Abstract
We recently reported a simple PCR procedure that targets a sequence variation of the virulence-correlated gene locus vcg. It was found that 90% of all clinical isolates possessed the vcgC sequence variant, while 93% of all environmental isolates possessed the vcgE sequence variant. Here we report that the clinical genotype of Vibrio vulnificus is significantly better able to survive in human serum than is the environmental genotype. The presence of a siderophore-encoding gene, viuB, influenced serum survivability among all isolates of V. vulnificus tested. Those strains positive for viuB (all C-type strains but very few E-type strains) showed greater serum survivability than those lacking viuB (most E-type strains). The addition of iron (in the form of ferric ammonium citrate) to human serum restored the survival of E-type strains lacking viuB to levels not significantly different from those of C-type and E-type strains that possess viuB. These findings suggest that viuB may dictate serum survival in both C- and E-type strains of V. vulnificus and may explain why some strains (C- and E-type strains) are pathogenic and others (predominately E-type strains) are not. Additionally, C-type strains exhibited a cross-protective response against human serum, not exhibited by E-type strains, after incubation under nutrient and osmotic downshift conditions that mimicked estuarine waters. This suggests that the nutrient/osmotic environment may influence the survival of V. vulnificus following entry into the human body, leading to selection of the C genotype over the E genotype.
Vibrio vulnificus is a gram-negative estuarine bacterium capable of causing fatal septicemia after ingestion of raw or undercooked seafood and infection of wounds following exposure to water containing this pathogen (14, 15). Primary septicemias result in mortality rates exceeding 50%, increasing to more than 90% for patients in shock, despite aggressive treatment (8, 9). Most patients suffering from septicemia have predisposing factors which result in elevated serum iron levels (liver dysfunction, alcohol-induced cirrhosis, hemochromatosis, and thalassemia major) or immunodeficiency (1, 2, 4, 8, 14, 15, 22, 23). The FDA estimates that between 12 and 30 million Americans are susceptible to infection, although only ca. 30 cases are reported yearly (14, 15).
While most clinical and environmental isolates of V. vulnificus possess several virulence factors, few environmental isolates appear capable of human pathogenesis. Virulent isolates of V. vulnificus are generally characterized as encapsulated, resistant to human serum, and able to utilize iron-saturated transferrin. Conversely, avirulent isolates lack one or more of these attributes. However, previous serum studies have indicated that all encapsulated strains, whether of clinical or of environmental origin, were to some extent resistant to human serum, while virulence varied drastically with 50% lethal dose (LD50) values ranging from 1.5 × 10° to 7.0 × 105 cells (10). Even so, of those strains with LD50 values of <4 × 104, 80% were of clinical origin. Virulence characterizations using LD50 studies, however, are confounded by predisposing host factors, especially serum iron content, which is known to significantly influence V. vulnificus virulence (20, 22, 23). Genetic analyses of environmental isolates also indicate much heterogeneity, while isolates from human infections appear limited to a few genetic types (3, 5). Taken together these studies suggest differing levels of virulence in V. vulnificus, although strains of a clinical origin tend to exhibit a higher degree of specialization for the host milieu.
As most fatal cases of V. vulnificus infection result from bacteremia, serum resistance is an essential aspect of surviving in the host environment. Iron availability seems to play a critical role in the pathogenesis of infection and constitutes the major bacteriostatic limitation of V. vulnificus in human serum. Wright et al. (23) directly correlated host iron availability with virulence, as the injection of mice with iron resulted in an LD50 reduction from 106 to ca. 1 cell of V. vulnificus. Studies by Kim et al. (7) concluded that V. vulnificus requires high levels of readily available non-transferrin-bound iron for the initiation of growth. V. vulnificus is known to produce two classes of iron-scavenging siderophores, catechol and hydroxymate, which allow for the use of transferrin-bound iron (7, 11, 13, 19, 20). Additionally, Simpson and Oliver (19) found that an avirulent strain of V. vulnificus did not produce the catechol siderophore, and Litwin et al. (11) found that a venB-cloned plasmid restored siderophore production and virulence in this mutant. Our laboratory recently reported a sequence variation of the virulence-correlated gene locus vcg, with 90% of all clinical isolates possessing the vcgC sequence variant while 93% of all environmental isolates possess the vcgE sequence variant (18). We have determined the avirulent isolate studied by Simpson and Oliver to be an E-type strain, supporting the C/E-genotype correlation with virulence for this gene. Multiplex PCR studies by Panicker et al. (16) found that the siderophore-encoding gene viuB was possessed by all 22 clinical isolates of V. vulnificus, while only 8 out of 33 environmental isolates tested positive. This suggests that viuB may be a necessary virulence factor for host survival by V. vulnificus.
