Abstract
Adenovirus is recognized as the most UV-resistant waterborne pathogen of concern to public health microbiologists. The U.S. EPA has stipulated that a UV fluence (dose) of 186 mJ cm−2 is required for 4-log inactivation credit in water treatment. However, all adenovirus inactivation data to date published in the peer-reviewed literature have been based on UV disinfection experiments using UV irradiation at 253.7 nm produced from a conventional low-pressure UV source. The work reported here presents inactivation data for adenovirus based on polychromatic UV sources and details the significant enhancement in inactivation achieved using these polychromatic sources. When full-spectrum, medium-pressure UV lamps were used, 4-log inactivation of adenovirus type 40 is achieved at a UV fluence of less than 60 mJ cm−2 and a surface discharge pulsed UV source required a UV fluence of less than 40 mJ cm−2. The action spectrum for adenovirus type 2 was also developed and partially explains the improved inactivation based on enhancements at wavelengths below 230 nm. Implications for water treatment, public health, and the future of UV regulations for virus disinfection are discussed.
UV disinfection is a well-accepted technology for inactivation of bacterial and protozoan pathogens. Until recently, UV was also considered a viable technology for disinfection of viruses. At UV fluences (doses) typically used in water disinfection, UV is very effective (>4-log inactivation) against almost all known pathogenic viruses, with the one exception of adenoviruses (5). Adenovirus has been recently listed on the U.S. EPA Candidate Contaminant List, which indicates that it is a high priority for possible future regulation and is known or anticipated to occur in public water systems, but significant data gaps need to be addressed before regulation can be invoked. According to recently published U.S. EPA regulations (17), the inactivation of adenoviruses to a level of 4 log requires a UV fluence of 186 mJ cm−2, based on an 80% credible interval, as presented in the Draft UV Disinfection Guidance Manual (16). The U.S. EPA-regulated UV fluence for inactivation of all viruses is now based on the conservative case of adenoviruses. Yates et al. (18) provide an excellent review of the issues surrounding the UV inactivation of adenovirus.
Although data sets for UV inactivation of adenovirus differ moderately, they all place adenovirus as the most UV-resistant health-related virus known. However, all peer-reviewed published studies to date have been performed using a low-pressure (LP) mercury vapor UV lamp source characterized by a monochromatic output in the UV range at 253.7 nm. Based on these LP UV irradiation studies, the UV fluence necessary to achieve 4-log inactivation of adenovirus varies from 120 to approximately 180 mJ cm−2. For adenovirus type 5 (Ad5), the required UV fluence is 160 to 170 mJ cm−2 (1), which is similar to those required for Ad1 (9), Ad2 (1, 5), Ad6 (9), and Ad40 and Ad41 (8). However, there are two studies that report higher resistance levels among the enteric adenoviruses, with UV fluences of 109 and 120 mJ cm−2 required for only 2-log inactivation of Ad40 and Ad41, respectively (1, 14), compared to Meng and Gerba's data (8) (120 mJ cm−2 for 4-log inactivation) for these same adenovirus types. No studies to date have been published on the inactivation of adenoviruses by use of polychromatic UV sources, such as medium-pressure (MP) and pulsed UV (PUV) lamps.
The goals of this research were to investigate the inactivation of enteric (Ad40) and respiratory (Ad2) adenovirus types by use of polychromatic UV irradiation and compare the results to data generated using an LP monochromatic UV source. The impacts of wavelength and high irradiance on the inactivation of adenoviruses were also studied.
MATERIALS AND METHODS
Adenovirus propagation and enumeration.
Two different types of adenovirus were investigated. Ad40 was propagated and enumerated according to previously reported procedures (14) at the USDA laboratories in Lincoln, NE. Briefly, virus propagation and infectivity assays were conducted using a PLC/PRF5 cell line and a most-probable-number method was used for determining viral concentrations before and after UV exposure. The Ad2 propagation and enumeration methods are detailed in Malley et al. (7) and were performed at the University of New Hampshire. In brief, virus was propagated in an A549 cell line, which was also used for infectivity assays. The 50% tissue culture infective dose method was used to determine the log inactivation.
Bench scale UV dosimetry.
