylori plasmid (45), was used as the backbone for pTM117. Although not specifically described in the work of Xiang et al., pHP666 was isolated in a 1995 study by these authors (45). The entire pHP666 sequence was amplified by PCR with primers F666NotI and R666NotI, which incorporated unique NotI sites for subsequent cloning steps. To facilitate replication of the plasmid in E. coli, the origin of replication and the rop gene from pBR322 (35) were amplified with primers BR322-F (PstI and HpaI sites) and BR322-R (NotI site), which incorporated the indicated restriction sites. The aphA-3 gene from C. coli was also amplified using primers aphA-F (XbaI, BamHI, SmaI, KpnI, and SacII sites) and aphA-R (NotI site) from pIP1433 (39). The aphA-F primer was designed to incorporate an MCS into its fragment, which eventually generated the MCS for pTM117. Finally, primers GF1 (XbaI site) and GFP-R (PstI site) were used to amplify a promoterless GFP mutant 3 (gfpmut3) derivative (9). Each resulting PCR fragment was restriction digested using standard procedures as follows: pHP666 was digested with NotI (New England Biolabs), the E. coli origin of replication was digested with PstI (Invitrogen)/NotI, aphA3 was digested with XbaI (Invitrogen)/NotI, and gfpmut3 was digested with XbaI/PstI. The fragments were all gel purified using a Qiagen gel purification kit and subsequently joined in a four-part ligation procedure. The resulting ligation products were transformed into E. coli DH5α, and transformants were selected with Kan. Plasmid isolates were restriction digested to confirm the presence of all four fragments, and one construct was designated pTM117. pTM117 was introduced into H. pylori via natural transformation with 1 to 1.5 μg of plasmid DNA as described previously (33), and transformants were selected on HBA plates containing Kan. Strain DSM215 is H. pylori WT strain G27 bearing pTM117.
Sequencing of pTM117.
To verify the complete sequence of pTM117, the plasmid was sequenced with each of the primers listed in Table 2. Sequencing reactions were performed using the BigDye Terminator 3.0 reagent (Applied Biosystems, Inc.) according to the manufacturer's directions with 345 ng of pTM117 and 30 pmol of primer (1 μl of 30 μM primer). The sequencing reaction protocol consisted of 25 cycles of 96°C for 30 s, 50°C for 15 s, and 60°C for 4 min. Sequencing reaction mixtures were cleaned using Performa DTR gel filtration cartridges (Edge Biosystems) and an Eppendorf 5415D centrifuge; columns were spun for 2 min at 1.5 × g, and samples were added and eluted for 1 min at the same speed. The reactions were performed with a 3130XL genetic analyzer (Applied Biosystems, Inc.), and the results were analyzed using the Invitrogen Vector NTI 10.0 software. Each sequencing reaction for each primer was independently performed three times, and all sequencing results were assembled to generate a 3× pass of the final vector sequence of pTM117.
Creation of promoter fusions.
Transcriptional fusions of amiE (HP0294) and pfr (HP0653) to the promoterless gfpmut3 gene in pTM117 were constructed by amplification of the promoters of each gene using primers amiE-F and amiE-R (319-bp product) and primers HP0653_Promoter_F and HP0653_Promoter_R (305-bp product), respectively. These primers incorporated SacII and BamHI restriction sites at the 5′ and 3′ ends, respectively, to facilitate directional ligation into pTM117. Each promoter fragment was subcloned into pGEM-T Easy (Promega), digested with SacII (New England Biolabs) and BamHI (Invitrogen), and ligated to appropriately digested pTM117 to create pDSM221 (amiE::gfpmut3) and pDSM368 (pfr::gfpmut3). Each promoter fusion was confirmed by sequencing with the apha3-2 primer. These plasmids were moved into WT strain G27 and DSM300 by natural transformation, and transformants were selected on plates containing 25 μg/ml Kan and 25 μg/ml Kan plus 25 μg/ml Cm, respectively. WT strain G27 containing pDSM221 was designated strain DSM235, and strain DSM300 containing pDSM221 was designated strain DSM305. Likewise, WT strain G27 containing pDSM368 was designated strain DSM369, and strain DSM300 containing pDSM368 was designated strain DSM370.
GFP expression reporter assays.
Flow cytometry was utilized to assess the ability of the amiE and pfr promoters to drive the expression of GFP in the corresponding plasmid constructs. Strains DSM235 and DSM369 were grown for 48 h in liquid culture as described above with and without 60 μM 2,2′-dipyridyl (dpp) (Sigma), an iron chelator. This concentration of the chelator was sufficient to slow but not severely hinder bacterial growth. For comparison, DSM305 and DSM370 were grown for 48 in liquid culture media without dpp. Then 1.5 ml of each culture was pelleted and resuspended in 1× sterile phosphate-buffered saline. The resuspended cultures were passed through a 1.2-μm Acrodisc PSF syringe filter (Pall) to remove any bacterial clumps or debris. The samples were then analyzed using a Beckman Coulter Epics XL-MCL flow cytometer. The laser was set at 750 V, and the instrument collected 100,000 events. Flow cytometry data were analyzed using WinList 3D, version 6.0 (Verity Software House).
Creation of Fur complementation vectors.
