Abstract
Knowledge of the spreading mechanism of honeybee pathogens within the hive is crucial to our understanding of bee disease dynamics. The aim of this study was to assess the presence of infectious chronic bee paralysis virus (CBPV) in bee excreta and evaluate its possible role as an indirect route of infection. Samples of paralyzed bees were (i) produced by experimental inoculation with purified virus and (ii) collected from hives exhibiting chronic paralysis. CBPV in bee heads or feces (crude or absorbed onto paper) was detected by reverse transcription-PCR. CBPV infectivity was assessed by intrathoracic inoculation of bees with virus extracted from feces and by placement of naive bees in cages previously occupied by contaminated individuals. CBPV RNA was systematically detected in the feces of naturally and experimentally infected bees and on the paper sheets that had been used to cover the floors of units containing bees artificially infected with CBPV or the floor of one naturally infected colony. Both intrathoracic inoculation of bees with virus extracted from feces and placement of bees in contaminated cages provoked overt disease in naive bees, thereby proving that the excreted virus was infectious and that this indirect route of infection could lead to overt chronic paralysis. This is the first experimental confirmation that infectious CBPV particles excreted in the feces of infected bees can infect naive bees and provoke overt disease by mere confinement of naive bees in a soiled environment.
The honeybee, Apis mellifera L., is a well-known honey producer and plays a major role in agriculture by assisting in the pollination of a wide variety of crops (29). Viral infections are the least understood of honeybee diseases, due to the lack of information on the mechanisms underlying potential disease outbreaks (20). Few data are available on their different modes of spread, transmission, and persistence.
Chronic paralysis is an infectious and contagious disease of adult honeybees caused by the chronic bee paralysis virus (CBPV) (10). CBPV is classified as a positive-sense, single-stranded RNA virus but has not been assigned to a genus or family. Chronic paralysis has been given a variety of names, such as “hairless black syndrome” and “little blacks,” and is the only common viral disease of adult bees that has well-described symptoms, which include abnormal trembling of wings and body. Some individuals become almost hairless and dark in appearance and suffer nibbling attacks from healthy bees of their colony. Affected bees become flightless, often crawling on the ground and on the stems of grass, sometimes in masses of thousands of individuals. The bloated abdomen is caused by distension of the honey sac with fluid, leading to the so-called “dysentery” symptom. Sick individuals die within a few days of the onset of symptoms (4, 5, 16). Many paralyzed bees from each colony die throughout the year, often in thousands during the summer (7). Many thousands of adult bees that die in the field may also be affected by paralysis (7). Severely affected colonies can suddenly collapse, particularly at the height of summer (16).
Diagnosis of the clinical disease has long been based on an agarose gel immunodiffusion (AGID) test which detects the large amounts of CBPV particles produced during paralysis outbreaks (14, 30). However, it has been demonstrated by sensitive infectivity tests and molecular detection that CBPV commonly persists throughout the year in apparently normal live individuals in colonies that are considered by beekeepers to be healthy (7, 31, 33).
Knowledge of the mechanism of CBPV spread is crucial to our understanding of the dynamics of the disease. Laboratory experiments show that adult workers are very susceptible to CBPV infection by injection. Inoculated bees usually show symptoms of paralysis at about 5 or 6 days postinoculation (p.i.), appearing feeble and exhibiting the same trembly movements as bees infected naturally in the field. These individuals remain alive for several more days (10). However, despite the efficiency of virus inoculation in laboratory trials, CBPV infections have never been related to Varroa destructor infestations (15), and the virus has not been reported in this parasite (14, 33). In 1983, Bailey et al. (9) demonstrated that paralysis virus can be easily transmitted experimentally to bees by topical application to the surface of cuticle freshly denuded of its hairs. They also showed that paralysis spread most efficiently among individual adult bees when they were crowded together. They concluded that paralysis may be transmitted in nature by direct contact of healthy bees crowded with paralyzed individuals (9). Thus, the transmission of CBPV by contact has been proved and is considered a factor of disease incidence (9, 15). One key issue that remains to be elucidated is whether infectious particles of the virus can persist outside the host and infect naive bees confined in contaminated environments.
In 1965, Bailey (2) indicated, without providing details, that fresh feces from chronically paralyzed bees contained CBPV particles that were infectious if injected into healthy bees. However, he did not check if these particles from feces were sufficient to infect healthy bees by mere contact. Environmental contamination by virus excreted in feces is consistent with the “dysentery” symptom described for this disease (16) and with our observations of experimentally infected bees in small cages. These observations led us to suspect that contaminated feces might be a factor of virus and disease transmission.