For the Ad2 testing, the LP and MP UV dosimetries were based on measurement of incident UV irradiation (253.7 for LP and the 200- to 300-nm range for MP) by use of a calibrated International Light radiometer (IL-1700, SED240/W detector), which was then corrected for various factors (see reference 2) to derive the average germicidal irradiance in the completely mixed batch system. A germicidal weighting of the UV wavelengths emitted from the polychromatic UV source relative to the DNA absorbance spectrum was applied. The average irradiance was multiplied by the exposure time to generate the desired UV fluence.
For Ad40 testing, the quantitative spectra of all lamps, including conventional LP and MP mercury lamps and a pulsed surface discharge (SD) lamp (11, 12, 13), were measured using a multichannel spectrometer, convenient for capturing the entire UV spectra of polychromatic lamps. The measurements used an Ocean Optics (model USB2000) multichannel spectrometer with 2,048 elements over the wavelength range of 200 to 800 nm. Calibration was with a deuterium lamp with NIST traceable calibrated spectral irradiance. The MP and PUV polychromatic UV sources were weighted similarly to those in the experiments with Ad2.
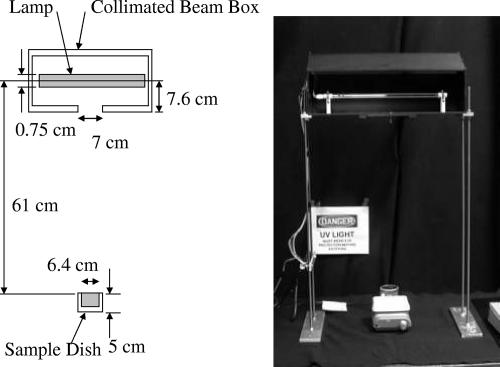
The test setups included both a standard collimated-beam (CB) and a high-intensity (HI) test cell, a new method for evaluating the effects of light intensity on inactivation (4). The CB setup was configured using the guidelines recommended by Bolton and Linden (2) and shown in Fig. 1.
FIG. 1.
Description of the geometry and a photograph of the CB apparatus used in the testing (with front cover removed).
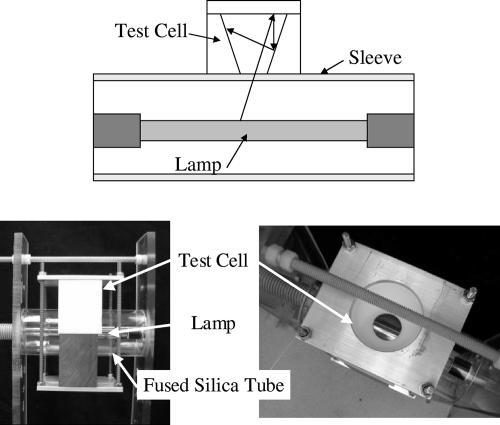
The HI cell pictured in Fig. 2 has the liquid in contact with the lamp envelope so that the irradiance of light entering the water sample is the same as that in a UV reactor. The UV fluence was determined using a Monte Carlo approach. This approach uses the geometric ray trace program OptiCAD, which includes the detailed geometry, the lamp emission spectrum, and all aspects of light propagation, absorption, and reflection, and which is detailed in Grapperhaus et al. (4). Inactivation differences between the CB and HI setups are a measure of irradiance-induced inactivation.
FIG. 2.
Description of the geometry for the test cell used to evaluate the effects of high-peak irradiance on disinfection of adenovirus. The test cell is in close proximity to the lamp, which provides substantially higher peak irradiance than that in the CB configuration.
Action spectra.
A complement of optical band-pass filters from Andover Corporation (Salem, NH) was used for action spectrum testing of Ad2. Band-pass filters provided a 10-nm half-peak bandwidth with peaks at 222, 228, 239, 260, 280, and 289 nm. The LP lamp was used without a filter for testing conducted at a wavelength of 254 nm. Details of the filters and the UV dosimetry are described in Linden et al. (6). All the samples for the action spectrum experiments were tested in phosphate-buffered laboratory water. Samples for LP and MP exposures were tested in both buffered laboratory water and finished filtered drinking water. An optical filter consisting of a solution of nitrate contained in a quartz vessel was used to generate light from an MP source, with the wavelengths below 240 nm cut off.
Statistical analyses.
Linear models were used to test the effects of UV fluence, lamp types, and UV wavelengths on the log inactivation of adenovirus. For analysis of the effects of UV fluence, lamp type (LP, MP, PUV), and water type on adenovirus inactivation, the statistical models included the inactivation as the response and the effects of UV fluence (continuous variable), lamp or water type (discrete nominal variable), and their interactions. In the model for the effects of the different wavelengths on adenovirus inactivation, the discrete nominal variable was wavelength. Analyses were performed using JMP software version 4.0.4 (SAS Institute Inc.), and all significance is reported at an α value of 0.05.