A 923-bp WT copy of the fur (HP1027) gene was amplified with primers FurCF (XbaI site) and FurCR (SalI site) and subcloned into pGEM-T Easy. The product encompassed the entire Fur coding sequence, as well as the predicted promoter region (13). The pHel2 complementation vector was constructed by liberating the fur gene from pGEM-T Easy by SalI (New England Biolabs) digestion (there was a SalI site within the vector's backbone). pHel2 was similarly digested and treated with calf intestinal phosphatase (New England Biolabs) to prevent pHel2 self-ligation (26). The SalI-digested fur fragment was then ligated to pHel2 to create pDSM227. The resulting plasmid was transformed into WT strain G27 and strain DSM145 (ΔHP1027 Kanr). These transformations yielded strains DSM279 and DSM281, respectively. Additionally, the fur gene was liberated from pGEM-T Easy by digestion with PstI and SacII (sites within the pGEM-T Easy vector MCS) and was ligated to appropriately digested pTM117, generating pDSM340 (digestion of pTM117 with PstI and SacII resulted in removal of the promoterless gfpmut3 gene). pDSM340 was sequenced with the FurCR (SalI site) primer to confirm that the correct construct was obtained. pDSM340 was then transformed into WT strain G27 and DSM300, and transformants were selected using the appropriate antibiotics (Table 1). DSM341 (pDSM340 in WT strain G27) and DSM343 (pDSM340 in strain DSM300) were the strains resulting from these transformations.
RPAs.
RNase protection assays (RPAs) were used to assess the ability of pDSM340 and pDSM227 to complement a fur deletion. Strains G27, DSM145, DSM300, DSM341, DSM343, DSM279, and DSM281 were grown overnight in liquid cultures with the appropriate antibiotics to maintain selection for the plasmids. The pHel2 fur complementation strains, DSM279 and DSM281, were grown with 8 μg/ml Cm and with 8 μg/ml Cm plus 25 μg/ml Kan, respectively. The pTM117 fur complementation strains, DSM341 and DSM343, were grown with 25 μg/ml Kan and 25 μg/ml Kan plus 8 μg/ml Cm, respectively. One half of each culture was removed for RNA isolation (time zero), while the other half was exposed to 200 μM dpp for 1 h. Addition of 200 μM dpp causes rapid chelation of the iron from the medium and results in an iron-depleted environment (31). RNA was extracted as previously described (37), and 1.5 or 2.0 μg of RNA was used in each RPA. Riboprobe templates for amiE, pfr, and fur were generated by PCR using the primer pairs listed in Table 2. The templates were ligated to pGEM-T Easy, and their orientations were subsequently determined by PCR. Probes were generated using a Maxiscript kit (Promega) and 50 μCi [32P]UTP (Perkin-Elmer), and RPAs were performed with an RPA III kit (Ambion) as previously described (20). RPA mixtures were resolved on 5% acrylamide-1× Tris-borate-EDTA-8 M urea denaturing gels, and the gels were exposed to Kodak phosphor screens. The phosphor screens were scanned using an FLA-5100 multifunctional scanner (Fujifilm) and were analyzed using the ImageGauge software (version 4.22; Fujifilm).
Plasmid copy number.
Southern blot analysis of total DNA was used to determine the plasmid copy number as previously described (26). Briefly, total genomic DNA was isolated from WT strain G27, DSM341, and DSM343 using an Easy DNA kit (Invitrogen). The DNA was digested with NotI or NotI and HindIII, which liberated the fur gene from pTM117 and infrequently cut the chromosomal DNA. Southern blotting was performed using an ECL direct nucleic acid labeling and detection system kit (Amersham Biosciences) according to standard procedures, with probing with end-labeled fur. The relative numbers of copies of fur present in WT strain G27, DSM341, and DSM343 were determined using an FLA-5100 multifunctional scanner (Fujifilm) and the ImageGauge software (version 4.22; Fujifilm). In addition, this experiment was repeated using WT strain G27, DSM235, and DSM305 total genomic DNA cut with NotI and probing for the amiE promoter.
Plasmid stability studies.
Strain DSM215 was cultured overnight in liquid medium supplemented with 25 μg/ml Kan. The resulting day 0 cultures were used to inoculate day 1 cultures in liquid medium without Kan. The day 1 cultures were grown overnight and subcultured into fresh liquid medium without Kan at a 1:20 dilution. This reinoculation cycle was repeated through the start of day 5 liquid cultures. Samples of the cultures from days 0, 1, 3, and 5 were plated on HBA plates to obtain single colonies. Four days later the colonies were replica plated onto HBA plates and HBA plates supplemented with 25 μg/ml Kan. Two days after the replica plating, the HBA plates supplemented with Kan were compared to the unsupplemented HBA plates to identify any Kan-sensitive colonies and to assess the stability of the pTM117 plasmid upon repeated passage in liquid medium in the absence of selection. This experiment was repeated as described above using strains DSM341 and DSM343 (pTM117 fur complementation vector in the WT strain G27 and Δfur backgrounds, respectively) with daily reinoculation until the start of day 3.
Nucleotide sequence accession numbers.
The nucleotide sequences of pHP666, pTM117, the strain G27 amiE promoter, and the strain G27 pfr promoter have been deposited in the GenBank database under accession numbers DQ198799, EF540942, EF537053, and EF537052, respectively.
RESULTS
Construction of pTM117.