In order to verify this hypothesis, we first checked that virus RNA could be detected by reverse transcription-PCR (RT-PCR) in honeybee fecal samples by artificially loading feces with purified virus.
Second, we used RT-PCR to investigate the excretion of CBPV in feces from experimentally and naturally infected bees and the presence of CBPV on surfaces soiled by bee feces. Third, we confirmed the infectivity of supernatants (i) from crude feces and (ii) from paper sheets soiled by feces by using them for intrathoracic inoculation of naive honeybees. The final step was to investigate effective CBPV transmission by keeping naive bees in cages previously occupied and soiled by experimentally infected bees.
MATERIALS AND METHODS
Virus stocks, propagation, and purification.
The CBPV isolate used in this study was obtained by infectivity test (2, 3) as previously described (31). Briefly, adult honeybees were sampled inside a hive that did not exhibit any symptoms of any infection but where CBPV had been detected by RT-PCR in bees sampled on the flight board. One month before, this colony from the laboratory apiary had been treated against Varroa destructor and nosemosis had been researched as described below but not detected. Bees, anesthetized with carbon dioxide, were infected by intrathoracic inoculation with 3 μl of a 10-fold-diluted supernatant, obtained by crushing the heads of bees from the same colony.
Typical trembling symptoms occurred on day 5 p.i., and all inoculated bees were dead at day 7 p.i. (11, 13). Chronic paralysis was diagnosed by AGID diagnosis (30), and CBPV was detected by RT-PCR (31) as described below. Negative controls, (i) inoculated with buffer and (ii) noninoculated, did not develop any paralysis symptoms, and their mortality rate did not exceed 2%. The virus was only weakly detected by RT-PCR in some of these controls, indicating a low virus level in bees from this hive (data not shown). CBPV was subsequently purified on sucrose density gradients (10 to 40%, wt/vol) (14, 17, 34). Purified virus was stored at −80°C in 0.01 M potassium phosphate buffer (PB), pH 6.7, and used as the inoculum for experimental infections. Acute bee paralysis virus (ABPV), which can induce early trembling symptoms and rapid mortality during infectivity tests (10), could not be detected by RT-PCR (12) either in bees from the hive or in inoculated bees.
Sample collection: honeybees and papers contaminated by feces.
All the live honeybee samples came from colonies maintained in the AFSSA laboratory apiary (Sophia Antipolis, France). The individual bees defined in this report as CBPV free, or naive, were sampled from the same colony, where (i) no chronic paralysis symptoms had ever been observed during the period of the experiments, (ii) infectivity tests had been negative, and (iii) CBPV remained undetected by RT-PCR. Infectivity tests and CBPV RT-PCR were always repeated at the same time as each sampling of adult naive bees for the experiments and were found negative. Positive (purified virus) and negative (buffer) controls were included for both tests to rule out any possibility of failures in the analytical techniques.
Apparently healthy but CBPV-infected bees from asymptomatic colonies were sampled. Their latent CBPV infection was assessed by performing infectivity tests and RT-PCR detection on sister bees from the same colonies. These bees are defined in this report as inapparently infected bees.
Naturally paralyzed bees, showing trembling symptoms, were collected either from the flight boards of hives in the laboratory apiary or from samples sent in by beekeepers for chronic paralysis diagnosis. These bees are referred to as naturally infected bees.
The floor of one of the laboratory hives with symptomatic bees was covered with a sheet of 3 chr chromatography paper (DEAE-cellulose; Whatman International Ltd., Maidstone, England) to collect feces excreted in the hive by naturally infected bees. This paper was placed under a clawed bottom board to prevent the bees from removing it from the hive and left during one rainy weekend.
Experimentally infected bees were produced by inoculating naive bees with 3 μl of a purified virus diluted 106-fold in physiological saline water. Control noninfected bees were produced by inoculating bees with 3 μl of buffer. Groups of 30 bees were kept in cages in a 30°C incubator and fed with sucrose syrup and sugar candy ad libitum (31). The floor of each cage was completely covered with a sheet of 3 chr chromatography paper to collect any feces. Trembling symptoms and mortalities were recorded daily until the ninth day p.i. Dead bees and papers were removed after each observation.
For the CBPV excretion analysis, dead bees were selected after the onset of symptoms and referred to as experimentally infected bees. Papers were collected for analysis between the fifth and eighth days p.i.
Head and feces preparation.
Heads and crude feces were both collected from individual naive, inapparently infected, or symptomatic bees (naturally or experimentally infected). Samples were analyzed individually or pooled in fives. Crude feces (approximately 10 to 30 μl of feces per bee) were collected from bees in a 0.5-ml microfuge tube by applying pressure to the honeybee abdomen (8). The feces were homogenized, diluted 10-fold, and centrifuged at 4,000 × g for 15 min at 4°C (17, 26). The bee heads were crushed at 4°C in 120 μl of PB each, and the supernatant centrifuged twice at 8,000 × g for 10 min.