Though well documented, differences in human serum resistance among isolates have yet to be fully explained. In this report, we attempt to rationalize these differences and further enhance the virulence predictive value of C/E genotyping by associating strain-specific serum resistance with clinical and environmental genotype classification. Our results suggest that (i) elevated serum iron levels influence serum resistance of both genotypes by alleviating the bacteriostatic effect of iron limitation and (ii) these differences in serum resistance between genotypes, as influenced by serum iron availability, are a physiological manifestation of the absence or presence of the siderophore-encoding gene viuB. Additionally, our studies found that environmental factors (osmotic and nutrient downshifts) influence serum survival of V. vulnificus via induction of a cross-protective response against human serum.
MATERIALS AND METHODS
Organisms, media, and growth conditions.
Five clinical C-genotype strains (YJ106, C7184K2, LSU1866, CMCP6, and SPRC10143) and five environmental E-genotype strains (JY1305, Env1, JY1701, SS108A3A, and 3001C1) of V. vulnificus were examined in each of the studies conducted in this report. In addition to Env1, an E-type (MP mussel 3) strain of viuB+ status was used in the serum iron survival studies. All strains were previously examined by our lab as regards their possession of the viuB gene (data not shown). Strains were grown to log phase in heart infusion (HI) broth (Difco, Detroit, MI) with aeration at room temperature (RT). Log phase was achieved after ca. 2 h of growth, with readings of optical density at 610 nm of between 0.15 and 0.25. Cells exposed to an osmotic and nutrient downshift were grown in HI broth with additions of sodium chloride to increase osmolarity from 300 mosM to 1,464 mosM (21). Upon inoculation into half-strength artificial seawater, this created an osmotic and nutrient downshift, typical of seasonal (spring/summer) estuarine waters.
Human serum survival studies.
The methods of serum sensitivity and complement inactivation employed were adopted from a previous study from this lab (6). A volume of 12 μl of log-phase cells was directly inoculated into 718 μl of complement-active or -inactive human serum (Sigma Chemical Co., St. Louis, MO) and incubated at 37°C. Complement-inactivated serum was generated by water bath incubation at 56°C for 30 min. Survival was determined following phosphate-buffered saline dilution of the cells taken from serum at 15-min intervals (up to 1 h) and then at 90-min intervals (to 6 h), plating to HI agar, and incubation at RT for 2 days. Serum iron availability studies were conducted in the same manner except that the serum iron content was modified with additions of ferric ammonium citrate (0.01 or 0.1 μg/ml, final concentration).
Serum survival following osmotic and nutrient downshifts.
Osmotic downshift methodology was adapted and modified from a previous study in our lab (21). Cells of each strain were grown in modified HI broth (1,464 mosM) and then inoculated into two 100-ml artificial seawater microcosms of 3.2% (1,000 mosM) or 1.6% (500 mosM) total salinity. Cells were incubated at RT with aeration for 0.25, 3, 24, and 48 h. After each interval cells were removed and exposed to normal human serum (300 mosM) for 1 h as described above.
Statistical analyses.