Since there is a relative lack of genetic tools for use with H. pylori and since currently there is only one plasmid system that is currently used for this organism (26), we sought to create an additional system to expand the repertoire of available genetic tools. Since the pHel system (26) had been shown to work well in some but not all H. pylori strains, we decided to adopt a similar strategy to develop our system, namely, modification of an endogenous H. pylori plasmid. However, to potentially expand the diversity of H. pylori strains in which the plasmid would be useful, we sought to use a plasmid backbone that was not too closely related to the pHel system. We had previously isolated and sequenced pHP666 from strain CCUG 17874 (45). Comparison of pHP666 to pHel1 (GenBank accession numbers DQ198799 and Z49272, respectively) revealed that the only significant conservation between the two plasmids was in the open reading frame predicted to encode RepA. The RepA replicase seems to be very well conserved among virtually all characterized H. pylori endogenous plasmids. Further analysis of the predicted origins of replication of pHP666 and pHel1 suggested that they are different since the iterons are not conserved. Given these distinct differences, we reasoned that the pHP666 backbone should be a good candidate for modification.
Our strategy was formulated with the idea that the resulting vector could be used to create transcriptional fusions to GFP, as well as serve as a shuttle vector for complementation. To this end, we constructed pTM117 as described in Materials and Methods. Briefly, this vector contains the origin of replication from pBR322 (35), an aphA-3 cassette from C. coli (39), and a promoterless gfpmut3 allele (9). These factors allow plasmid replication in E. coli, selection on Kan in E. coli and H. pylori, and creation of transcriptional fusions to enhanced GFP to monitor promoter activity in H. pylori, respectively. A pTM117 vector map is shown in Fig. 1.
FIG. 1.
Physical and genetic map of E. coli-H. pylori shuttle vector pTM117. pHP666 comprises the backbone of the pTM117 vector and contains the H. pylori origin of replication. Kan resistance is encoded by the aphA-3 gene, and an MCS lies upstream of the promoterless gfpmut3 allele. Unique multiple cloning restriction enzyme sites are present in the indicated area upstream of gfpmut3. Predicted open reading frames (with the designations of the closest gene homologues) are represented by arrows, and the directions of transcription are indicated by the directions of the arrows.
pTM117 GFP reporter assays.
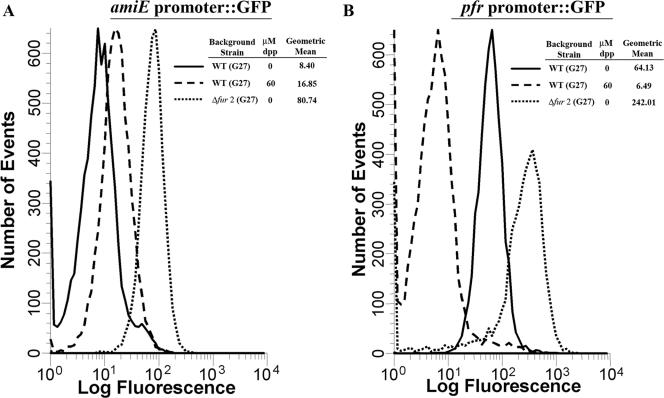
pTM117 was engineered with the idea that promoters of interest could easily be studied by transcriptionally fusing them to the promoterless gfpmut3 gene within the vector. In this way, relative levels of fluorescence could be used as a reporter of promoter activity, and GFP expression could be monitored by flow cytometry. To determine if pTM117 could be utilized in this fashion, amiE and pfr promoter fusions were constructed. These promoters were chosen since a number of studies have shown that they are regulated by Fur inversely; amiE is repressed by iron-bound Fur (43), and pfr is repressed by apo Fur (14, 43). Although we were unable to detect GFP expression using a hand-held UV light, examination of H. pylori strains carrying either of these fusions by fluorescence microscopy confirmed that there was detectable GFP fluorescence (data not shown). Additionally, flow cytometry showed distinct peaks of fluorescence that were significantly higher than the background (i.e., the fluorescence of an isogenic strain carrying a promoterless plasmid) (Fig. 2 and data not shown). We next examined whether we could visualize iron- and Fur-dependent regulation of amiE and pfr. Based on previously published results, we expected to see an increase in GFP expression driven by the amiE promoter (43) and a decrease in pfr promoter-driven GFP expression (14) when bacteria were iron limited. Changes in GFP expression under the control of the promoter of amiE are shown in Fig. 2A, and alterations in GFP expression under the control of the pfr promoter are shown in Fig. 2B. As expected, amiE promoter-driven expression was increased by iron limitation, while pfr promoter-driven expression was decreased. Moreover, both promoter fusions were deregulated when they were carried in a Δfur H. pylori strain. These data suggested that pTM117 can be used to create transcriptional fusions to GFP, which can then be monitored by flow cytometry.
FIG. 2.
Flow cytometry analysis of pTM117 GFP transcriptional fusions. Strains bearing amiE::gfpmut3 or pfr::gfpmut3 promoter fusions were grown in iron-replete or -depleted medium for 48 h, and fluorescence was analyzed as described in Material and Methods. (A) Results for the amiE::gfpmut3 fusions. (B) Results for the pfr::gfpmut3 fusions. In both panels, the solid line indicates the results for the plasmid in H. pylori WT strain G27 grown in iron-replete conditions, the dashed line indicates the results for the plasmid in WT bacteria grown in iron-depleted conditions, and the dotted line indicates the results for the plasmid in Δfur bacteria grown in iron-replete conditions. Fluorescence is expressed in relative units, and the data are representative of multiple independent flow analyses.
Utilization of pTM117 for complementation.