RNA preparation from feces for CBPV detection by RT-PCR was validated by artificially loading negative feces with purified virus. The feces from 30 naive bees were pooled and diluted 10-fold in PB. Half of this sample was loaded with 104-fold diluted purified virus, cleared, and then used as the “artificially loaded” positive control. The remaining half was used as the negative control.
The feces naturally dropped by bees onto the paper sheets that covered the bottoms of cages or the hive were assessed by checking all sheets for feces, counting the fecal impacts, and noting the appearance of the feces. Round, 1-cm-diameter pieces were cut out and individually crushed in 300 μl of PB at 4°C. The supernatant was cleared twice at 12,000 × g for 2 min. For the positive-control samples, 10 μl of artificially loaded feces was applied to 3 chr chromatography paper. The negative controls consisted of (i) feces from healthy bees loaded onto paper and (ii) papers from control cages of bees inoculated with buffer.
Nosema spore quantification.
Because an infection with Nosema apis can cause digestive disorders, the nosemosis diagnostic methods described by Ruijter were used to quantify the level of Nosema sp. infection in pools of five bees (32).
Chronic paralysis virus infection diagnosis by AGID.
Chronic paralysis virus infection was assessed by AGID analysis of each sample collected from experimentally infected or naturally diseased bees. AGID diagnosis was performed with pooled bee heads using antiserum raised against the original CBPV isolate as previously described by Ribière et al. (30).
RNA preparation.
Two methods were tested to validate RNA preparation for CBPV detection by RT-PCR in honeybee fecal specimens: a direct method and RNA extraction. In each method, 4 aliquots of 100 μl of the artificially loaded feces supernatant were analyzed. The direct method of RT-PCR without RNA extraction was adapted from the method for Kashmir bee virus detection in feces (26). Samples were heated at 95°C for 2 min, centrifuged briefly, cooled to 50°C, and then used directly in reverse transcription. RNA extraction was performed using a HighPure viral RNA purification kit (Roche Diagnostics, Meylan, France) based on combined guanidine hydrochloride and glass fiber filter methods. The extracted RNAs were used in reverse transcription.
RT-PCR.
RT-PCR detection was performed as previously described by Ribière et al. (31): 12.5 μl of each sample was reverse transcribed and subjected to PCR amplification using primer pair CBPV1-CBPV2 designed on the RNA-dependent RNA polymerase which gives a predicted product of 455 bp. PCR products were separated by electrophoresis in 1% agarose gel and visualized and photographed under UV light. The specificity of RT-PCR detection in the feces was validated by sequencing amplicons from one experimentally infected bee (head and feces), one naturally infected bee (head and feces), and one feces sample absorbed onto paper. The PCR products were purified using a QIAquick PCR purification kit (QIAGEN, France). The nucleotide sequences were determined from both forward and reverse directions by the MilleGen Biotechnologies Company (Labège, France), and alignment was done on 358-nucleotide deduced sequences and 119-amino-acid deduced sequences using MegAlign (DNASTAR Lasergene software) with a reference virus isolate (GenBank reference AF375659).
Quantitative real-time two-step RT-PCR.
A two-step real-time RT-PCR assay based on TaqMan technology using a fluorescent probe (6-carboxyfluorescein-6-carboxytetramethylrhodamine) was performed as previously described (18) to quantify the CBPV genomic load in crude feces and on papers with or without feces.
Infectivity tests with virus-contaminated feces.
Infectivity tests were performed on groups of 30 naive bees (two groups for each treatment) confined to cages supplied with sucrose syrup and candy in a 30°C incubator.
First, the infectivity of the virus contained in feces was verified by intrathoracic inoculation using pooled fecal preparations obtained from (i) experimentally infected bees, (ii) naturally infected bees, and (iii) paper extracts collected in the infection experiments. Positive controls were inoculated with the artificially loaded sample. Negative controls were inoculated with preparations from healthy bee feces, either (i) crude or (ii) absorbed onto paper. Trembling symptoms and mortalities were recorded daily until the ninth day p.i.
Second, the effective indirect transmission of CBPV was investigated by placing naive bees in cages previously occupied and soiled by experimentally infected bees. On the seventh day of a preliminary experimental inoculation using purified virus, naive bees were placed in four soiled cages in which the last 2-day-old contaminated papers had been kept. These cages were supplied with new sucrose syrup and sugar candy food sources and placed in a decontaminated incubator at 30°C. Live paralyzed bees were kept in two of the cages and removed 5 days later (3 days after death). The contaminated papers were not changed throughout the entire test. For the negative control, naive bees were kept in cages previously occupied by bees inoculated with buffer. Trembling symptoms and mortalities were recorded daily until day 15. The dead bees in these two infectivity tests were removed after each observation. For each group, AGID diagnosis and RT-PCR CBPV and ABPV (12) RNA detection were performed on pools of dead bees and of survivors collected on the last day of the experiment.