Serum survival studies with/without iron additions were performed in triplicate, while osmotic/nutrient downshift studies were performed in duplicate. Cell count data in all cases were log transformed (log10 [CFU]) prior to statistical analyses. Serum survival and cross-protection studies were analyzed via two-way non-repeated-measure analysis of variance (ANOVA) with Bonferroni posttest comparisons. The rate of serum killing for each genotype was analyzed via two-way ANOVA with Dunnett's posttest comparisons against the untreated (control) cells.
RESULTS AND DISCUSSION
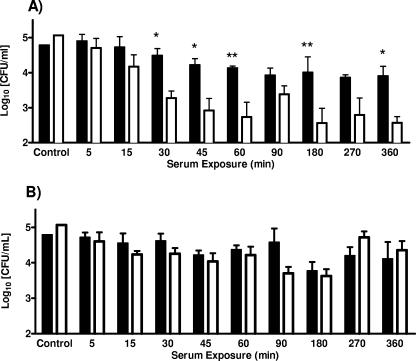
Human serum survival studies (Fig. 1A) confirmed C-type strains to be highly resistant to human serum (no significant reduction), while rapid inactivation of E-type strains (>2-log CFU reduction by 60 min; P < 0.01) occurred via complement-mediated killing. Conversely, both C- and E-type cells exposed to complement-inactivated human serum (Fig. 1B) were found to survive equally well, with no significant decrease in CFU over time. Thus, the ability to resist the bactericidal and bacteriostatic effects of human serum may account, in part, for the prevalence of C-type strain infections in humans compared to E-type strain infections. Individually, the five C-type strains examined behaved similarly, while the five E-type strains studied exhibited differences in human serum survival. In particular, while four of the five E-type strains lacked resistance, strain Env1 was strongly resistant and exhibited serum survival comparable to that of C-type strains.
FIG. 1.
Survival of clinical (solid bars) and environmental (open bars) genotypes of V. vulnificus exposed to normal human serum (A) or complement-inactivated human serum (B). Each bar represents the average survival in CFU for the five strains tested of each genotype. Control columns represent cells grown in HI prior to serum exposure. Asterisks denote significant differences between genotypes at each time interval based on results of two-way ANOVA with Bonferroni posttests (*, P < 0.05; **, P < 0.01).
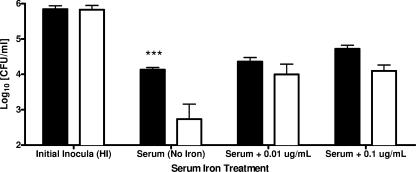
Serum iron availability studies (Fig. 2) indicated that serum survival of the E-genotype strains of V. vulnificus could be increased to levels not significantly different from that of C-genotype strains by the addition of as little as 0.01 μg/ml ferric ammonium citrate. Survivability of the C-type strains was slightly but significantly increased (P < 0.01) by the addition of 0.1 μg/ml of free iron to human serum. These results suggest that serum iron availability is the main growth-limiting factor in human serum, agreeing with the prevalence of V. vulnificus infection in persons with predisposing factors that elevate serum iron levels (14).
FIG. 2.
Serum survival of clinical (solid bars) and environmental (open bars) genotypes of V. vulnificus exposed to normal human serum for 1 h with or without additions of ferric ammonium citrate. Each bar represents the average survival in CFU for the five strains tested of each genotype. Columns marked “Initial Inocula” represent cells grown in HI prior to serum exposure. Asterisks denote significant differences in survivability between genotypes based on results of two-way ANOVA with Bonferroni posttests (***, P < 0.001).