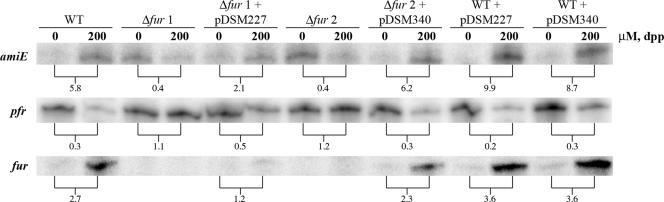
Since complementation in trans is often difficult in H. pylori, we next assessed whether pTM117 could be used as a complementation vector. In these studies, we examined complementation of a fur mutation. For comparison, we concurrently constructed a fur complementation vector using pHel2 (26). RPAs were then used to assess the abilities of both vectors to complement iron-bound and apo Fur regulation in Δfur G27. Once again, we monitored amiE and pfr expression as these genes are iron bound and apo Fur regulated, respectively. We also analyzed fur expression in these strains to compare the basal levels of fur expression in these strains under normal and iron-chelated conditions and to assess the ability of the fur-bearing vectors to complement the iron-bound autoregulation of Fur. RNA was isolated for each test (complemented) and control (WT and Δfur) strain prior to and after an additional hour of exposure to 200 μM dpp. This concentration of dpp rapidly chelates the available iron and does not affect the viability of bacterial cells (31). As shown in Fig. 3 and as expected, addition of dpp to WT bacteria resulted in a large increase in amiE expression (5.8-fold). This increase was not seen in the Δfur strains. When WT bacteria carried an additional copy of the fur gene in the context of pHel2 or pTM117, we again saw a large increase in amiE expression upon exposure to dpp. Iron-bound Fur regulation of amiE was partially restored in the fur mutant carrying pHel2 (2.1-fold) and was fully restored in the strain carrying pTM117 (6.2-fold). These data suggest that while either pHel2 or pTM117 can be used to complement iron-bound Fur regulation in a fur-deficient strain, full complementation is achieved only with pTM117.
FIG. 3.
Determination of the ability of pTM117 and pHel2 to complement iron-bound and apo Fur regulation. The indicated strains were grown overnight in iron-replete liquid medium. On the following day, one half of each culture was used for RNA isolation. The other half was exposed to iron-depleted conditions for 1 h by addition of 200 μM dpp prior to isolation of the RNA. In the top panel, an amiE riboprobe was used to determine iron-bound Fur complementation. In the middle panel, a pfr riboprobe was used to quantitate apo Fur complementation. In the bottom panel, a fur riboprobe was used to quantitate Fur complementation. Fold changes are indicated below each pair and were calculated by comparing the relative amount of protected riboprobe in the iron-depleted environment (200 μM dpp) to the relative amount in the iron-replete lane (0 μM dpp). The data are representative of multiple independent experiments.
To determine if both vectors could also complement apo Fur regulation, we next monitored pfr expression. As expected, addition of dpp to WT bacteria resulted in a decrease in pfr expression (0.3-fold), as shown in Fig. 3. Once again, this change in expression was not seen in the Δfur mutants. WT bacteria carrying both complementation vectors exhibited the expected decrease in pfr expression upon iron chelation. Once again, fur carried on pHel2 showed partial complementation (0.5-fold), while the pTM117 derivative showed full complementation of apo regulation (0.3-fold). In order to verify that there were no mutations in the pHel2 and pTM117 constructs that could account for the difference in the levels of complementation, we sequenced the fur insert from both vectors (data not shown). No mutations in either the promoter or coding sequence of fur in either vector were identified; thus, the complementation differences were not due to introduced mutations.
In order to assess the ability of the complementation vectors to complement fur autoregulation and to compare the basal levels of fur expression in the strains, RPAs were also performed using a fur riboprobe. Addition of dpp to WT bacteria resulted in a 2.7-fold increase in fur expression, as shown in Fig. 3. This increase was what was expected based on previous findings (12, 13). In the Δfur background, pTM117 was able to restore fur regulation to a level similar to that in WT bacteria (2.3-fold), while fur complementation with pHel2 resulted in only a modest increase in fur expression under iron-chelated conditions. This mirrors the trends seen with both amiE and pfr; i.e., we achieved partial complementation with pHel2 and full complementation with pTM117 (Fig. 3). Taken together, the RPA data indicate that pTM117 can be used as an effective complementation vector.
Plasmid characterization.
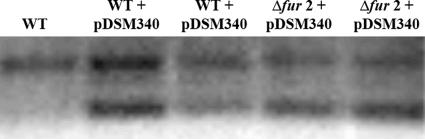
To expand our understanding of pTM117 and its derivatives, we next examined some of the basic vector characteristics to determine the copy number and plasmid stability. The copy number was determined using Southern blot analysis to examine the relative copy number of fur found in the chromosome (one copy) compared to the copy number on the plasmid in strains carrying the pTM117 fur complementation vector. Additionally, since we reasoned that the relative copy number of the plasmid could be artificially depressed if carrying too many copies of fur was deleterious to the bacteria, we examined the relative copy number of the amiE promoter found on the chromosome compared to the copy number on the plasmids in strains carrying the amiE::gfpmut3 transcriptional fusions. In each case, we determined that there were one or two copies of pTM117 per bacterial cell (Fig. 4 and data not shown), suggesting that pTM117 is a very-low-copy-number plasmid.
FIG. 4.