RESULTS
Numeration and definition of feces, chronic paralysis, and nosema spore detection.
During experimental infections by inoculation of diluted purified virus, some infected bees defecated after onset of the trembling symptom on day 5 p.i. All infected bees had died by day 8 p.i., as previously described (11, 13). The deposits of feces excreted per day and per cage were 0 to 4 in number, brown, and approximately 1 cm in diameter. In comparison, the feces excreted by caged bees inoculated with buffer consisted of 0 to 2 small impact spots (<0.4 cm in diameter) of clear feces per day, the mortality rate did not exceed 2%, and no trembling symptoms were observed. RT-PCR and AGID tests showed that virus-inoculated bees were infected and developed chronic paralysis and that bees inoculated with buffer were healthy.
The nosema spore count at day 8 p.i. was 8 × 106 spores/ml per CBPV-infected bee and 10 × 106 spores/ml per bee inoculated with buffer.
Moreover, numerous small impacts of brown feces were noted on the paper that for two days had covered the floor of a hive with chronically paralyzed bees.
Molecular detection of CBPV in feces.
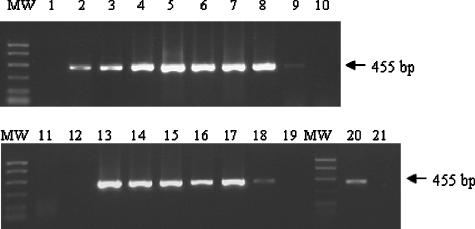
The results of CBPV detection in feces by RT-PCR are summarized in Fig. 1. A preliminary test was carried out to ascertain whether CBPV RNA in feces could be detected by molecular tests. Direct preparation without RNA extraction allowed the detection of CBPV RNA in negative feces loaded with purified virus in only one of the four analyses, and the detected PCR product signal was weak (Fig. 1, lane 2). This direct RNA preparation was not suitable, probably because of the great fragility of RNA (19), which requires appropriate extraction methods (24). Moreover, RT-PCR experiments on insects (21) and feces (28, 36) are often hampered by problems of inhibitory components which compromise reverse transcription and PCRs. These problems were avoided by RNA extraction: detection of the CBPV genome was positive in all four analyses, and the signal was as intense as that obtained for virus diluted in buffer (Fig. 1, lanes 3 and 4). Therefore, the RNAs for subsequent analyses were extracted according to the extraction protocol.
FIG. 1.
Detection of CBPV in honeybee heads and feces by RT-PCR. Lanes: MW, PCR molecular weight markers (Promega); 1, feces from healthy bees (with RNA extraction); 2, virus artificially loaded into negative feces (without RNA extraction); 3, virus diluted in buffer (with RNA extraction); 4, virus artificially loaded into negative feces (with RNA extraction); 5, pool of heads from experimentally infected bees; 6, pool of feces from experimentally infected bees; 7, pool of heads from naturally infected bees; 8, pool of feces from naturally infected bees; 9, pool of heads from inapparently infected bees; 10, pool of feces from inapparently infected bees; 11, pool of heads from healthy bees; 12, pool of feces from healthy bees; 13, head from one experimentally infected bee; 14, feces from one experimentally infected bee; 15, head from one naturally infected bee; 16, feces from one naturally infected bee; 17, paper with feces from a cage of experimentally infected bees; 18, paper without feces from a cage of experimentally infected bees; 19, paper with feces from a negative-control cage (bees inoculated with water); 20, paper with feces from a colony with paralyzed bees; 21, paper without feces from a colony with paralyzed bees.
CBPV excretion in feces.
RT-PCR was performed to assess the simultaneous presence of CBPV in crude feces and bee heads. CBPV was detected in pooled feces and in pooled heads from the same individuals (experimentally and naturally infected bees) (Fig. 1, lane 5 to 8). Virus was systematically detected in the six pools of feces from experimentally infected bees and in the three pools of feces from naturally infected bees. Virus was not found in pooled heads or pooled feces from healthy bees (Fig. 1, lanes 11 and 12). Virus was weakly detected in the pooled heads of inapparently infected bees but not in their feces (Fig. 1, lanes 9 and 10). Virus was also systematically detected in individual samples of heads and feces (Fig. 1, lanes 13 to 16) from two experimentally and six naturally infected bees. The CBPV loads, evaluated by real-time two-step RT-PCR, ranged from 108 to 1010 CBPV RNA copies per μl of feces.
