Abstract
Protein kinase A (PKA) and the nuclear A-kinase–anchoring protein AKAP95 have previously been shown to localize in separate compartments in interphase but associate at mitosis. We demonstrate here a role for the mitotic AKAP95–PKA complex. In HeLa cells, AKAP95 is associated with the nuclear matrix in interphase and redistributes mostly into a chromatin fraction at mitosis. In a cytosolic extract derived from mitotic cells, AKAP95 recruits the RIIα regulatory subunit of PKA onto chromatin. Intranuclear immunoblocking of AKAP95 inhibits chromosome condensation at mitosis and in mitotic extract in a PKA-independent manner. Immunodepletion of AKAP95 from the extract or immunoblocking of AKAP95 at metaphase induces premature chromatin decondensation. Condensation is restored in vitro by a recombinant AKAP95 fragment comprising the 306–carboxy-terminal amino acids of the protein. Maintenance of condensed chromatin requires PKA binding to chromatin-associated AKAP95 and cAMP signaling through PKA. Chromatin-associated AKAP95 interacts with Eg7, the human homologue of Xenopus pEg7, a component of the 13S condensin complex. Moreover, immunoblocking nuclear AKAP95 inhibits the recruitment of Eg7 to chromatin in vitro. We propose that AKAP95 is a multivalent molecule that in addition to anchoring a cAMP/PKA–signaling complex onto chromosomes, plays a role in regulating chromosome structure at mitosis.
Keywords: AKAP, cAMP, chromosome condensation, mitosis, PKA
Proper packaging of DNA into chromosomes is an essential process in preparation for mitosis. Chromosome condensation at mitosis requires DNA topoisomerase II (Adachi et al. 1991) and a family of proteins of highly conserved ATPases called SMCs (structural maintenance of chromosomes) (Hirano and Mitchison 1994; Saitoh et al. 1994; Hirano et al. 1997). Evidence that SMCs promote chromosome condensation was provided by the purification of Xenopus 8S and 13S multiprotein complexes, termed condensins (Hirano et al. 1997), and the demonstration that two of these proteins are required for chromosome condensation and maintenance of condensed chromatin (Hirano and Mitchison 1994). Another component of the 13S condensin complex, pEg7, was also recently shown to be implicated in mitotic chromosome condensation in vitro (Cubizolles et al. 1998).
cAMP-dependent protein kinase A (PKA) has been proposed to be a negative regulator of mitosis. PKA activity oscillates in cycling Xenopus egg extracts (Grieco et al. 1994). Onset of mitosis correlates with a decrease in cAMP level and PKA activity, whereas cAMP level and PKA activity rise during metaphase to peak at early interphase (Grieco et al. 1996). Consistent with this finding, downregulation of PKA mediated by microinjection of the PKA inhibitor PKI was shown together with activation of cyclin-dependent kinase 1 (CDK1) to be required for mitotic nuclear envelope disassembly and chromatin condensation in cultured mammalian cells (Lamb et al. 1991). In contrast, PKA activation is necessary for nuclear reassembly upon exit from mitosis (Grieco et al. 1996). These results suggest a requirement for a downregulation of cAMP/PKA signaling for entry into mitosis, but they do not explain the gradual rise in PKA activity during mitosis.
Biological effects of cAMP are mainly mediated by PKA types I and II in eukaryotic cells. The PKA type II holoenzyme complex consists of two catalytic (C) and two regulatory (RIIα or RIIβ) subunits, which modulate the catalytic activity of PKA by binding and inactivating C (Scott 1991). PKA is activated by binding of two cAMP molecules to each R subunit that promotes release of the C subunits from the R–cAMP complex. Active C subunits phosphorylate specific substrates and can be translocated to the nucleus, where they play a role in gene activation (Riabowol et al. 1988).
The specificity of cellular and nuclear responses to cAMP is mediated by targeting of the RII subunit of PKA to discrete subcellular loci through associations with A-kinase–anchoring proteins or AKAPs (Colledge and Scott 1999). A 95-kD AKAP, designated AKAP95, has been cloned and characterized in the rat (Coghlan et al. 1994) and human (Eide et al. 1998). AKAP95 has been localized exclusively in the nucleus of interphase rat and human fibroblasts (Coghlan et al. 1994; Eide et al. 1998); however, as no RIIα has been detected in interphase nuclei (Eide et al. 1998), the role of AKAP95 in the nucleus remains elusive. At mitosis, AKAP95 interacts with RIIα apparently in the vicinity of the metaphase plate (Eide et al. 1998). These observations suggest that interaction between AKAP95 and RIIα may be cell cycle–regulated, but the significance of the AKAP95–RIIα complex at mitosis remains unknown.
We demonstrate here in in vivo and in vitro immunoblocking and rescue experiments the formation of an AKAP95–PKA signaling complex onto mitotic chromosomes, and a role of AKAP95 in chromatin condensation and maintenance of condensed chromosomes during mitosis. The latter process also requires cAMP/PKA signaling and anchoring of PKA to chromatin by AKAP95. The data also suggest that one function of AKAP95 is to promote the recruitment of components of the condensin complex onto chromatin. The results argue towards a critical role of AKAP95 in the regulation of chromatin structure at mitosis and provide a functional significance for increasing PKA activity during mitosis.
Materials and Methods
Buffers, Reagents, and Antibodies
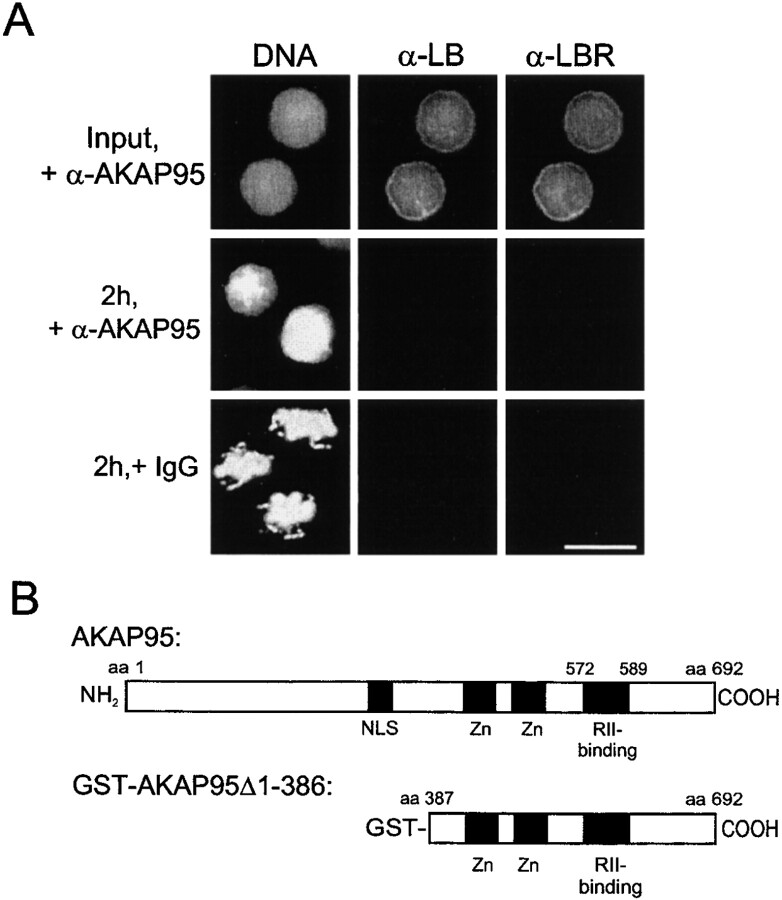
Nuclear isolation buffer (buffer N) consisted of 10 mM Hepes, pH 7.5, 2 mM MgCl2, 250 mM sucrose, 25 mM KCl, 1 mM DTT, 1 mM PMSF, and 10 μg/ml each of aprotinin, leupeptin, and pepstatin A. Cell lysis buffer consisted of 20 mM Hepes, pH 8.2, 5 mM MgCl2, 10 mM EDTA, 1 mM DTT, and 20 μg/ml cytochalasin B and protease inhibitors. A GST-AKAP95 fragment covering amino acids 387–692 and including the RII-binding domain of human AKAP95 (designated GST-AKAP95Δ1-386) was described previously (Eide et al. 1998; see Fig. 5 C). A synthetic peptide derived from the AKAP Ht31 was described previously and contained the amphipathic helix structure of Ht31 required for RII binding (Carr et al. 1991); thus, Ht31 was used as a specific inhibitor of AKAP–RII interaction. Control Ht31-P peptides corresponded to Ht31 with two isoleucine residues mutated to prolines, disrupting the amphipathic helix structure of AKAPs (Carr et al. 1991). Affinity-purified rabbit polyclonal antibodies against AKAP95 (Upstate Biotechnology Inc.) were described elsewhere (Coghlan et al. 1994; Eide et al. 1998). Two novel mAbs against human AKAP95 (mAb36 and mAb47) were developed in conjunction with Transduction Laboratories. Each mAb was produced by immunizing mice with the GST-AKAP95Δ1-386 fusion peptide. Anti–human RIIα mAbs (Transduction Laboratories) were described earlier (Eide et al. 1998). Affinity-purified rabbit antibodies against human lamin B receptor (LBR) or heterochromatin protein 1α, rabbit antiserum against a peptide of human lamin B, and human anti–lamin B autoantibodies (gifts from J.-C. Courvalin, Institut Jacques Monod, Paris, France) were described previously (Chaudhary and Courvalin 1993; Collas et al. 1996; Buendia and Courvalin 1997; Minc et al. 1999). Anti–human Eg7 polyclonal antibodies were generated by immunizing rabbits with a peptide comprising the last 15 amino acids of human Eg7 (KTTPILRASARRHRS) (Cubizolles et al. 1998).