Southern blot analysis to determine plasmid copy number. Total genomic DNA was isolated from the indicated strains, digested with NotI and HindIII, and subjected to Southern blot analysis with an end-labeled fur probe. The top band represents the chromosomal copy of fur, while the bottom band represents the plasmid-borne copy of the gene. The data are representative of multiple independent experiments.
To determine plasmid stability, we serially passaged WT strain G27 carrying pTM117 for up to 5 days in the absence of antibiotic selection. The results of three independent experiments (experiments A to C) are shown in Table 3. This table shows the percentage of colonies that remained Kan resistant when cultures were grown in the absence of antibiotics for the indicated times relative to the results for the day 0 starting inocula. For each experiment, we consistently obtained >99% stability for each passage. Similar stability rates were also found when we used the pTM117 fur complementation plasmid in the WT strain G27 and Δfur backgrounds (data not shown). These data demonstrate that pTM117 and its derivatives are stable in the absence of antibiotic selection.
TABLE 3.
Percentages of H. pylori Kanr CFU in the absence of Kan selectiona
| Expt | % H. pylori Kanr CFU
|
||
|---|---|---|---|
| Day 1 | Day 3 | Day 5 | |
| A | 99.87 | 99.30 | 99.73 |
| B | 100 | 99.71 | 100 |
| C | 99.98 | 99.97 | NDb |
The data are the results of three independent experiments and indicate the percentage of Kanr colonies based on the total number of colonies on the indicated days.
ND, not determined.
Finally, we investigated whether pTM117 remained episomal within the H. pylori cell. We reasoned that if pTM117 was integrated into the chromosome, then we should be unable to recover the plasmid from H. pylori strains bearing the pTM117 fusions. Therefore, we prepared plasmids from H. pylori strains bearing pTM117 (empty vector), as well as strains bearing the pfr and amiE reporter fusions and the fur complementation vector (from both WT and Δfur G27). The resulting DNA was then used to transform E. coli. In each case we recovered Kan-resistant E. coli strains that bore the pTM117 plasmid derivatives, showing that pTM117 does remain episomal in the H. pylori cell. These data are supported by the fact that we obtained bands of the expected sizes for chromosomal and plasmid-borne fragments by Southern blotting (Fig. 4).
DISCUSSION
There are few tools for genetic study of H. pylori compared to the tools that are available for many other bacteria, and given that H. pylori infection is the most common bacterial infection of humans, there is a real need for continued study of this pathogen. Here we describe creation of pTM117, its use as a GFP transcriptional reporter, and its use as a complementation vector. We chose pHP666 (45) as the backbone for our system based on the previous successes in modification of cryptic plasmids for use in H. pylori (26, 30). Moreover, since pHP666 appears to be present in a large number of H. pylori strains (45), we reasoned that it might be broadly useful. pTM117 was therefore created by addition of the apha-3 gene for selection on Kan in E. coli and H. pylori, the promoterless gfpmut3 allele for creation of transcriptional fusions to the enhanced GFP protein to monitor promoter activity in H. pylori, and an E. coli origin of replication to allow replication of the plasmid in this organism. Basic characterization of pTM117 showed that it is a low-copy-number vector which is stably maintained in G27.
Using two well-studied Fur regulated promoters, amiE and pfr, we found that pTM117 can be used as a GFP reporter plasmid. As our plasmid design incorporated an MCS upstream of the promoterless gfpmut3, in theory, virtually any promoter of interest could be cloned into pTM117 and GFP expression could be monitored as a reporter of transcriptional activity. While flow cytometry has been broadly used in other bacteria, to our knowledge, this is the first time that this technique has been used to analyze GFP expression in H. pylori. Previously, GFP expression in H. pylori has been monitored by fluorescent microscopy (26) or by Western blotting (27). In these previous studies GFP was expressed from the flaA (27) or flaB (26) promoters in the context of pHel2 and from the flaA or flaB promoters by chromosomal integration into these loci (27). pTM117 is the first easily usable transcriptional reporter plasmid designed for use in H. pylori. We predict that this system can be used to monitor the activity of most clonable H. pylori promoters, and it should be possible to use it for differential fluorescence induction (40) to monitor changes in expression of promoters of interest upon exposure to diverse environmental conditions. To this end, our laboratory has successfully made cagA, vacA, ureA, and napA fusions to gfpmut3. Moreover, differential fluorescence induction has been performed with strains carrying these reporters in an effort to identify regulators of these important virulence factors (K. Jones and D. S. Merrell, unpublished data).
There are many advantages of using flow cytometry to study bacterial pathogenesis; the main advantage is that flow cytometry is very efficient and allows large numbers of samples to be analyzed in a relatively short period of time. Additionally, sample preparation is simple. For workers not versed in flow cytometry or requiring alternative antibiotic selection, it is conceivable that the GFP reporter and the aphA-3 cassette from pTM117 could readily be replaced by other reporter genes and resistance markers, respectively. For instance, lacZ or luciferase could likely replace the gfpmut3 gene, and a Cm resistance gene has been shown to function in place of the aphA-3 cassette (N. Salama, personal communication). Thus, pTM117 can be further adapted to suit the needs of the individual researcher and project. Taken together, these data suggest that pTM117 could be a useful tool for the study of gene regulation in H. pylori.