CBPV was clearly detected in 12 feces deposits absorbed onto pieces of paper collected from cages of infected bees (Fig. 1, lane 17). The signals from the PCR products on two pieces of paper without feces collected from the same experimental units were weak (Fig. 1, lane 18). The virus was not found in any heads, feces, or papers from control cages of bees inoculated with buffer (Fig. 1, lanes 11, 12, and 19). Weak CBPV PCR product signals were obtained from the four pieces of paper with feces from the paper that had been left for one rainy weekend in a colony with confirmed chronic paralysis (Fig. 1, lane 20). The signal for the paper pieces without feces was very weak and not visible in the photograph (Fig. 1, lane 21) but became visible when the agarose gel was visualized directly under UV light. The real-time PCR results indicated that the virus loads detected on papers from experimental units and from the diseased colony exceeded 105 CBPV RNA copies per square centimeter of paper with feces and 103 CBPV RNA copies per square centimeter of paper without feces.
Sequence analysis showed that the sequences of RT-PCR products obtained from honeybee heads and feces, crude as well as absorbed onto paper, matched the sequence of the CBPV reference isolate published in GenBank (AF375659), indicating that the amplification in feces was specific.
Infectivity of virus excreted in feces.
The results of infectivity tests by the inoculation of CBPV-contaminated feces are summarized in Table 1. In this experiment, naive bees were inoculated with the supernatant of feces in which CBPV had been confirmed by molecular detection, i.e., feces artificially loaded with CBPV or feces (crude or absorbed onto paper) from naturally or experimentally infected bees. Negative controls were performed using feces (crude and absorbed onto paper) from healthy bees inoculated with buffer and checked as free of CBPV by RT-PCR. During this infectivity test, the trembling symptoms began on the fifth day after inoculation with contaminated fecal preparations. The mortality rate at day 8 p.i. was 70% for individuals inoculated with feces artificially loaded with CBPV, 100% for those inoculated with crude feces from naturally infected bees, and 53% and 76%, respectively, for bees inoculated with crude feces or feces absorbed onto paper from experimentally infected bees. Paralysis and virus were assessed in both dead and live bees 8 days after inoculation. The caged control bees inoculated with virus-free crude feces and with virus-free feces absorbed onto paper did not develop any trembling symptoms, and their mortality rates were 20% and 4%, respectively, on day 8 p.i. Disease and CBPV could not be detected in either surviving or dead bees. ABPV could not be detected in either bees inoculated with CBPV-contaminated feces or control bees.
TABLE 1.
Infectivity of feces samples tested by inoculation
| Origin of feces used as inoculum | Type of feces | Day p.i. of trembling symptom onseta | % Mortality (range) at day 8 p.i. | Live/dead bees pos or neg by AGID and RT-PCRb |
|---|---|---|---|---|
| Healthy bee feces artificially loaded with virus | Crude | 5 | 70 (56-83) | +/+ |
| Naturally paralyzed bees | Crude | 5 | 100 (100-100) | ns/+ |
| Experimentally infected bees | Crude | 5 | 53 (53-53) | +/+ |
| On paper | 5 | 76 (53-100) | +/+ | |
| Healthy bees inoculated with buffer | Crude | None | 20 (20)c | −/− |
| On paper | None | 3.3 (3.3-3.3) | −/− |
None, no symptoms observed during the experiment.
The AGID and RT-PCR results were 100% identical, so the results are presented in the same column. Bees were sampled at day 8 p.i. ns, no sample; pos, positive; neg, negative.
For this treatment, only one experimental cage was usable.
The results obtained for naive bees kept in soiled cages are presented in Table 2. Bees placed in CBPV-contaminated experimental cages with or without five paralyzed bees for 5 days developed paralysis symptoms from day 10 postencaging, and their mortality rates were 61.6% and 75%, respectively, on the 15th day. Control bees kept in virus-free experimental units remained without symptoms throughout the experiment and had a survival rate of 31.6% after 15 days. Disease and virus were detected only in live and dead bees kept in contaminated cages.
TABLE 2.
Results of CBPV contamination test for bees encaged in a contaminated environmenta
| Cages previously occupied by: | Contaminant | Day p.e. of trembling symptom onsetb | % Mortality (range) at day 15 p.e. | Live/dead bees pos or neg by AGID and RT-PCRc |
|---|---|---|---|---|
| Experimentally infected bees | 2-day-old paper and 5 paralyzed bees for 5 days | 10 | 75 (56.6-93.3) | +/+ |
| 2-day-old paper | 10 | 61.6 (60-63) | +/+ | |
| Healthy bees inoculated with buffer | 2-day-old paper | None | 31.6 (30-33) | −/− |
p.e., postencaging.