Figure 5.
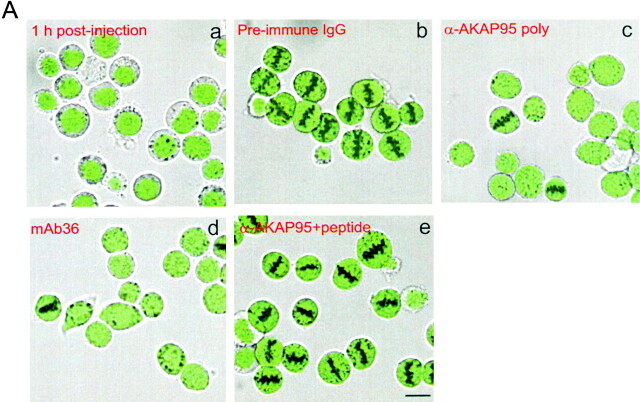
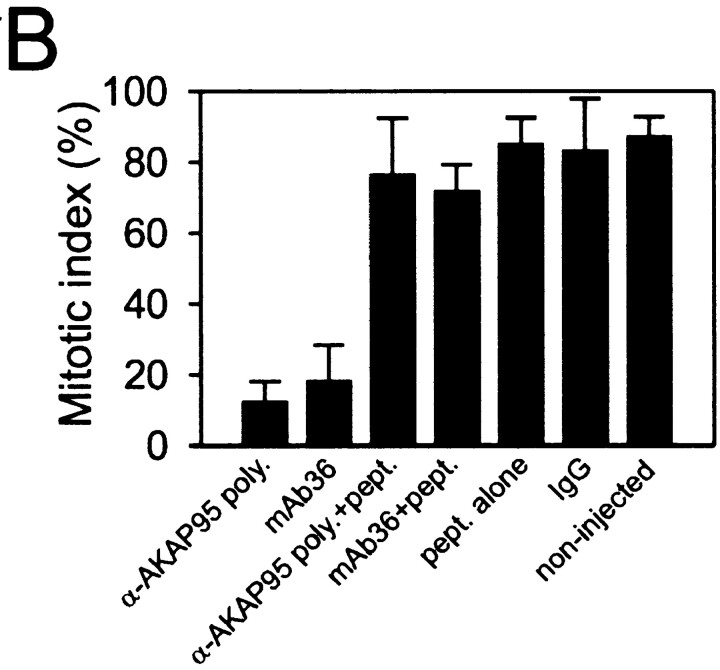
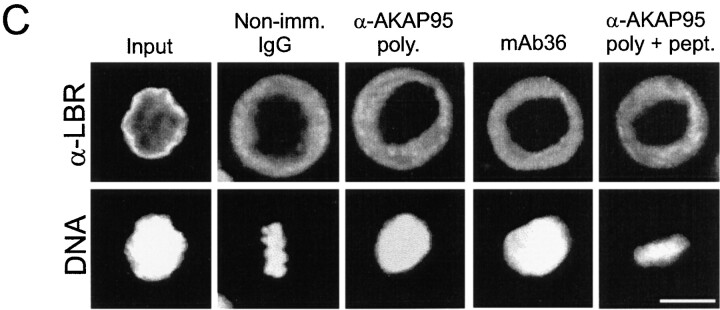
Injection of anti–AKAP95 antibodies into HeLa cell nuclei prevents mitotic chromosome condensation but not nuclear membrane disassembly. (A) Cells were synchronized in S phase by double thymidine block (a, input cells), and nuclei were microinjected with ∼2 pg of either preimmune IgGs (b), affinity-purified anti–AKAP95 polyclonal antibodies (c), mAb 36 (d), or anti–AKAP95 polyclonal antibodies with ∼250 pg competitor GST-AKAP95Δ1-386 peptide (e). In each case, injection solution contained 10 μg/ml of a 150-kD FITC-dextran (green) to verify injections into nuclei (a). Subsequently, injected cells were synchronized in metaphase with nocodazole for 18 h and examined by bright field and fluorescence microscopy (b–e). (B) Mitotic index of cells injected with indicated reagents or control noninjected cells was determined after labeling DNA with Hoechst 33342. (C) Immunofluorescence detection of LBR in noninjected interphase cells, or cells injected with indicated reagents as in A. DNA was labeled with Hoechst 33342. Bars, 10 μm.
Cell Synchronization and Microinjection
HeLa cells were grown in EMEM medium (GIBCO BRL) as described in Eide et al. 1998. For microinjections, cells were synchronized in S phase by a double thymidine (2.5 mM) block (Keryer et al. 1998). Cells were synchronized in M phase by subsequent exposure to 1 μM nocodazole for 18 h (Eide et al. 1998). Mitotic indexes were typically ∼95%.
For nuclear microinjections, HeLa cells were plated onto glass coverslips at 106 cells per 28-cm2 dish and synchronized in S phase by double thymidine block. Nuclei were microinjected with 25–50 pl of solution using a Narishige micromanipulator and picoinjector. Injection solution consisted of EMEM containing 10 μg/ml of a 150-kD FITC-dextran (Sigma Chemical Co.) to visualize the injections. Nuclei were injected with either 2–5 pg affinity-purified anti–AKAP95 polyclonal antibodies (at ∼0.1 mg/ml IgG), mAb36 (diluted 1/10), 2–5 pg preimmune IgGs (at ∼0.1 mg/ml), or ∼250 pg GST-AKAP95Δ1-386 peptide together with anti–AKAP95 antibodies. Inhibitors, antagonists, or catalytic peptides were injected at concentrations indicated in Results. Successful nuclear injections were assessed by retention of the FITC-dextran within the nucleus 1 h after injection (see Results). After a resting period of 6 h, injected cells were synchronized in M phase with 1 μM nocodazole. Mitotic cells were microinjected as described above while arrested in M phase with nocodazole. Injected cells remained in nocodazole for up to 2 h after injection during the period of observation.
Mitotic and Interphase Cell Extracts
Mitotic HeLa cells were washed once in ice-cold PBS, once in 20 vol of ice-cold lysis buffer, and packed at 800 g for 10 min. The cell pellet was resuspended in 1 vol of lysis buffer and incubated for 30 min on ice. Cells were homogenized by a 2× 2-min sonication on ice and the lysate was centrifuged at 10,000 g for 15 min at 4°C. The supernatant was cleared at 200,000 g for 3 h at 4°C in a Beckman SW55 rotor. The clear supernatant (mitotic cytosolic extract) was aliquoted, frozen in liquid nitrogen, and stored at −80°C. Protein concentration of the extract was usually ∼15 mg/ml. Interphase cytosolic extracts were prepared as above from unsynchronized HeLa cells (95–98% in interphase) except that EDTA was omitted from the cell lysis buffer.