RESULTS AND DISCUSSION
Experiments were performed with both Ad2 and Ad40, under either filtered UV irradiation, LP and MP continuous-wave UV sources or a PUV source.
Action spectrum of Ad2.
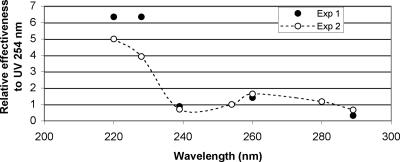
The effectiveness of different UV wavelengths was evaluated for Ad2. Two sets of experiments were performed at different times. In the first set, filters producing six different parts of the MP UV spectra were used, and UV fluences of 30, 60, and 90 mJ·cm−2 were delivered in duplicate. In the second set, the two lower wavelengths were repeated, but with UV fluences of 8, 16, and 24 mJ cm−2 performed in duplicate, and exposures with other filters were repeated once at the same fluences previously used. From these data, the log inactivation was plotted as a function of UV fluence and regression equations were developed for the UV fluence response of each wavelength investigated. The UV fluences required for 3-log inactivation were determined from the regression equations as a function of wavelength and form the action spectrum reported in Fig. 3.
FIG. 3.
Action spectrum for the inactivation of Ad2 with UV light. Data are from two experiments, each performed in duplicate, and represent the effectiveness for a 3-log inactivation relative to the response at 254 nm. Significant differences were found between the relative effectiveness of the 220 and 228-nm wavelengths compared to all the others and between the 260-nm and both the 239- and 289-nm wavelengths, based on the Tukey HSD test. In experiment 1, the 280- and 289-nm wavelengths were also significantly different.
The results illustrated in Fig. 3 represent the effectiveness of different wavelengths for a 3-log inactivation of adenovirus relative to the response at 254 nm and indicate that the germicidal effectiveness against adenovirus differs at different wavelengths. Wavelengths around 220 and 228 nm were most effective for inactivation of Ad2 and significantly different from the other wavelengths in both experiments, based on the Tukey honestly significant difference (HSD) test. There was no significant difference between the effectiveness of the 254-nm exposure and that of the 239-, 260-, 280-, or 289-nm wavelengths in either experiment. In experiments 1 and 2, the differences were significant between the 260-nm and both the 239- and 289-nm wavelengths, and in experiment 1, the 280- and 289-nm wavelengths were significantly different.
Continuous LP and MP UV inactivation of Ad2.
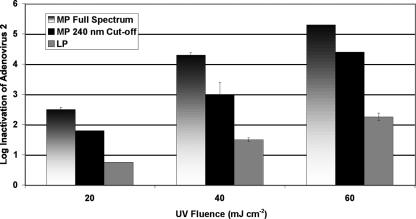
LP and full-spectrum MP UV sources were examined for inactivation of Ad2. A set of experiments was also performed using the MP UV source with a 240-nm cutoff filter to eliminate the wavelengths below 240 nm from the MP spectrum. This method simulated the use of “doped” UV lamps or sleeves to minimize the production of unwanted nitrite or the production of other by-products, as stipulated under the German and Austrian guidelines (3, 10), and was compared to the full-spectrum MP UV irradiation. As indicated in Fig. 4, the LP UV inactivation of Ad2 was similar to other data previously reported in the literature (cited above), with approximately 30 mJ cm−2 required for each log inactivation. The full-spectrum MP UV source was significantly more effective than the LP UV source or any other wavelength range in the action spectrum, aside from the two lowest wavelengths tested. This result indicates that a majority of the effectiveness of the MP UV source for adenovirus was likely due to the lower wavelengths.
FIG. 4.
Inactivation of Ad2 with UV light by MP, MP without wavelengths below 240 nm, and LP UV (254 nm). Error bars indicate 1 standard deviation. Note that MP and MP cutoff fluences are germicidally weighted by the DNA absorbance spectrum.
When the lower wavelengths (<240 nm) were eliminated from the full-spectrum MP irradiation, the inactivation was approximately 1 log lower than that without the lower wavelengths cut off, as indicated in Fig. 4. The filtered MP irradiation was also approximately doubly more effective than the LP irradiation alone, while the full-MP-spectrum light was almost three times more effective than the LP source.