None, no symptoms observed during the experiment.
The AGID and RT-PCR results were 100% identical, so the results are presented in the same column. pos, positive; neg, negative.
DISCUSSION
The present study demonstrated that both naturally and experimentally infected bees with active disease systematically excreted CBPV in their feces. The virus could be detected in the crude feces and in feces absorbed onto paper. Infectivity tests showed that the virus detected in feces, whether crude or absorbed onto paper, was sufficiently infectious to contaminate and induce overt disease when it was injected into naive bees and also when naive bees were kept in soiled cages.
Chronic paralysis virus has been detected in many colonies throughout the year (7). Disease occurs irregularly in apiaries, sometimes leading to losses of thousands of individuals (3, 16). The means by which CBPV disseminates within the population is a key component of the dynamics of disease development.
In this study, we observed that paralyzed experimentally infected bees excreted more and browner feces than control bees inoculated with buffer. This suggests that one route of CBPV spread could be excretion and is consistent with the “dysentery” description of the disease and with the observation of CBPV particles in feces (2, 16). Nosema spores were also detected but with no significant difference in spore amount between CBPV-infected and noninfected bees. An infection by Nosema apis, which can cause digestive disorders, might have explained the difference in number and quality of the excreted feces. However, “dysentery” symptoms were observed during another experimental infection with CBPV but with no detection of nosema spores (data not shown). We concluded that, in our experiments, the excretion of brown feces was due to chronic paralysis disease but the use of nosema-infected bees might have increased virus excretion or the virus load in feces. Further study is needed to see if a relationship exists between CBPV excretion and nosema infections, paying special attention to distinguish between N. apis and N. ceranae, which are both present in European colonies but may induce different disorders in colony health (23, 25, 27).
The genomic load of 1010 CBPV RNA copies per feces, evaluated by real-time RT-PCR, was consistent with the viral titer of 109 particles per bee fecal deposit determined by electronic microscopy (2) and with the estimated genomic CBPV load per symptomatic paralyzed bee, which was up to 1013 CBPV copies (18). Moreover, the spread of virus within the hive by paralyzed bees was confirmed by detecting the virus in feces from a symptomatic colony that were absorbed onto paper and in apparently unsoiled pieces of this paper.
When infectivity was tested by inoculation, the observed differences in paralyzed-bee mortality rates at day 8 p.i. (ranging from 53% to 100% mortality) were probably due to differences in infectious virus titers among the different fecal samples used as inocula. ABPV infection might have explained a higher mortality rate, but ABPV was not detected. Moreover, bees infected with ABPV die earlier (acute paralysis) than bees infected with CBPV (chronic paralysis) (10). Kashmir bee virus infection was also excluded because it is rapidly lethal and does not provoke symptoms typical of paralysis (6). The mean incubation period before the onset of trembling symptoms was longer (10 days) in bees kept in contaminated cages than in inoculated bees (5 days). This delay is a function of the route of infection. Virus contamination of bees by spraying is less effective than that by inoculation (2).
In our experimental conditions, the outbreak of paralysis was induced with similar efficiencies when naive bees were simply kept in soiled cages and when they were kept in soiled cages together with five paralyzed bees for 5 days (Table 2). We concluded that paralyzed bees spread enough infectious particles in their feces to contaminate naive bees by mere confinement in a soiled environment. Contamination by contact with and/or ingestion of fecal deposits inside the beehive is supposed to play a significant role in the spread of many other bee pathogens, such as bee virus X, bee virus Y, filamentous virus, and the two protozoa Nosema apis and Malpighamoeba mellificae (1, 8, 22, 35). The results of this study indicated a similar risk of CBPV contamination when individual bees remove feces or dead bees from the hive. As described for nosemosis (22), the occurrence of dysentery may effectively aggravate chronic paralysis disease and enhance its spread. These routes of dissemination and contamination are consistent with the density-dependent mechanism suggested by Bailey et al. for transmission of the disease but are independent of direct contact of epidermal cytoplasm between healthy and paralyzed bees (9).
This study provides the first experimental confirmation that infectious CBPV particles, excreted in the feces of paralyzed bees, can infect and provoke overt disease in naive bees by mere confinement of these latter in a CBPV-contaminated environment.
Virus spread via the feces of paralyzed bees may increase the incidence of paralysis when adult bees are confined to their colonies by local events at times of the year when they would normally be foraging (7, 9). These investigations into the mechanisms of CBPV spread, by identifying some of the factors that initiate dissemination, may provide options for reducing virus incidence (13). Further information about CBPV spread and other disease parameters is required to improve models for the prediction of disease outbreak. Better knowledge of virus half-lives outside the host is needed to see if decontamination could provide a resource-efficient means of disease control.