Preparation of Nuclei and Chromatin
Confluent unsynchronized HeLa cells were harvested, washed in PBS, sedimented at 400 g, and resuspended in 20 vol of ice-cold buffer N containing 10 μg/ml cytochalasin B. After a 30-min incubation on ice, cells were homogenized on ice by 140–170 strokes of a tight fitting (B-type) glass pestle in a 7-ml homogenizer. Nuclei were sedimented at 400 g for 10 min at 4°C and washed twice by resuspending in buffer N and centrifuging as above. Essentially, all nuclei recovered were morphologically intact, as judged by phase-contrast microscopy and labeling with 10 μg/ml FITC-conjugated ConA (data not shown) (Collas 1999). Nuclei were used fresh or frozen at −80°C in buffer N containing 70% glycerol. High salt (2 M NaCl)–extracted nuclear matrices were prepared from purified nuclei as described previously (Reyes et al. 1997). Matrices were solubilized in 8 M urea/10 mM Tris-HCl, pH 8.0.
To prepare interphase chromatin, 108 HeLa nuclei suspended in 200 μl buffer N containing 1% Triton X-100 were incubated with 5 U of micrococcal nuclease (Sigma Chemical Co.) at 37°C for 5 min. Digestion was terminated by adding EDTA to 5 mM and the mixture was centrifuged at 10,000 g for 5 min. The supernatant (S1) was collected and the pellet was resuspended in 2 mM EDTA and incubated for 15 min at 4°C. After sedimentation as above, the supernatant (S2) was combined with S1 to yield the chromatin fraction, proteins were precipitated with 10% TCA and dissolved in SDS sample buffer. To solubilize mitotic condensed chromatin, mitotic cell lysates were centrifuged at 10,000 g for 10 min. The pellet was resuspended in 500 μl buffer and sedimented at 1,000 g for 20 min in a swingout rotor (Eppendorf) through a 1-M sucrose cushion made in buffer N. Mitotic chromosomes were recovered from the pellet. This pellet was extracted with 1% Triton X-100 at 4°C for 30 min, sedimented at 15,000 g for 15 min, and the detergent-insoluble fraction was digested with 5 U/ml micrococcal nuclease at 37°C for 5 min. After sedimentation as above, proteins of the supernatant (soluble chromatin) were precipitated in 10% TCA and dissolved in SDS-sample buffer.
Loading of Nuclei with Anti–AKAP95 Antibodies
Purified HeLa cell nuclei (2,000 nuclei/μl) were permeabilized in 500 μl buffer N containing 0.75 μg/ml lysolecithin for 15 min at room temperature. Excess lysolecithin was quenched by adding 1 ml of 3% BSA made in buffer N and incubating for 5 min on ice, before sedimenting the nuclei and washing once in buffer N. Nuclei were resuspended at 2,000 nuclei/μl in 100 μl buffer N containing affinity-purified anti–AKAP95 antibodies (1:40 dilution; 2.5 μg) or 2.5 μg of preimmune rabbit IgGs. After a 1-h incubation on ice with gentle agitation, nuclei were sedimented at 500 g through 1 M sucrose for 20 min and held in buffer N on ice until use. Antibody loading of nuclei was verified by immunofluorescence. AKAP95 was not proteolyzed during permeabilization and antibody loading procedures, and lysolecithin-treatment of nuclei did not affect nuclear envelope disassembly or chromatin condensation in mitotic extract (data not shown).
Nuclear Disassembly and Chromatin Condensation Assay
A nuclear disassembly/chromatin condensation reaction consisted of 20 μl mitotic extract, 1 μl nuclear suspension (∼2 × 104 nuclei), and 0.6 μl of an ATP-generating system (1 mM ATP, 10 mM creatine phosphate, 25 μg/ml creatine kinase; Collas 1998). The reaction was started by addition of the ATP-generating system and allowed to proceed at 30°C for up to 2 h. The extract supported nuclear envelope disassembly and chromatin condensation without apoptotic proteolysis of nuclear envelope proteins or DNA degradation (data not shown; see Lazebnik et al. 1993; Duband-Goulet et al. 1998). Chromatin condensation was monitored by after staining samples with 0.1 μg/ml Hoechst 33342. Chromatin was considered to condense when it acquired an irregular and compact morphology sometimes with distinct chromosomes or chromosome fragments. In some instances, extracts were preincubated with inhibitors for 30 min on ice before adding nuclei and the ATP-generating system. Percent chromatin condensation was calculated as the ratio of condensed chromatin masses per number of nuclei examined. For biochemical analyses, condensed chromatin was sedimented at 1,000 g through 1 M sucrose as described above and recovered from the pellet.
Premature Chromatin Decondensation Assay
HeLa chromatin condensed in mitotic extract was recovered by sedimentation at 1,000 g through 1 M sucrose, washed by resuspension and sedimentation in lysis buffer, and incubated for up to 2 h in fresh mitotic extract, either intact or immunodepleted of AKAP95 or RIIα. In some experiments, this extract contained antibodies or inhibitors. DNA was labeled with Hoechst and examined as above. Premature chromatin decondensation (PCD) referred to swelling of the chromatin into ovoid or spherical objects, with no discernible chromosomes. Kinetics of PCD was evaluated by measurement of the proportion of chromatin masses undergoing decondensation at regular time intervals.
Histone H1 Kinase Assay
5 μl of HeLa cytosolic extract was mixed with 5 μl of a 2× histone kinase buffer (160 mM β-glycerophosphate, 20 mM EGTA, 30 mM MgCl2, 2 mM DTT, 20 μg/ml each of aprotinin, leupeptin, and pepstatin A, and 100 nM PKI). The kinase reaction was initiated by addition of 10 μl of a cocktail containing 2.5 mg/ml histone H1, 0.7 mM ATP, and 50 μCi of γ-[32P]ATP. The reaction was carried out at room temperature for 15 min and stopped by addition of 15 μl of 2× SDS loading buffer and boiling. Proteins were resolved by 15% SDS-PAGE, the gel was dried, and phosphorylation of histone H1 was visualized by autoradiography.
Immunological Procedures
Immunoblotting of nuclei, chromatin, and reaction supernatants was performed as described (Collas et al. 1996) using the following antibodies: rabbit anti-AKAP95 (1:250 dilution), rabbit anti-lamin B (1,000), anti-LBR (1:500), RIIα mAb (1:250), anti-Eg7 (1:500), and HRP-conjugated secondary antibodies. Blots were revealed with chloronaphtol/hydrogen peroxide (Collas et al. 1996) or by enhanced chemiluminescence (Amersham). For immunoprecipitations, whole mitotic or interphase cells (as indicated) were sonicated twice for 2 min on ice in immunoprecipitation (IP) buffer (10 mM Tris-HCl, pH 7.5, 50 mM KCl, 1% Triton X-100, and protease inhibitors), and the lysate was centrifuged at 15,000 g for 15 min. The supernatant was precleared with protein A/G agarose (1:25 dilution) at 4°C for 1 h. Immunoprecipitations were carried out with relevant antibodies (anti–RIIα mAb, anti–AKAP95 polyclonal, and anti-Eg7, each at a 1:50 dilution) from the supernatant at room temperature for 2.5 h, followed by incubation with protein A/G agarose (1:25 dilution) for 1.5 h and centrifugation at 4,000 g for 10 min. Immune complexes were washed three times in IP buffer and proteins were eluted in 2× SDS boiling sample buffer. RIIα was also immunoprecipitated from mitotic extract, or chromatin, or reaction supernatant fractions diluted 10× in IP buffer and sonicated (chromatin fraction only). AKAP95 or RIIα was also immunodepleted from undiluted cytosolic extracts using affinity-purified anti–AKAP95 antibodies or anti–RIIα mAbs at a 1:50 dilution. For immunofluorescence analysis (Collas et al. 1996), cells, nuclei, or condensed chromatin masses were settled onto poly-l-lysine–coated coverslips, fixed with 3% paraformaldehyde, permeabilized with 0.5% (cells) or 0.1% (nuclei) Triton X-100, and proteins were blocked with 2% BSA in PBS/0.01% Tween 20 (PBST). Primary antibodies (anti–AKAP95 polyclonal, anti-LBR, anti–RIIα mAb, anti–lamin B human autoantibody) and secondary antibodies were used at a 1:100 dilution. DNA was stained with 0.1 μg/ml Hoechst 33342. Observations were made on an Olympus AX70 epifluorescence microscope using a 100× objective, and photographs were taken with a Photonic Science CCD camera and OpenLab software (Improvision Co.).