There are two basic methods for achieving complementation within bacteria: complementation from a nonnative locus within the chromosome and complementation by a gene carried on a plasmid. In H. pylori, disruption of rdxA has been described as a way of complementing genes within the chromosome (11). While this method clearly takes advantage of the inherent properties of the rdxA locus, it nonetheless has a few potential confounders. First, Mtz is a mutagen, and prolonged exposure to this drug likely results in second-site mutations within the chromosome. Second, the rate of spontaneous Mtz resistance is often very high; it can range from 2.6 × 10−5 to 3.5 × 10−8 for various strains (24). Such a high rate could potentially require screening of hundreds of transformants to identify one that is Mtz resistant due to complementation within the rdxA locus. Therefore, while complementation within the rdxA locus can be beneficial, these potential confounders make it less than ideal. Thus, there is a real need for plasmid systems in H. pylori that can be used for complementation.
We have shown that pTM117 can be used to complement both iron-bound and apo Fur regulation in a Δfur mutant. Additionally, the pHel2 vector was able to partially complement iron-bound and apo Fur regulation in a Δfur background, although to a lesser extent than pTM117. The difference can be partially explained by the levels of fur expression obtained for each of the vectors. Less fur is expressed from pHel2 than from pTM117, as shown by the fainter bands in the pHel2 lanes of Fig. 3 (bottom panel) than in the WT lane and the pTM117 lanes. This is intriguing since pHel2 is predicted to have a higher copy number (four copies per cell [26] compared to the one to two copies of pTM117 per cell). Sequencing of the promoter regions and fur genes carried on both plasmids revealed that they were both identical to WT fur. Thus, the reason for this trend is currently unclear.
For pTM117 to be a truly useful tool for the study of H. pylori, ideally it would be utilizable in H. pylori strains in addition to strain G27. To this end, we have successfully transformed pTM117 or one of its derivatives into the gerbil-colonizing strain B128 (isolate 7.13 [19]), the sequenced strain HPAG1 (32), and the monkey-colonizing strain J166 (isolate 316-3.4; J. Solnick Lab Collection). In addition, pTM117 or its derivatives have successfully been moved into strains SS1 (29), 26695 (38), and J99 (1; N. Salama and K. Ottemann, personal communication). This fact, combined with the results described here, indicates that pTM117 should be a useful option for complementation and transcriptional studies in a wide variety of strain backgrounds.
Acknowledgments
Research in the laboratory of D. Scott Merrell is supported by grant R073LA from USUHS and by grant AI065529 from the NIH. We thank Lucy Tompkins for generous monetary support from her NIH grant.
We thank K. Jones for her help in developing the flow cytometry protocol, S. Miles for sequencing assistance, N. Ami-rav for technical assistance, N. Salama, K. Ottemann, K. Guillemin, and D. Berg for useful discussions, A. Covacci for his continued support and advice, A. Camilli for critical reading of the manuscript, members of the Falkow lab for useful discussions, and J. Solnick for providing J166.
Footnotes
Published ahead of print on 5 October 2007.
REFERENCES
- 1.Alm, R. A., L. S. Ling, D. T. Moir, B. L. King, E. D. Brown, P. C. Doig, D. R. Smith, B. Noonan, B. C. Guild, B. L. deJonge, G. Carmel, P. J. Tummino, A. Caruso, M. Uria-Nickelsen, D. M. Mills, C. Ives, R. Gibson, D. Merberg, S. D. Mills, Q. Jiang, D. E. Taylor, G. F. Vovis, and T. J. Trust. 1999. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature 397:176-180. [DOI] [PubMed] [Google Scholar]
- 2.Bauerfeind, P., R. M. Garner, and L. T. Mobley. 1996. Allelic exchange mutagenesis of nixA in Helicobacter pylori results in reduced nickel transport and urease activity. Infect. Immun. 64:2877-2880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bereswill, S., F. Lichte, T. Vey, F. Fassbinder, and M. Kist. 1998. Cloning and characterization of the fur gene from Helicobacter pylori. FEMS Microbiol. Lett. 159:193-200. [DOI] [PubMed] [Google Scholar]
- 4.Bereswill, S., R. Schonenberger, A. H. van Vliet, J. G. Kusters, and M. Kist. 2005. Novel plasmids for gene expression analysis and for genetic manipulation in the gastric pathogen Helicobacter pylori. FEMS Immunol. Med. Microbiol. 44:157-162. [DOI] [PubMed] [Google Scholar]
- 5.Bijlsma, J. J., B. Waidner, A. H. Vliet, N. J. Hughes, S. Hag, S. Bereswill, D. J. Kelly, C. M. Vandenbroucke-Grauls, M. Kist, and J. G. Kusters. 2002. The Helicobacter pylori homologue of the ferric uptake regulator is involved in acid resistance. Infect. Immun. 70:606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaser, M. J. 1990. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J. Infect. Dis. 161:626-633. [DOI] [PubMed] [Google Scholar]