Tests were performed with both laboratory and filtered drinking waters (data not shown), but the water matrix did not significantly change the adenovirus inactivation rate for the LP (P = 0.92) or MP (P = 0.80) UV lamp. There was a significant difference in adenovirus inactivation between the LP and MP UV sources (P < 0.001) for the inactivation of Ad2 in these two water types.
Inactivation of Ad40 with LP, MP, and PUV irradiation.
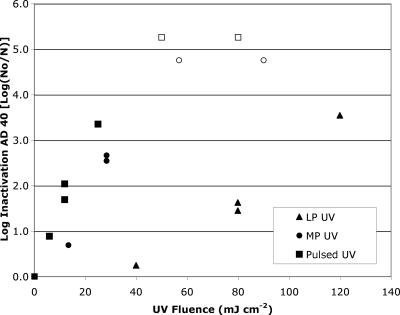
Ad40 was exposed to UV irradiation from LP, MP, and PUV irradiation under similar CB conditions. The LP and MP sources were conventional continuous-wave UV sources, while the PUV system utilized an SD lamp developed by Phoenix Science and Technology (Chelmsford, MA) (11, 12, 13). The UV inactivation data are presented in Fig. 5. The inactivation of adenovirus under LP UV irradiation was similar to data in the literature, with a fluence of 80 mJ cm−2 required for approximately 1.5-log inactivation and a fluence of over 120 mJ cm−2 required for 4-log inactivation. Both the MP and PUV sources outperformed the LP UV source by over fourfold, and the inactivations of Ad40 were determined to be significantly different (P < 0.001) between the monochromatic and polychromatic lamp types. Based on the Tukey HSD test, the adenovirus inactivation achieved using the LP lamp was significantly lower than that achieved using MP or SD lamp; however, the fluence responses of the MP and SD lamps did not differ significantly. The response of the SD lamp with the HI cell appeared to outperform those of all lamp types in the CB setups.
FIG. 5.
Inactivation of Ad40 with UV light by LP, MP, and PUV. Open symbols indicate that the log inactivation is greater than the value listed. Polychromatic UV fluences were germicidally weighted according to the DNA absorbance spectrum. The LP UV fluence response was significantly different from the MP and PUV data.
For 3-log inactivation of Ad40 by use of a polychromatic source, a UV fluence of approximately 30 mJ cm−2 was required and a fluence of 40 mJ cm−2 was estimated to achieve 4-log inactivation. These data for polychromatic UV sources were more consistent with the UV fluence requirements for 4-log inactivation of most enteric viruses previously studied (e.g., reference 5) and matched those generated for Ad2 in this study. In the test with the pulsed SD lamp and the HI cell, one pulse achieved inactivation below the detection limit (5.2-log inactivation) (data not shown), at a fluence of 30 mJ cm−2. Thus, the high irradiance level increased the log inactivation by at least 2 log compared to that for the CB SD test.
Implications.
Recent data showing the high level of resistance of adenovirus to UV light were developed using LP UV sources at a wavelength of 254 nm (1, 5, 9, 14). Based on the action spectrum information and the MP and PUV results, it appears that other wavelengths emitted by the polychromatic UV lamps are more effective than the 254 nm emitted by LP UV. One caveat here is that the UV transmittance of a drinking water or wastewater sample may not allow lower wavelengths, such as those below 230 nm, to penetrate deep into a water column. Therefore, in relation to disinfection effectiveness, these lower wavelengths may be rendered less significant. However, even when wavelengths below 240 nm were eliminated, the polychromatic MP UV source still outperformed the monochromatic LP source. In order to assess the importance of polychromatic UV sources for inactivation of adenovirus, a flowthrough challenge test should be performed using MP or PUV reactors.
Currently, the U.S. EPA has set a fluence level of 186 mJ cm−2 for 4-log reduction of all viruses, based on adenovirus data for drinking water. If virus inactivation by use of UV is required, the UV system will be much larger than what would be needed for most other viruses as well as bacteria and protozoan parasites. It is possible that when MP and PUV systems are used, the required fluences for obtaining virus inactivation may prove to be significantly lower, resulting in a considerable savings for water utilities in disinfection costs and protection of public health to a greater degree than that achieved by comparable LP UV systems at similar fluences.