Results
Subcellular Distribution of Human AKAP95 during the Cell Cycle
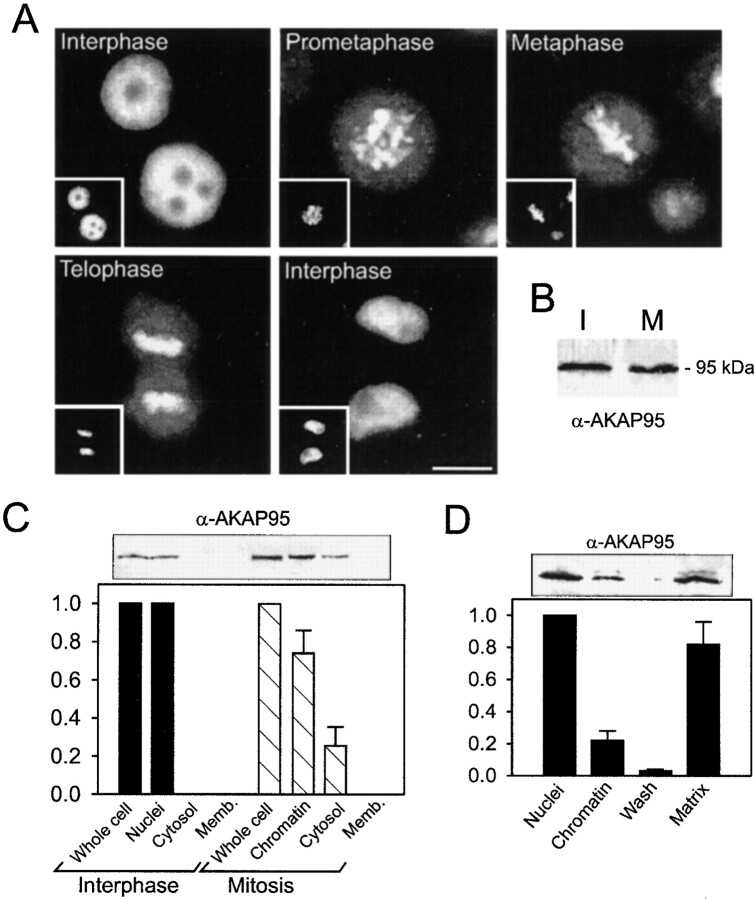
The subcellular localization of human AKAP95 was examined throughout the HeLa cell cycle. Immunofluorescence analysis using an affinity-purified anti–AKAP95 antibody confirmed that AKAP95 was exclusively nuclear in interphase, colocalized with DNA, and was excluded from nucleoli (Fig. 1 A). As early as prometaphase and until telophase, AKAP95 staining colocalized with chromosomes over a diffuse cytoplasmic labeling (Fig. 1 A). In late telophase/interphase, AKAP95 labeling became excluded from the cytoplasm and restricted to the daughter nuclei (Fig. 1 A). Similar results were obtained with two newly developed anti–AKAP95 mAbs, mAb36 and mAb47, and in human 293T fibroblasts with all three antibodies (data not shown).
Figure 1.
Subcellular and subnuclear localization of AKAP95 in HeLa cells. (A) The distribution of AKAP95 during the HeLa cell cycle was examined by immunofluorescence analysis of unsynchronized cells using an affinity-purified polyclonal anti–AKAP95 antibody. DNA (insets) was labeled with Hoechst 33342. Bar, 10 μm. (B) Interphase (I) and mitotic (M) HeLa cells (107 cells each) were dissolved in SDS-sample buffer, proteins separated by SDS-PAGE, and immunoblotted using the affinity-purified anti–AKAP95 antibody. (C) Interphase and mitotic HeLa cells (107 and 5 × 107, respectively) were fractionated into nuclei or chromatin, cytosol, and cytoplasmic membranes, and proteins of each fraction were immunoblotted using anti–AKAP95 antibodies. Relative amounts of AKAP95 in each fraction were determined by densitometric analysis of duplicate blots. (D) Purified HeLa nuclei (108) were fractionated into chromatin and high salt–extracted nuclear matrices, proteins were immunoblotted using anti–AKAP95 antibodies, and duplicate blots analyzed by densitometry.
Immunoblotting analysis of AKAP95 revealed similar levels of AKAP95 in whole interphase and mitotic cell lysates (Fig. 1 B). Fractionation of interphase and mitotic cells confirmed that AKAP95 was exclusively nuclear in interphase (Fig. 1 C). At mitosis, ∼75% of AKAP95 cofractionated with chromatin, whereas ∼25% was detected in a 200,000-g supernatant fraction (Fig. 1 C, cytosol). No AKAP95 was detected in membrane fractions of interphase or mitotic cells (Fig. 1 C). Furthermore, fractionation of purified HeLa nuclei into chromatin and high salt–extracted nuclear matrices revealed that ∼80% of AKAP95 partitioned with the matrix, whereas ∼20% cofractionated with chromatin (Fig. 1 D). Thus, AKAP95 is restricted to the nuclei of interphase cells, where it associates primarily with the nuclear matrix. At mitosis, AKAP95 redistributes into a major chromatin-associated fraction and a minor soluble pool. Upon nuclear reassembly at the end of mitosis, AKAP95 is sequestered into the daughter nuclei where it re-acquires an interphase distribution.
AKAP95 Associates with the RIIα Regulatory Subunit of PKA at Mitosis and in the Mitotic Extract
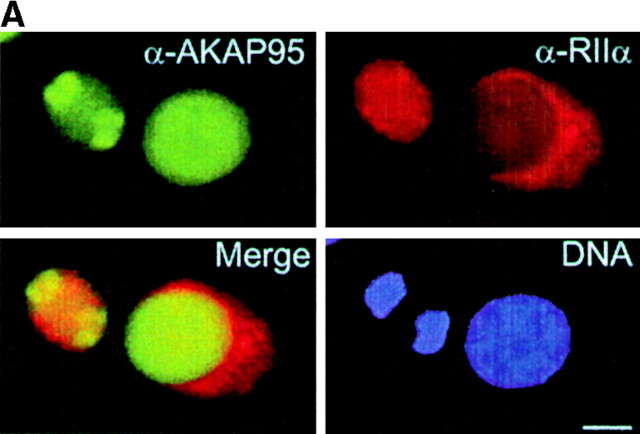
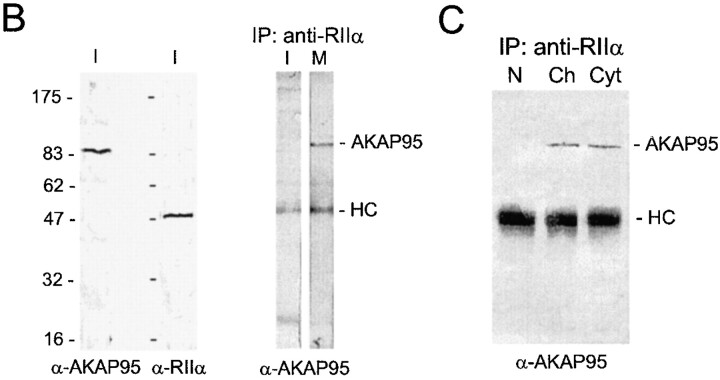
Association of AKAP95 with RIIα in interphase and mitosis was investigated in dual immunofluorescence and immunoprecipitation experiments. In interphase, AKAP95 labeling was clearly distinct from the cytoplasmic staining of RIIα (Fig. 2 A, right cell in all panels). At mitosis, RIIα exhibited a more uniform cytoplasmic labeling that mostly overlapped with that of AKAP95 (Fig. 2 A, left cell in all panels). Although both AKAP95 and RIIα were detected by immunoblotting in interphase whole cell lysates (Fig. 2 B, left), immunoprecipitation of RIIα from such lysates did not coprecipitate AKAP95 in interphase, but did coprecipitate AKAP95 at mitosis (Fig. 2 B, right). Immunoprecipitation of RIIα also coprecipitated AKAP95 from both a mitotic cytosolic fraction and from mitotic chromatin solubilized by micrococcal nuclease digestion (Fig. 2 C). This illustrates the assembly of both soluble and chromatin-associated AKAP95–RIIα complexes at mitosis.