Acknowledgments
This work was supported by the French Ministère de l'Agriculture et de l'Alimentation and by funds from the Fonds Européens d'Orientation et de Garantie Agricole (FEOGA), in accordance with the French program for the improvement of the production and commercialization of beekeeping products.
We are grateful to M.-P. Chauzat and Michel Aubert (AFSSA, Sophia Antipolis, France) for their help and valuable comments on the manuscript. The help of Diana Warwick in improving the English of the manuscript is gratefully acknowledged.
Footnotes
Published ahead of print on 12 October 2007.
REFERENCES
- 1.Allen, M., and B. V. Ball. 1996. The incidence and world distribution of honeybee viruses. Bee World 77:141-162. [Google Scholar]
- 2.Bailey, L. 1965. Paralysis of the honeybee, Apis mellifera Linnaeus. J. Invertebr. Pathol. 7:132-140. [DOI] [PubMed] [Google Scholar]
- 3.Bailey, L. 1967. The incidence of virus diseases in the honeybee. Ann. Appl. Biol. 60:43-48. [DOI] [PubMed] [Google Scholar]
- 4.Bailey, L. 1976. Viruses attacking the honeybee, p. 271-304. In M. A. Lauffer, F. B. Bang, K. Maramorosch, and K. M. Smith (ed.), Advances in virus research, vol. 20. Academic Press, New York, NY. [Google Scholar]
- 5.Bailey, L., and B. V. Ball. 1991. Honeybee pathology. Harcourt Brace Jovanovich, Sidcup, United Kingdom.
- 6.Bailey, L., J. M. Carpenter, and R. D. Woods. 1979. Egypt bee virus and Australian isolates of Kashmir bee virus. J. Gen. Virol. 43:641-647. [Google Scholar]
- 7.Bailey, L., B. V. Ball, and J. N. Perry. 1981. The prevalence of viruses of honeybees in Britain. Ann. Appl. Biol. 97:109-118. [Google Scholar]
- 8.Bailey, L., B. V. Ball, and J. N. Perry. 1983. Association of viruses with two protozoal pathogens of the honeybee. Ann. Appl. Biol. 103:13-20. [Google Scholar]
- 9.Bailey, L., B. V. Ball, and J. N. Perry. 1983. Honeybee paralysis: its natural spread and its diminished incidence in England and Wales. J. Apic. Res. 22:191-195. [Google Scholar]
- 10.Bailey, L., A. J. Gibbs, and R. D. Woods. 1963. Two viruses from adult honeybees (Apis mellifera Linnaeus). Virology 21:390-395. [DOI] [PubMed] [Google Scholar]
- 11.Bailey, L., and R. D. Woods. 1977. Two more small RNA viruses from honeybees and further observations on sacbrood and acute bee-paralysis viruses. J. Gen. Virol. 37:175-182. [Google Scholar]
- 12.Bakonyi, T., R. Frakas, A. Szendroi, M. Dobos-Kovacs, and M. Rusvai. 2002. Detection of acute bee paralysis virus by RT-PCR in honeybee and Varroa destructor field samples: rapid screening of representative Hungarian apiaries. Apidologie 33:63-74. [Google Scholar]
- 13.Ball, B. V. 1996. Honeybee viruses: a cause for concern? Bee World 77:117-119. [Google Scholar]
- 14.Ball, B. V. 1999. Paralysis, p. 81-89. In M. E. Colin, B. V. Ball, and M. Kilani (ed.), Bee disease diagnosis. Options Mediterannéennes, Zaragoza, Spain.
- 15.Ball, B. V., and M. F. Allen. 1988. The prevalence of pathogens in honeybee (Apis mellifera) colonies infested with the parasitic mite Varroa jacobsoni. Ann. Appl. Biol. 113:237-244. [Google Scholar]
- 16.Ball, B. V., and L. Bailey. 1997. Viruses, p. 11-32. In R. A. Morse and K. Flottum (ed.), Honeybee pests, predators, and diseases. A. I. Root Company, Medina, OH.