Acknowledgments
This research was supported by the American Water Works Association Research Foundation, project no. 2593 (James P. Malley, Jr., principal investigator, and Albert Ilges, project manager), and by the Environmental Protection Agency, contract no. 68-D-01-057 (Raymond Schaefer, principal investigator).
We thank Sharleen Johnson, a masters student at Duke University; Amy Moore, a postdoctoral student at the University of New Hampshire; and Mike Grapperhaus, a Senior Researcher at Phoenix Science and Technology, for their assistance in this study and Zuzana Bohrerova for help with the statistical analyses.
Mention of trade names or commercial products in this article is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture.
Footnotes
Published ahead of print on 12 October 2007.
REFERENCES
- 1.Baxter, C. S., R. Hoffman, M. R. Templeton, M. Brown, and R. Andrews. 2007. Inactivation of adenovirus 2, 5, and 41 in drinking water by UV light, free chlorine, and monochloramine. ASCE J. Environ. Eng. 133:95-103. [Google Scholar]
- 2.Bolton, J. R., and K. G. Linden. 2003. Standardization of methods for fluence (UV dose) determination in bench-scale UV experiments. ASCE J. Environ. Eng. 129:209-215. [Google Scholar]
- 3.DVGW. 2006. UV disinfection devices for drinking water supply—requirements and testing. DVGW W294-1, -2, and -3. German Gas and Water Management Union (DVGW), Bonn, Germany.
- 4.Grapperhaus, M., R. B. Schaefer, and K. Linden. 2007. Modeling of a new UV test cell for evaluation of lamp fluence rate effects in regard to water treatment, and comparison to collimated beam tests. J. Environ. Eng. Sci. 6:271-276. [Google Scholar]
- 5.Gerba, C. P., D. M. Gramos, and N. Nwachuku. 2002. Comparative inactivation of enteroviruses and adenovirus 2 by UV light. Appl. Environ. Microbiol. 68:5167-5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Linden, K. G., G. A. Shin, and M. D. Sobsey. 2001. Relative efficacy of UV wavelengths for the inactivation of Cryptosporidium parvum. Water Sci. Technol. 43:171-174. [PubMed] [Google Scholar]
- 7.Malley, J. P., K. G. Linden, and A. A. Mofidi. 2004. Inactivation of pathogens with innovative UV technologies. Final report. AWWA Research Foundation, Denver, CO.
- 8.Meng, Q. S., and C. P. Gerba. 1996. Comparative inactivation of enteric adenoviruses, poliovirus, and coliphage by ultraviolet irradiation. Water Res. 30:2665-2668. [Google Scholar]
- 9.Nwachuku, N., C. P. Gerba, A. Oswald, and F. D. Mashadi. 2005. Comparative inactivation of adenovirus serotypes by UV light disinfection. Appl. Environ. Microbiol. 71:5633-5636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.ÖNORM. 2003. Plants for the disinfection of water using ultraviolet radiation—requirements and testing—part 2: medium pressure mercury lamp plants. ÖNORM M 5873-2. Österreichisches Normungsinstitut, Vienna, Austria.
- 11.Schaefer, R. August 1999. Surface discharge lamps. U.S. patent 5,945,790.
- 12.Schaefer, R. April 2004. Surface discharge lamp and system. U.S. patent 6,724,134.
- 13.Schaefer, R., M. Grapperhaus, I. Schaefer, and K. Linden. 2007. Pulsed UV lamp performance and comparison with UV mercury lamps. J. Environ. Eng. Sci. 6:303-310. [Google Scholar]
- 14.Thurston-Enriquez, J. A., C. Haas, J. Jacangelo, K. Riley, and C. P. Gerba. 2003. Inactivation of feline calicivirus and adenovirus type 40 by UV radiation. Appl. Environ. Microbiol. 69:577-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reference deleted.
- 16.U.S. Environmental Protection Agency. 2003. Draft ultraviolet disinfection guidance manual. EPA 815-D-03-007. U.S. Environmental Protection Agency Office of Water, Washington, DC.
- 17.U.S. Environmental Protection Agency. 2006. Long term 2 enhanced surface water treatment rule. Federal Register, 5 January 2006. U.S. Environmental Protection Agency, Washington, DC.
- 18.Yates, M., J. Malley, P. Rochelle, and R. Hoffman. 2006. Effect of adenovirus resistance on UV disinfection requirements: report on the state of adenovirus science. Am. Water Works Assoc. J. 98:93-106. [Google Scholar]