Figure 2.
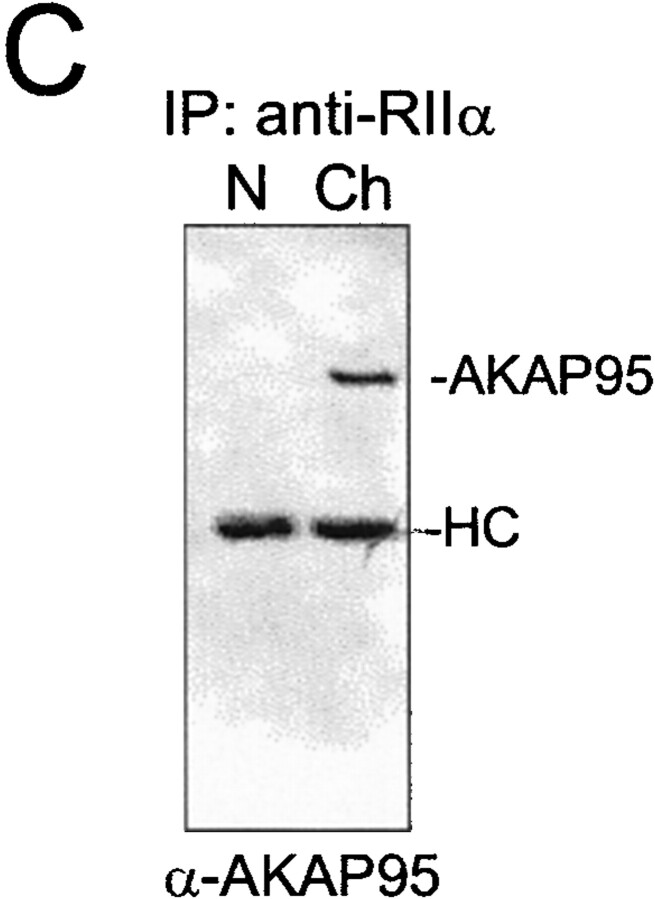
Dynamic association of AKAP95 with the RIIα regulatory subunit of PKA in HeLa cells. (A) Double immunofluorescence analysis of AKAP95 and RIIα using affinity-purified anti–AKAP95 antibodies and an mAb against human RIIα. DNA was labeled with Hoechst 33342. In each panel, the left cell is in mitosis, whereas the right cell is in interphase. Bar, 10 μm. (B, left two panels) Immunoblotting of AKAP95 and RIIα in interphase (I) cells using antibodies described in A. (right) Coimmunoprecipitation of AKAP95 with RIIα from mitotic (M) but not interphase (I) cells. Cells were lysed by sonication, the lysate was cleared at 15,000 g, and RIIα was immunoprecipitated (IP) from the supernatant using anti–RIIα mAbs. Precipitates were immunoblotted with anti–AKAP95 antibodies. (C) Anti-RIIα immunoprecipitation from interphase nuclei (N), mitotic condensed chromatin (Ch), or mitotic extract (Cyt). Precipitates were immunoblotted with anti–AKAP95 antibodies. HC, IgG heavy chain.
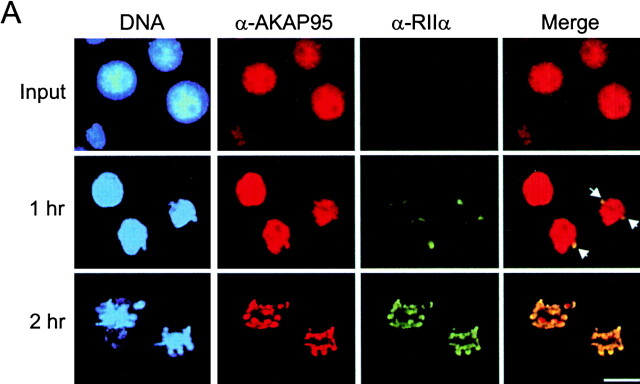
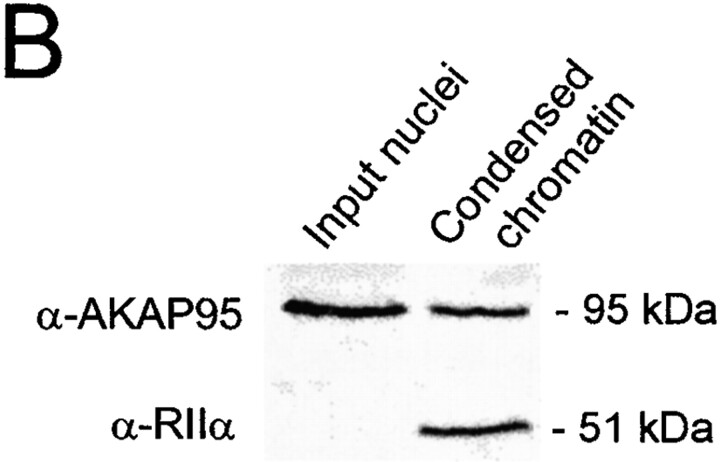
The dynamics of formation of the chromatin-associated mitotic AKAP95–RIIα complex was investigated using a cytosolic extract prepared from mitotic HeLa cells. The extract supports disassembly of exogenous purified interphase HeLa nuclei, including nuclear envelope breakdown and chromatin condensation (see Materials and Methods). Immunofluorescence and immunoblotting analysis of input nuclei and condensed chromatin showed that AKAP95 was associated with the condensing chromatin (Fig. 3 A, α-AKAP95, and B, top panel). As in vivo, a minor portion of AKAP95 was also released into the cytosol during chromatin condensation (data not shown). Concomitantly, RIIα, undetected in input interphase nuclei, was recruited from the extract onto the chromatin surface where it colocalized and cofractionated with AKAP95 (Fig. 3 A, α-RIIα and Merge, and B, bottom panel). Furthermore, immunoprecipitation of RIIα from condensed chromatin coprecipitated AKAP95 (Fig. 3 C), illustrating the formation of a chromatin-associated AKAP95–RIIα complex during chromatin condensation in vitro. It is noteworthy that association of AKAP95 with chromatin also occurred in mitotic extract immunodepleted of RIIα (data not shown), indicating that mitotic redistribution of AKAP95 is independent of RIIα.
Figure 3.
Association of the RIIα regulatory subunit of PKA with AKAP95 during chromatin condensation. (A) Purified HeLa nuclei were disassembled in mitotic extract and double-labeled with anti–AKAP95 and anti–RIIα antibodies at indicated time points. Arrows in merged images point to initial sites of RIIα accumulation on the chromatin. Bar, 10 μm. (B) Input nuclei and condensed chromatin (after 2 h in the extract as in A) were immunoblotted using anti–AKAP95 and anti–RIIα antibodies. (C) Immunoprecipitation of RIIα from lysates of input nuclei (N) or condensed chromatin (Ch). Immune complexes were immunoblotted using anti-AKAP95 antibodies. HC, IgG heavy IgG chain.
Altogether, these results indicate that in mitotic extract, nuclear AKAP95 is redistributed and primarily associates with condensing chromosomes in an RIIα-independent manner. Concomitantly, AKAP95 recruits RIIα from a cytosolic pool onto chromatin. Thus, one function of chromatin-associated AKAP95 at mitosis is to target PKA, via RIIα, to condensing chromosomes.