- 17.Ball, B. V., H. A. Overton, K. W. Buck, L. Bailey, and J. N. Perry. 1985. Relationships between the multiplication of chronic bee-paralysis virus and its associate particle. J. Gen. Virol. 66:1423-1429. [Google Scholar]
- 18.Blanchard, P., M. Ribière, O. Celle, P. Lallemand, F. Schurr, V. Olivier, A. L. Iscache, and J. P. Faucon. 2007. Evaluation of a real-time two step RT-PCR assay for quantitation of chronic bee paralysis virus (CBPV) genome in experimentally infected bee tissues and in life stages of a symptomatic colony. J. Virol. Methods 141:7-13. [DOI] [PubMed] [Google Scholar]
- 19.Chen, Y., J. Evans, M. Hamilton, and M. Feldlaufer. 2007. The influence of RNA integrity on the detection of honeybee viruses: molecular assessment of different sample storage methods. J. Apic. Res. 46:81-87. [Google Scholar]
- 20.Chen, Y. P., J. S. Pettis, A. Collins, and M. F. Feldlaufer. 2006. Prevalence and transmission of honeybee viruses. Appl. Environ. Microbiol. 72:606-611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chungue, E., C. Roche, M. F. Lefevre, P. Barbazan, and S. Chanteau. 1993. Ultra-rapid, simple, sensitive, and economical silica method for extraction of dengue viral RNA from clinical specimens and mosquitoes by reverse transcriptase-polymerase chain reaction. J. Med. Virol. 40:142-145. [DOI] [PubMed] [Google Scholar]
- 22.Fries, I. 1997. Protozoa, p. 56-76. In R. A. Morse and K. Flottum (ed.), Honeybee pests, predators and diseases. A. I. Root Company, Medina, OH.
- 23.Fries, I., R. Martín, A. Meana, P. García-Palencia, and M. Higes. 2006. Natural infections of Nosema ceranae in European honeybees. J. Apic. Res. 45:230-233. [Google Scholar]
- 24.Hale, A. D., J. Green, and D. W. Brown. 1996. Comparison of four RNA extraction methods for the detection of small round structured viruses in faecal specimens. J. Virol. Methods 57:195-201. [DOI] [PubMed] [Google Scholar]
- 25.Higes, M., R. Martín, and A. Meana. 2006. Nosema ceranae, a new microsporidian parasite in honeybees in Europe. J. Invertebr. Pathol. 92:93-95. [DOI] [PubMed] [Google Scholar]
- 26.Hung, A. C. F. 2000. PCR detection of Kashmir bee virus in honeybee excreta. J. Apic. Res. 39:103-106. [Google Scholar]
- 27.Klee, J., A. M. Besana, E. Genersch, S. Gisder, A. Nanetti, D. Q. Tam, T. X. Chinh, F. Puerta, J. M. Ruz, P. Kryger, D. Message, F. Hatjina, S. Korpela, I. Fries, and R. J. Paxton. 2007. Widespread dispersal of the microsporidian Nosema ceranae, an emergent pathogen of the western honeybee, Apis mellifera. J. Invertebr. Pathol. 96:1-10. [DOI] [PubMed] [Google Scholar]
- 28.Lou, Q., S. K. Chong, J. F. Fitzgerald, J. A. Siders, S. D. Allen, and C. H. Lee. 1997. Rapid and effective method for preparation of fecal specimens for PCR assays. J. Clin. Microbiol. 35:281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Morse, R. A., and N. W. Calderone. 2000. The value of honeybees as pollinators of US crops in 2000. Bee Cult. 128:2-15. [Google Scholar]
- 30.Ribière, M., J. P. Faucon, and M. Pépin. 2000. Detection of chronic bee paralysis virus infection: application to a field survey. Apidologie 31:567-577. [Google Scholar]
- 31.Ribière, M., C. Triboulot, L. Mathieu, C. Aurières, J. P. Faucon, and M. Pépin. 2002. Molecular diagnosis of chronic bee paralysis virus infection. Apidologie 33:339-351. [Google Scholar]
- 32.Ruijter, A. 2000. Nosemosis of bees, p. 793-795. In Manual of standards for diagnostic tests and vaccines. O. I. E. Standards Commission, Paris, France.
- 33.Tentcheva, D., L. Gauthier, N. Zappulla, B. Dainat, F. Cousserans, M. E. Colin, and M. Bergoin. 2004. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 70:7185-7191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tompkins, G. J. 1991. Purification of invertebrate viruses, p. 31-40. In J. R. Adams and J. R. Bonami (ed.), Atlas of invertebrate viruses. CRC Press, Boca Raton, FL.
- 35.Varis, A. L., B. V. Ball, and M. Allen. 1992. The incidence of pathogens in honeybee (Apis mellifera L) colonies in Finland and Great Britain. Apidologie 23:133-137. [Google Scholar]
- 36.Wilde, J., J. Eiden, and R. Yolken. 1990. Removal of inhibitory substances from human fecal specimens for detection of group A rotaviruses by reverse transcriptase and polymerase chain reactions. J. Clin. Microbiol. 28:1300-1307. [DOI] [PMC free article] [PubMed] [Google Scholar]