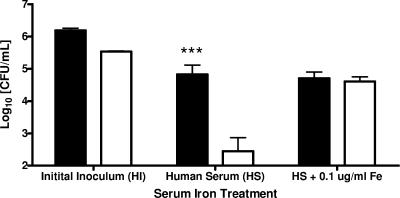
The results shown in Fig. 2 may reflect differences in siderophore production between genotypes of V. vulnificus, with E-genotype strains not producing the type or amount of siderophore necessary for growth and survival in human serum. PCR detection of viuB was conducted on 12 C-type strains and seven E-type strains, including those strains in this study, and the results were similar to those reported by Panicker et al. (16) in that 100% of the C-genotype strains were found to be viuB+ while only two of the E-type strains possessed this siderophore gene. Data shown in Fig. 3 indicate that viuB strongly influenced serum survival among the strains of V. vulnificus tested. Those strains positive for viuB (all C-type strains but very few E-type strains) showed significantly higher serum survival (P < 0.001) than did those lacking viuB (the majority of E-type strains). The addition of ferric ammonium citrate (0.1 μg/ml) to the E-type strains lacking viuB restored serum survival to levels not significantly different from those of the E-type strains possessing viuB. These findings suggest that viuB may dictate human serum survival in both C- and E-type strains of V. vulnificus and may hold the answer to why some strains are virulent and others (predominately E-type strains) are not. This also likely explains why strain Env1, which was one of only two E-type strains in our collection that possessed the viuB gene, behaved more like a C-type isolate than an E-type isolate in its ability to resist serum killing. Most reported cases of vibriosis are the result of a C-type strain infection; however, E-type strains have occasionally been recovered from clinical sources as well (3, 18). Our results offer an explanation for these instances of E-type strain infection, as elevated serum iron content in predisposed individuals and/or possession of siderophore-encoding viuB may alleviate the bacteriostatic inhibition of human serum. The result would be growth of V. vulnificus in human serum that is higher than the rate of bactericidal action of the human complement system.
FIG. 3.
Serum survival of viuB+ (▪) and viuB-negative (□) E- genotype strains of V. vulnificus exposed to normal human serum for 1 h with or without addition of ferric ammonium citrate. Each bar represents the average survival in CFU for each of the viuB+ or viuB-negative E-genotype strains tested. Initial inoculum columns represent cells grown in HI prior to serum exposure. Asterisks denote significant differences in survivability between genotypes based on results of two-way ANOVA with Bonferroni posttests (***, P < 0.001).
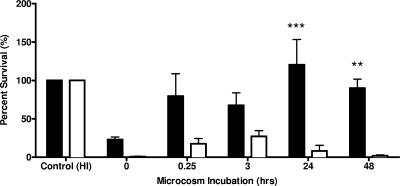
As a ubiquitous and opportunistic estuarine pathogen, V. vulnificus must be able to respond and adapt to constantly fluctuating estuarine waters. In responding to the variety of stressors encountered in such an environment, marine bacteria adapt and may gain an increased capacity for human pathogenicity. Both Nyström et al. (12) and Smith (21) found starvation survival stress responses in marine Vibrio spp. and Vibrio vulnificus strains, respectively, to confer significantly increased resistance to potentially lethal stresses such as heat, UV, and oxidation. Rosche et al. (17) further characterized this cross-protective response in V. vulnificus, with nutrient downshifts and osmotic shock inducing production of stress-alleviating proteins. Upon host entrance and introduction to serum, V. vulnificus is exposed to decreased osmotic pressures (∼300 mosM) relative to normal seawater (1,000 mosM) and increases in both nutrient availability and oxidative stress. Jarecki (6) showed that carbon starvation of V. vulnificus strain C7184 in artificial seawater induces a starvation survival response that significantly increases resistance to human serum. When transferred directly from HI into serum (no starvation or osmotic downshift), the C-type strain showed a 77% reduction in CFU (Fig. 4). However, after 24 h of microcosm incubation, the C-type strain exposed to human serum showed a >20% increase in CFU relative to the initial inoculum. On the other hand, the E-type strain showed rapid human complement-mediated killing (>93%) under both conditions. The clinical genotype of V. vulnificus thus exhibited significantly (P < 0.001) increased serum resistance relative to the environmental genotype following 24 h of incubation in estuarine salts (500 mosM). Thus, the C-type strain undergoes a nutrient and osmotic downshift-mediated cross-protective response against human serum, while no such response was evident in the E-type strain of V. vulnificus even after 48 h under the downshift conditions. These results also suggest that the osmotic and nutrient conditions of the estuarine environment may influence the survival of V. vulnificus following entry into the human body, leading to selection of the C genotype over the E genotype.