Mitotic Chromosome Condensation Requires Functional AKAP95
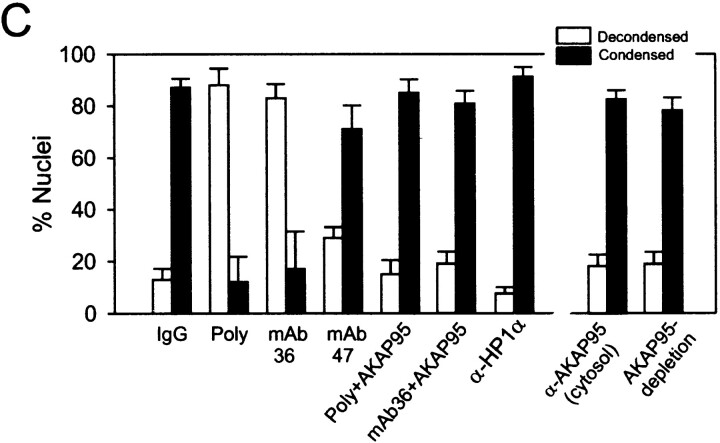
The functional significance of the chromatin-associated AKAP95–RIIα complex was first investigated using the mitotic extract. To this end, we attempted to block the function of nuclear AKAP95 by introducing affinity-purified anti–AKAP95 antibodies (or control preimmune rabbit IgGs) into purified HeLa nuclei as described in Materials and Methods. Antibody-loaded nuclei (Fig. 4 A, Input) were exposed to mitotic extract and nuclear disassembly assessed after 2 h by DNA staining and immunofluorescence analysis using anti–lamin B and anti–LBR antibodies. Fig. 4 A shows that anti–AKAP95 antibodies, but not preimmune IgGs, inhibited chromatin condensation although nuclear envelope disassembly took place as judged by the absence of lamin B or LBR labeling. Inhibition of chromatin condensation occurred with most (∼90%) chromatin masses examined (see Fig. 4 C, Poly) and was reproduced by mAb36, whereas mAb47 was little effective (Fig. 4 C). Inhibition of chromatin condensation was specific for anti–AKAP95 antibodies since it did not occur with the following: anti–AKAP95 antibodies introduced into nuclei with 0.1 mg/ml of the GST-AKAP95Δ1-386 peptide (Fig. 4 B) used to generate the antibodies (Fig. 4 C, Poly + AKAP95, mAb36 + AKAP95); and affinity-purified polyclonal antibodies against heterochromatin proteins HP1α (Fig. 4 C), HP1β, or HP1γ (not shown) (Minc et al. 1999).
Figure 4.
Anti–AKAP95 antibodies introduced into nuclei inhibit chromatin condensation in mitotic extract. (A) HeLa nuclei were loaded with either affinity-purified anti–AKAP95 antibodies (+α-AKAP95) or preimmune IgGs (+IgGs) and incubated for 2 h in a cytosolic extract prepared from mitotic HeLa cells. Aliquots of extracts were immunofluorescently labeled with human anti–lamin B (α-LB) and rabbit anti–LBR (α-LBR) antibodies. DNA was stained with Hoechst 33342. Note the lack of chromatin condensation with anti–AKAP95 antibodies (middle). (B) Schematic representation of full-length human AKAP95 (top; Eide et al. 1998) and of the GST-AKAP95Δ1-386 peptide (bottom). (C) HeLa nuclei were loaded with either preimmune IgGs (IgG), affinity-purified anti–AKAP95 antibodies (Poly) alone, or together with a competitor GST-AKAP95Δ1-386 peptide (Poly + AKAP95), mAb36 alone or with GST-AKAPΔ951-386 (mAb36 + AKAP95), mAb47 or affinity-purified anti–HP1α antibodies (α-HP1α). Nuclei were incubated in mitotic extract for 2 h as in A, the DNA was stained, and proportions (percent ± SD) of decondensed (open bars) and condensed (filled bars) chromatin masses determined. Alternatively, untreated nuclei were incubated in mitotic extract containing affinity-purified anti–AKAP95 antibodies or immunodepleted of soluble AKAP95, and proportions of decondensed and condensed chromatin masses were determined (right two data sets).
Preincubation of anti–AKAP95 antibodies in the extract or immunodepletion of soluble AKAP95 did not inhibit condensation (Fig. 4 C). This illustrates a role for nuclear rather than cytosolic AKAP95 in chromatin condensation. Additionally, loading nuclei with anti–AKAP95 antibodies did not block the recruitment of RIIα onto the chromatin, as judged by immunofluorescence and immunoblotting analyses of condensed chromatin (data not shown). This argues that inhibition of chromatin condensation with anti–AKAP95 antibodies does not result from inhibition of the PKA-anchoring activity of AKAP95.
To determine whether inhibiting nuclear AKAP95 function in vivo affected mitotic chromosome condensation, nuclei of HeLa cells synchronized in S phase by a double thymidine block were microinjected with ∼2–5 pg of anti–AKAP95 polyclonal antibodies or mAb36, each with or without 250 pg of competitor GST-AKAP95Δ1-386 peptide, or with preimmune IgGs. Successful nuclear injections were verified by coinjection of a 150-kD FITC-dextran and scored by retention of the dextran within the nuclei 1 h after injection (Fig. 5 A, a, green). Injected cells were released from the thymidine block, exposed to 1 μM nocodazole, and the proportion of cells arrested at mitosis was determined after 17 h. A fraction of injected cells was also analyzed by immunofluorescence using antibodies against LBR, a marker of the inner nuclear membrane. Fig. 5 shows that, after nocodazole treatment, cells injected with anti–AKAP95 antibodies did not undergo chromosome condensation, as shown by the absence of detectable metaphase chromosomes under bright field microscopy (Fig. 5 A, c and d) and after DNA staining in most cells (Fig. 5 C), and by mitotic indexes of 15–20% (Fig. 5 B). In contrast, preimmune IgGs or anti–AKAP95 antibodies injected with GST-AKAP95Δ1-386 did not prevent chromosomes from forming a metaphase plate (Fig. 5 A, b and e, and B; see Table ). In addition, despite the lack of chromosome condensation in antibody-injected cells, the release of FITC-dextran from the nucleus into the cytoplasm of these cells after nocodazole treatment (Fig. 5 A, c and d, green) argued that the nuclear envelope was disassembled. This was verified by immunofluorescence analysis of LBR (Fig. 5 C). Breakdown of the nuclear envelope implies that immunoblocking of nuclear AKAP95 does not prevent entry into mitosis per se, but rather affects chromosome structure at this stage of the cell cycle. However, it should be noted that microinjection of a 25-fold higher concentration of anti–AKAP95 antibodies blocked nuclear envelope breakdown in the majority of injected cells (data not shown).
Table 1.
Disruption of AKA95-RII Anchoring or cAMP/PKA Inhibition Does Not Inhibit Mitotic Chromosome Condensation
| Inhibitor | Mitotic index |
|---|---|
| (Percent) | |
| None (∼25 pl FITC-dextran) | 88 ± 8 |
| α-AKAP95 (polyclonal; ∼2 pg) | 17 ± 7 |
| α-AKAP95 (∼2 pg) + GST-AKAP95Δ1-386 (250 pg) | 80 ± 6 |
| Ht31 (50 nM) | 79 ± 8 |
| Ht31-P (50 nM) | 82 ± 6 |
| PKI (10 nM) | 85 ± 5 |
| Rp-8-Br-cAMPS (10 μM) | 90 ± 4 |
Nuclei of HeLa cells synchronized in interphase were injected with either 2–5 pg affinity-purified polyclonal anti–AKAP95 antibodies, 2–5 pg anti–AKAP95 antibodies with 250 pg GST-AKAP95Δ1-386 peptide, 50 nM RII-anchoring inhibitor peptide Ht31, 50 nM control Ht31-P peptide, 10 nM PKI, or 10 μM cAMP antagonist Rp-8-Br-cAMPS. Injection volumes were 25–50 pl. Control nuclei were injected with 25–50 pl FITC-dextran only. Cells were released from the thymidine block and synchronized with 1 μM nocodazole to induce M phase arrest. After 17 h in nocodazole, mitotic indexes were determined. Data from 30–50 injected cells per treatment are reported. (Similar results were obtained regardless of the site of injection within the cells; data not shown).