- 7.Bury-Mone, S., J. M. Thiberge, M. Contreras, A. Maitournam, A. Labigne, and H. De Reuse. 2004. Responsiveness to acidity via metal ion regulators mediates virulence in the gastric pathogen Helicobacter pylori. Mol. Microbiol. 53:623-638. [DOI] [PubMed] [Google Scholar]
- 8.Chalker, A. F., H. W. Minehart, N. J. Hughes, K. K. Koretke, M. A. Lonetto, K. K. Brinkman, P. V. Warren, A. Lupas, M. J. Stanhope, J. R. Brown, and P. S. Hoffman. 2001. Systematic identification of selective essential genes in Helicobacter pylori by genome prioritization and allelic replacement mutagenesis. J. Bacteriol. 183:1259-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cormack, B. P., R. H. Valdivia, and S. Falkow. 1996. FACS-optimized mutants of the green fluorescent protein (GFP). Gene 173:33-38. [DOI] [PubMed] [Google Scholar]
- 10.Covacci, A., S. Censini, M. Bugnoli, R. Petracca, D. Burroni, G. Macchia, A. Massone, E. Papini, Z. Xiang, N. Figura, et al. 1993. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc. Natl. Acad. Sci. USA 90:5791-5795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Croxen, M. A., G. Sisson, R. Melano, and P. S. Hoffman. 2006. The Helicobacter pylori chemotaxis receptor TlpB (HP0103) is required for pH taxis and for colonization of the gastric mucosa. J. Bacteriol. 188:2656-2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delany, I., G. Spohn, A. B. Pacheco, R. Ieva, C. Alaimo, R. Rappuoli, and V. Scarlato. 2002. Autoregulation of Helicobacter pylori Fur revealed by functional analysis of the iron-binding site. Mol. Microbiol. 46:1107-1122. [DOI] [PubMed] [Google Scholar]
- 13.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2003. An anti-repression Fur operator upstream of the promoter is required for iron-mediated transcriptional autoregulation in Helicobacter pylori. Mol. Microbiol. 50:1329-1338. [DOI] [PubMed] [Google Scholar]
- 14.Delany, I., G. Spohn, R. Rappuoli, and V. Scarlato. 2001. The Fur repressor controls transcription of iron-activated and -repressed genes in Helicobacter pylori. Mol. Microbiol. 42:1297-1309. [DOI] [PubMed] [Google Scholar]
- 15.de Vries, N., E. J. Kuipers, N. E. Kramer, A. H. van Vliet, J. J. Bijlsma, M. Kist, S. Bereswill, C. M. Vandenbroucke-Grauls, and J. G. Kusters. 2001. Identification of environmental stress-regulated genes in Helicobacter pylori by a lacZ reporter gene fusion system. Helicobacter 6:300-309. [DOI] [PubMed] [Google Scholar]
- 16.Dunn, B. E., H. Cohen, and M. J. Blaser. 1997. Helicobacter pylori. Clin. Microbiol. Rev. 10:720-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ernst, F. D., S. Bereswill, B. Waidner, J. Stoof, U. Mader, J. G. Kusters, E. J. Kuipers, M. Kist, A. H. van Vliet, and G. Homuth. 2005. Transcriptional profiling of Helicobacter pylori Fur- and iron-regulated gene expression. Microbiology 151:533-546. [DOI] [PubMed] [Google Scholar]
- 18.Ferrero, R. L., V. Cussac, P. Courcoux, and A. Labigne. 1992. Construction of isogenic urease-negative mutants of Helicobacter pylori by allelic exchange. J. Bacteriol. 174:4212-4217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Franco, A. T., D. A. Israel, M. K. Washington, U. Krishna, J. G. Fox, A. B. Rogers, A. S. Neish, L. Collier-Hyams, G. I. Perez-Perez, M. Hatakeyama, R. Whitehead, K. Gaus, D. P. O'Brien, J. Romero-Gallo, and R. M. Peek, Jr. 2005. Activation of beta-catenin by carcinogenic Helicobacter pylori. Proc. Natl. Acad. Sci. USA 102:10646-10651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gancz, H., S. Censini, and D. S. Merrell. 2006. Iron and pH homeostasis intersect at the level of Fur regulation in the gastric pathogen Helicobacter pylori. Infect. Immun. 74:602-614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goodwin, A., D. Kersulyte, G. Sisson, S. J. Veldhuyzen van Zanten, D. E. Berg, and P. S. Hoffman. 1998. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol. Microbiol. 28:383-393. [DOI] [PubMed] [Google Scholar]
- 22.Guo, B. P., and J. J. Mekalanos. 2001. Helicobacter pylori mutagenesis by mariner in vitro transposition. FEMS Immunol. Med. Microbiol. 30:87-93. [DOI] [PubMed] [Google Scholar]
- 23.Guo, B. P., and J. J. Mekalanos. 2002. Rapid genetic analysis of Helicobacter pylori gastric mucosal colonization in suckling mice. Proc. Natl. Acad. Sci. USA 99:8354-8359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Haas, C. E., D. E. Nix, and J. J. Schentag. 1990. In vitro selection of resistant Helicobacter pylori. Antimicrob. Agents Chemother. 34:1637-1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heuermann, D., and R. Haas. 1995. Genetic organization of a small cryptic plasmid of Helicobacter pylori. Gene 165:17-24. [DOI] [PubMed] [Google Scholar]
- 26.Heuermann, D., and R. Haas. 1998. A stable shuttle vector system for efficient genetic complementation of Helicobacter pylori strains by transformation and conjugation. Mol. Gen. Genet. 257:519-528. [DOI] [PubMed] [Google Scholar]
- 27.Josenhans, C., S. Friedrich, and S. Suerbaum. 1998. Green fluorescent protein as a novel marker and reporter system in Helicobacter sp. FEMS Microbiol. Lett. 161:263-273. [DOI] [PubMed] [Google Scholar]