FIG. 4.
Percent survival of C-genotype (solid bars) and E-genotype (open bars) strains of V. vulnificus incubated in artificial estuarine water and then removed and exposed to human serum for 1 h. Each bar represents the average survival in CFU for the five strains tested of each genotype. Results are relative to initial inoculum (control), which represent cells grown in modified HI (1,464 mosM) prior to microcosm inoculation and serum exposure. Asterisks denote significance based on results of two-way ANOVA with Bonferroni posttests (**, P < 0.01; ***, P < 0.001).
Taken as a whole, our studies further support the virulence predictive value of C/E genotyping by associating strain-specific serum resistance with clinical and environmental source type classification. Additionally, these studies may aid in the development of a combined C/E-genotype-viuB screening method for the detection of clinically important isolates of V. vulnificus, which would be of great benefit in reducing associated illness and death.
Acknowledgments
We thank Tom Rosche, Alan Buck, Brett Froelich, and Rebecca Powell for their assistance in collecting and processing oyster and water samples. We also thank Melissa Jones and Liza Warner for conducting PCR analyses of genotype and viuB status for the strains employed in this study, as well as for helpful discussions in the preparation of the manuscript.
This report was prepared under award NA05N054781244 from NOAA, Department of Commerce.
The statements, findings, conclusions, and recommendations are those of the authors and do not necessarily reflect the views of NOAA or the U.S. Department of Commerce.
Footnotes
Published ahead of print on 12 October 2007.
REFERENCES
- 1.Brennt, D. E., A. C. Writhe, S. K. Dutta, and J. G. Morris, Jr. 1991. Growth of Vibrio vulnificus in serum from alcoholics: association with high transferring iron saturation. J. Infect. Dis. 164:1030-1032. [DOI] [PubMed] [Google Scholar]
- 2.Bullen, J. J., P. B. Spalding, C. G. Ward, and J. M. Gutteridge. 1991. Hemochromatosis, iron and septicemia caused by Vibrio vulnificus. Arch. Intern. Med. 151:1606-1609. [PubMed] [Google Scholar]
- 3.Chatzidaki-Livanis, M., M. A. Hubbard, K. Gordon, V. J. Harwood, and A. C. Wright. 2006. Genetic distinctions among clinical and environmental strains of Vibrio vulnificus. Appl. Environ. Microbiol. 72:6136-6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hor, L.-I., T.-T. Chang, and S.-T. Wang. 1999. Survival of Vibrio vulnificus in whole blood from patients with chronic liver diseases: association with phagocytosis by neutrophils and serum ferritin levels. J. Infect. Dis. 179:275-278. [DOI] [PubMed] [Google Scholar]
- 5.Jackson, J. K., R. L. Murphree, and M. Tamplin. 1997. Evidence that mortality from Vibrio vulnificus infection results from single strains among heterogeneous populations in shellfish. J. Clin. Microbiol. 35:2098-2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jarecki, A. 1995. The role of starvation in the resistance of Vibrio vulnificus to the bactericidal activity of human serum. M.S. thesis. University of North Carolina at Charlotte, Charlotte.