Mitotic Chromosome Condensation Does Not Require Anchoring of PKA to AKAP95 nor PKA Activity
The recruitment of RIIα by AKAP95 onto condensing chromatin led us to determine whether the putative role of AKAP95 in chromatin condensation required association with RIIα and anchoring of PKA. To this end, the RII-anchoring inhibitor peptide Ht31 (500 nM) was preincubated in mitotic extract before adding nuclei. Chromatin condensed to the same extent with Ht31 and control Ht31-P peptides that do not compete binding of RII to AKAPs (data not shown). Thus, the role of AKAP95 in chromosome condensation in vitro is independent of its RII-anchoring function. Furthermore, neither chromatin condensation nor nuclear envelope disassembly was affected by inhibiting PKA activity in the extract with 1 μM of the PKA inhibitor PKI, or by downregulating cAMP signaling with 100 μM of the cAMP antagonists Rp-8-Br-cAMPS or Rp-8-CPT-cAMPS (data not shown). Thus, chromosome condensation in mitotic extract does not require cAMP signaling or PKA activity.
The effects of disrupting AKAP-RII anchoring and downregulating cAMP/PKA signaling on chromosome condensation at mitosis were investigated by microinjecting nuclei of interphase HeLa cells with 50 nM Ht31, 50 nM Ht31-P, 10 nM PKI, or 10 μM Rp-8-Br-cAMPS, and assessing mitotic indexes after nocodazole treatment as done previously after immunoblocking of AKAP95. As shown in Table , neither reagent inhibited nuclear envelope breakdown (data not shown) nor chromosome condensation, as judged by mitotic indexes of 79–90%. Therefore, disruption of AKAP95-RII anchoring or cAMP/PKA inhibition does not impair mitotic chromosome condensation.
AKAP95 Function Is Required to Maintain Chromosomes Condensed during Mitosis
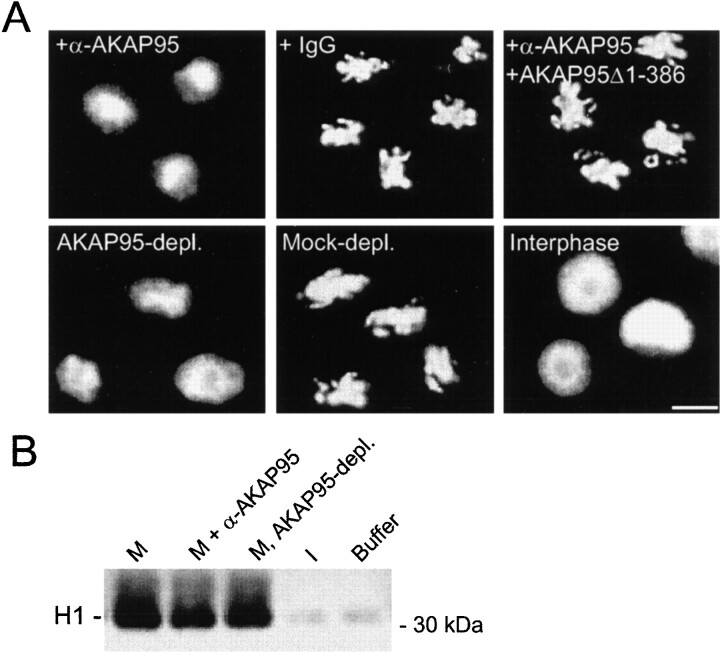
Although chromatin condensation occurred normally in the mitotic extract containing anti–AKAP95 antibodies, we consistently observed a phase of decondensation of the chromatin upon prolonged (>4 h) incubation in the extract (data not shown). This raised the hypothesis that inhibition of AKAP95 might affect the morphology of the condensed chromatin. To test this possibility, chromatin was allowed to condense for 2 h in mitotic extract, anti–AKAP95 antibodies were added (1:50 dilution) and chromatin morphology was examined by DNA staining after another 2 h of incubation. Chromatin decondensation was observed in extract containing anti–AKAP95 antibodies (Fig. 6 A, +α-AKAP95), but not in the extract containing preimmune IgGs or anti–AKAP95 antibodies together with 0.1 mg/ml of GST-AKAP95Δ1-386 competitor peptide (Fig. 6 A). Thus, immunoblocking AKAP95 in the extract prevented the chromatin from remaining in a condensed form. To determine whether immunodepletion of AKAP95 had a similar effect, chromatin condensed in mitotic extract was purified by sedimentation and further incubated in a fresh extract immunodepleted of AKAP95. Chromatin exposed to AKAP95-depleted, but not mock-depleted, extract underwent decondensation within 2 h (Fig. 6 A), indicating that AKAP95 is implicated in maintaining chromatin in a condensed form. Moreover, the extent of chromatin decondensation after immunoblocking or immunodepletion of AKAP95 was more limited than decondensation of mitotic chromatin exposed to an interphase extract (Fig. 6 A, Interphase), suggesting the involvement of distinct decondensation pathways. Consequently, chromatin decondensation in mitotic extract was referred to as premature chromatin decondensation (PCD).
Figure 6.
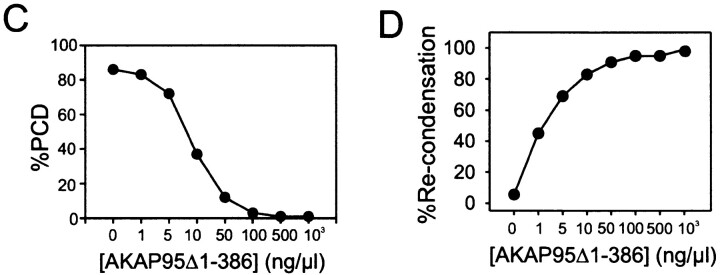
AKAP95 is required for the maintenance of condensed chromatin in mitotic extract. (A) Immunoblocking or immunodepletion of AKAP95 promotes PCD. Morphology of in vitro–condensed chromatin exposed for 2 h to mitotic extract containing either affinity-purified anti–AKAP95 antibodies (+α-AKAP95), preimmune IgGs (+IgG), or anti–AKAP95 antibodies with 0.1 mg/ml GST-AKAP95Δ1-386 competitor peptide (+α-AKAP95 + GST-AKAP95Δ1-386). Alternatively, chromatin was also exposed to mitotic extract immuno- or mock-depleted of AKAP95 (AKAP95-depl. and Mock-depl., respectively). Chromatin was also incubated in interphase extract for 1 h (Interphase). Bar, 10 μm. (B) Histone H1 kinase activity of either mitotic extracts after 2 h of incubation at 30°C without (M) or with anti–AKAP95 polyclonal antibodies (M, + α-AKAP95), mitotic extract immunodepleted of AKAP95 (M, AKAP95-depl.), interphase cytosolic extract to assess baseline H1 kinase activity (I), or cell lysis buffer used to prepare the mitotic extracts (Buffer). (C) Inhibition of PCD with GST-AKAP95Δ1-386. Purified condensed chromatin was incubated for 2 h in mitotic extract immunodepleted of AKAP95 and containing increasing concentrations of GST-AKAP95Δ1-386. Proportions of PCD were determined after DNA staining with Hoechst. (D) Restoration of condensation of prematurely decondensed chromatin with GST-AKAP95Δ1-386. Purified condensed chromatin was allowed to undergo PCD for 2 h in mitotic extract immunodepleted of AKAP95. Increasing concentrations of GST-AKAP95Δ1-386 were added, and proportions of recondensed chromatin units determined after another hour of incubation.
As PCD might be interpreted as a consequence of premature exit of the extract from the M phase, we tested this possibility by measuring the level of histone H1 kinase activity in the mitotic extract incubated for 2 h without or with anti–AKAP95 antibodies and in AKAP95-depleted extract. Fig. 6 B shows that elevated (mitotic) levels of H1 kinase activity were maintained in antibody-containing and immunodepleted extracts (compare with basal H1 kinase activity in interphase HeLa cell extract). This result indicates that immunoblocking or immunodepleting AKAP95 did not release the extract from the M phase, and lends further support to the hypothesis that PCD and interphase chromatin decondensation involve distinct processes.