- 28.Langford, M. L., J. Zabaleta, A. C. Ochoa, T. L. Testerman, and D. J. McGee. 2006. In vitro and in vivo complementation of the Helicobacter pylori arginase mutant using an intergenic chromosomal site. Helicobacter 11:477-493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 30.Lee, W. K., Y. S. An, K. H. Kim, S. H. Kim, J. Y. Song, B. D. Ryu, Y. J. Choi, Y. H. Yoon, S. C. Baik, K. H. Rhee, and M. J. Cho. 1997. Construction of a Helicobacter pylori-Escherichia coli shuttle vector for gene transfer in Helicobacter pylori. Appl. Environ Microbiol. 63:4866-4871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Merrell, D. S., L. J. Thompson, C. C. Kim, H. Mitchell, L. S. Tompkins, A. Lee, and S. Falkow. 2003. Growth phase-dependent response of Helicobacter pylori to iron starvation. Infect. Immun. 71:6510-6525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oh, J. D., H. Kling-Backhed, M. Giannakis, J. Xu, R. S. Fulton, L. A. Fulton, H. S. Cordum, C. Wang, G. Elliott, J. Edwards, E. R. Mardis, L. G. Engstrand, and J. I. Gordon. 2006. The complete genome sequence of a chronic atrophic gastritis Helicobacter pylori strain: evolution during disease progression. Proc. Natl. Acad. Sci. USA 103:9999-10004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salama, N. R., G. Otto, L. Tompkins, and S. Falkow. 2001. Vacuolating cytotoxin of Helicobacter pylori plays a role during colonization in a mouse model of infection. Infect. Immun. 69:730-736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salama, N. R., B. Shepherd, and S. Falkow. 2004. Global transposon mutagenesis and essential gene analysis of Helicobacter pylori. J. Bacteriol. 186:7926-7935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sutcliffe, J. G. 1979. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harbor Symp. Quant. Biol. 43(Pt. 1):77-90. [DOI] [PubMed] [Google Scholar]
- 36.Tan, S., C. D. Fraley, M. Zhang, D. Dailidiene, A. Kornberg, and D. E. Berg. 2005. Diverse phenotypes resulting from polyphosphate kinase gene (ppk1) inactivation in different strains of Helicobacter pylori. J. Bacteriol. 187:7687-7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson, L. J., D. S. Merrell, B. A. Neilan, H. Mitchell, A. Lee, and S. Falkow. 2003. Gene expression profiling of Helicobacter pylori reveals a growth-phase-dependent switch in virulence gene expression. Infect. Immun. 71:2643-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomb, J. F., O. White, A. R. Kerlavage, R. A. Clayton, G. G. Sutton, R. D. Fleischmann, K. A. Ketchum, H. P. Klenk, S. Gill, B. A. Dougherty, K. Nelson, J. Quackenbush, L. Zhou, E. F. Kirkness, S. Peterson, B. Loftus, D. Richardson, R. Dodson, H. G. Khalak, A. Glodek, K. McKenney, L. M. Fitzegerald, N. Lee, M. D. Adams, E. K. Hickey, D. E. Berg, J. D. Gocayne, T. R. Utterback, J. D. Peterson, J. M. Kelley, M. D. Cotton, J. M. Weidman, C. Fujii, C. Bowman, L. Watthey, E. Wallin, W. S. Hayes, M. Borodovsky, P. D. Karp, H. O. Smith, C. M. Fraser, and J. C. Venter. 1997. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature 388:539-547. [DOI] [PubMed] [Google Scholar]
- 39.Trieu-Cuot, P., G. Gerbaud, T. Lambert, and P. Courvalin. 1985. In vivo transfer of genetic information between gram-positive and gram-negative bacteria. EMBO J. 4:3583-3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Valdivia, R. H., and S. Falkow. 1996. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol. Microbiol. 22:367-378. [DOI] [PubMed] [Google Scholar]
- 41.van Amsterdam, K., A. H. van Vliet, J. G. Kusters, and A. van der Ende. 2006. Of microbe and man: determinants of Helicobacter pylori-related diseases. FEMS Microbiol. Rev. 30:131-156. [DOI] [PubMed] [Google Scholar]
- 42.van Vliet, A. H., E. J. Kuipers, J. Stoof, S. W. Poppelaars, and J. G. Kusters. 2004. Acid-responsive gene induction of ammonia-producing enzymes in Helicobacter pylori is mediated via a metal-responsive repressor cascade. Infect. Immun. 72:766-773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Vliet, A. H., J. Stoof, S. W. Poppelaars, S. Bereswill, G. Homuth, M. Kist, E. J. Kuipers, and J. G. Kusters. 2003. Differential regulation of amidase- and formamidase-mediated ammonia production by the Helicobacter pylori Fur repressor. J. Biol. Chem. 278:9052-9057. [DOI] [PubMed] [Google Scholar]
- 44.Wang, G., T. J. Wilson, Q. Jiang, and D. E. Taylor. 2001. Spontaneous mutations that confer antibiotic resistance in Helicobacter pylori. Antimicrob. Agents Chemother. 45:727-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiang, Z., S. Censini, P. F. Bayeli, J. L. Telford, N. Figura, R. Rappuoli, and A. Covacci. 1995. Analysis of expression of CagA and VacA virulence factors in 43 strains of Helicobacter pylori reveals that clinical isolates can be divided into two major types and that CagA is not necessary for expression of the vacuolating cytotoxin. Infect. Immun. 63:94-98. [DOI] [PMC free article] [PubMed] [Google Scholar]