- 7.Kim, C.-M., R.-Y. Park, M.-H. Choi, H.-Y. Sun, and S.-H. Shin. 2007. Ferrophilic characteristics of Vibrio vulnificus and potential usefulness of iron chelation therapy. J. Infect. Dis. 195:90-98. [DOI] [PubMed] [Google Scholar]
- 8.Klontz, K. C., L. Spencer, M. Schreiber, H. T. Janowski, L. M. Baldy, and R. A. Gunn. 1988. Syndromes of Vibrio vulnificus infections: clinical and epidemiologic features in Florida cases, 1981-1987. Ann. Intern. Med. 109:318-323. [DOI] [PubMed] [Google Scholar]
- 9.Koenig, K. L., J. Mueller, and T. Rose. 1991. Vibrio vulnificus: hazard on the half shell. West. J. Med. 155:400-403. [PMC free article] [PubMed] [Google Scholar]
- 10.Linkous, D. A. 1998. Comparison of virulence among Vibrio vulnificus strains of varying capsular and LPS serotypes. M.S. thesis. University of North Carolina at Charlotte, Charlotte.
- 11.Litwin, C. M., T. W. Rayback, and J. Skinner. 1996. Role of catechol siderophore synthesis in Vibrio vulnificus virulence. Infect. Immun. 64:2834-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nyström, T., R. M. Olsson, and S. Kjelleberg. 1992. Survival, stress resistance, and alterations in protein expression in the marine Vibrio sp. strain S14 during starvation for different individual nutrients. Appl. Environ. Microbiol. 58:55-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okujo, N., T. Akiyama, S. Miyoshi, S. Shinoda, and S. Yamamoto. 1996. Involvement of vulnibactin and extracellular protease in utilization of transferrin- and lactoferrin-bound iron by Vibrio vulnificus. Microbiol. Immunol. 40:595-598. [DOI] [PubMed] [Google Scholar]
- 14.Oliver, J. D. 2006. Vibrio vulnificus, p. 349-366. In F. L. Thompson, B. Austin, and J. Swing. (ed.), Biology of vibrios. ASM Press, Washington, DC.
- 15.Oliver, J. D., and J. B. Kaper. 2007. Vibrio species, p. 343-379. In M. P. Doyle and L. R. Beuchat (ed.), Food microbiology: fundamentals and frontiers, 3rd ed. ASM Press, Washington, DC.
- 16.Panicker, G., M. Vickery, and A. K. Bej. 2004. Multiplex PCR detection of clinical and environmental strains of Vibrio vulnificus in shellfish. Can. J. Microbiol. 50:911-922. [DOI] [PubMed] [Google Scholar]
- 17.Rosche, T. M., D. J. Smith, E. E. Parker, and J. D. Oliver. 2005. RpoS involvement and requirement for exogenous nutrient for osmotically induced cross protection in Vibrio vulnificus. FEMS Microbiol. Ecol. 53:455-462. [DOI] [PubMed] [Google Scholar]
- 18.Rosche, T. M., Y. Yano, and J. D. Oliver. 2005. A rapid and simple PCR analysis indicates there are two subgroups of Vibrio vulnificus which correlate with clinical or environmental isolation. Microbiol. Immunol. 49:381-389. [DOI] [PubMed] [Google Scholar]
- 19.Simpson, L. M., and J. D. Oliver. 1983. Siderophore production by Vibrio vulnificus. Infect. Immun. 41:644-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simpson, L. M., and J. D. Oliver. 1987. Ability of Vibrio vulnificus to obtain iron from transferrin and other iron-binding proteins. Curr. Microbiol. 15:155-157. [Google Scholar]
- 21.Smith, D. J. 1999. Nutrient requirement for osmotically-induced cross protection in Vibrio vulnificus. M.S. thesis. University of North Carolina at Charlotte, Charlotte.
- 22.Starks, A. M., T. R. Schoeb, M. L. Tamplin, S. P. Parveen, T. J. Doyle, P. E. Bomeisl, G. M. Escudero, and P. A. Gulig. 2000. Pathogenesis of infection by clinical and environmental strains of Vibrio vulnificus in iron-dextran-treated mice. Infect. Immun. 68:5785-5793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright, A. C., L. M. Simpson, and J. D. Oliver. 1981. Role of iron in the pathogenesis of Vibrio vulnificus infections. Infect. Immun. 34:503-507. [DOI] [PMC free article] [PubMed] [Google Scholar]