Conclusive evidence that AKAP95 was required for maintenance of condensed chromatin in vitro was provided in a rescue experiment. Purified condensed chromatin was exposed to mitotic extract immunodepleted of AKAP95. Increasing concentrations of GST-AKAP95Δ1-386 were added and after 2 h proportions of PCD were determined by DNA staining. GST-AKAP95Δ1-386 dramatically inhibited PCD in a concentration-dependent manner (Fig. 6 C). We determined whether GST-AKAP95Δ1-386 was also able to promote recondensation of prematurely decondensed chromatin. Purified condensed chromatin was allowed to undergo PCD for 2 h in AKAP95-depleted mitotic extract. Subsequent addition of GST-AKAP95Δ1-386 to the extract restored chromatin condensation also in a concentration-dependent manner (Fig. 6 D). Thus, GST-AKAP95Δ1-386 was capable of inhibiting PCD and recondensing prematurely decondensed chromatin in AKAP95-depleted extract, indicating that AKAP95 is required for maintaining chromatin in a condensed form. Furthermore, since anti–AKAP95 antibodies do not block binding of RIIα to AKAP95, the data also argue that AKAP95 has an intrinsic function in the maintenance of condensed chromatin.
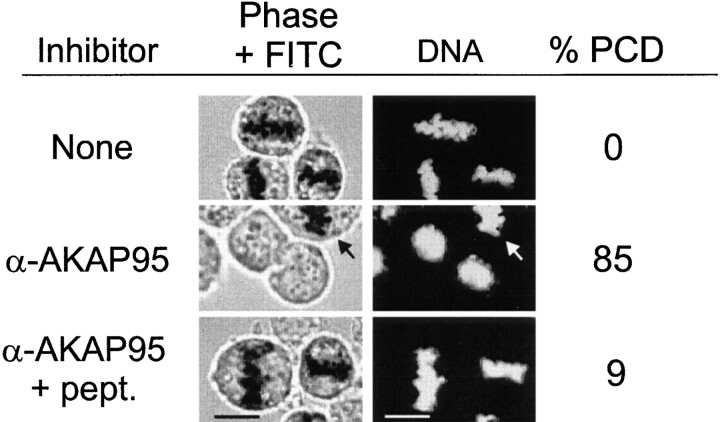
The relevance of these in vitro observations on chromosome structure during mitosis was investigated. HeLa cells arrested in the M phase with 1 μM nocodazole were injected with affinity-purified anti–AKAP95 antibodies and, after 1 h, chromatin morphology was assessed by phase-contrast and DNA staining while cells remained in nocodazole. Anti–AKAP95 antibodies readily induced decondensation of chromosomes that could no longer be distinguished by phase-contrast (Fig. 7, α-AKAP95, arrow points to the metaphase chromosomes of a mitotic cell that was not injected). Furthermore, no nuclear envelope was detected, arguing that decondensation was reminiscent of PCD observed previously in vitro and distinct from interphase nuclear reformation. In contrast, coinjection of anti–AKAP95 antibodies with 250 pg GST-AKAP95Δ1-386 did not alter the state of chromosome condensation (Fig. 7, α-AKAP95 + pept.). These results indicate that chromosome decondensation is not an artefactual consequence of exposure of cells to nocodazole. Rather, they show that as in in vitro, immunoblocking AKAP95 at mitosis induces PCD, indicating that functional AKAP95 is needed for maintaining chromosomes condensed throughout mitosis.
Figure 7.
Induction of PCD in mitotic HeLa cells by immunoblocking of AKAP95. HeLa cells were synchronized in metaphase by thymidine block and nocodazole treatment. Mitotic cells were injected with either 2–5 pg affinity-purified polyclonal anti–AKAP95 antibodies or anti–AKAP95 antibodies together with 250 pg competitor GST-AKAP95Δ1-386 peptide. Control cells were injected with FITC-dextran only (top). Cells remained in nocodazole after injection and were examined after 1 h by phase-contrast and fluorescence (FITC) microscopy. DNA was labeled with Hoechst 33342. Percentage of PCD was calculated from 30–40 cells injected per treatment. Arrow points to a noninjected cell that did not undergo PCD. Bars, 10 μm.
Anchoring of RIIα to AKAP95 and PKA Activity Are Required for Maintenance of Condensed Chromatin during Mitosis
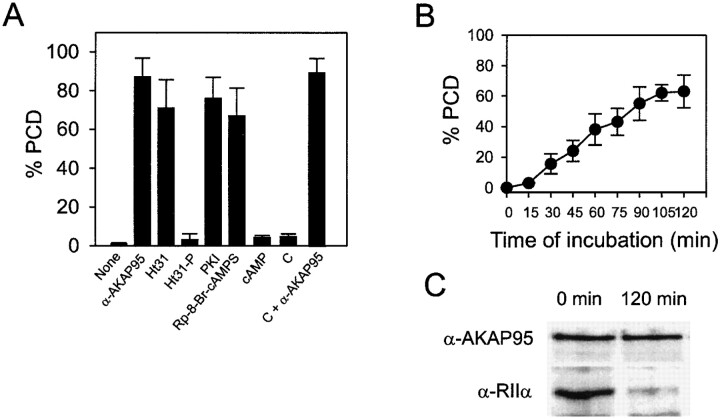
Whether AKAP95–RIIα association and cAMP signaling through PKA were required for maintenance of condensed chromatin during M phase was examined. Purified condensed chromatin was added to a mitotic extract preincubated with 500 nM Ht31 (or control Ht31-P) peptides to disrupt AKAP95–RII interactions (data not shown) and PCD was assessed after 1 h by DNA staining. Fig. 8 A shows that while chromatin remained condensed with Ht31-P, Ht31 induced PCD, indicating a requirement for AKAP95–RII interaction to maintain chromatin condensed. Whether PKA activity and cAMP signaling were involved in this process was determined using PKA inhibitor PKI (1 μM) and the cAMP antagonist Rp-8-Br-cAMPS (100 μM). Both reagents induced PCD, whereas control activation of PKA with 1 μM cAMP had no effect (Fig. 8 A). Adding free C subunits together with anti–AKAP95 antibodies also induced PCD (Fig. 8 A), suggesting that only PKA bound to chromatin-associated AKAP95 is implicated in maintaining condensed chromatin. Altogether, these results indicate that maintenance of condensed chromatin in mitotic extract requires functional AKAP95, cAMP signaling events mediated by PKA and anchoring of PKA (via RIIα) to chromosomes by AKAP95.
Figure 8.
AKAP95–RIIα interaction, cAMP signaling, and PKA activity are required for maintenance of condensed chromatin in mitotic extract. (A) Chromatin condensed in mitotic extract was purified and exposed to fresh mitotic extract containing either anti–AKAP95 antibodies (1:50 dilution), 500 nM Ht31, 500 nM Ht31-P, 1 μM PKI, 100 μM Rp-8-Br-cAMPS, 1 μM cAMP, 15 ng/μl recombinant catalytic subunit of PKA (C), or C plus anti–AKAP95 antibodies. Proportions (percent ± SD) of PCD were determined by DNA labeling after 90 min. (B) Chromatin condensed in mitotic extract was purified and exposed to fresh mitotic extract immunodepleted of RIIα. Proportions (percent ± SD) of PCD were determined by DNA staining of sample aliquots at regular intervals. (C) Chromatin fractions at the start (Input) and at the end (120 min) of incubation in RIIα-depleted mitotic extract were sedimented and proteins were immunoblotted using anti–AKAP95 and anti–RIIα antibodies.
The requirement for RIIα binding to AKAP95 and PKA activity for maintenance of condensed chromatin was tested further by assessing chromatin morphology after immunodepleting RIIα from the extract. Up to 60% of condensed chromatin masses incubated in the mitotic extract depleted of RIIα underwent PCD over a 2-h period (Fig. 8 B). Moreover, immunoblotting analysis of chromatin at the beginning (0 h) and at the end (120 min) of incubation in an RIIα-depleted extract showed that whereas input chromatin harbored RIIα, most RIIα was solubilized by 120 min (Fig. 8 C, bottom). In contrast, no obvious change in the amount of AKAP95 bound to chromatin was detected under these conditions (Fig. 8 C, top). Together with our previous observation that RIIα is recruited from the cytosol to the chromatin via AKAP95, these results reflect a dissociation of RIIα from the chromatin and suggest that, as with AKAP95, keeping RIIα on condensed chromatin involves an equilibrium between soluble and chromatin-associated pools of PKA.
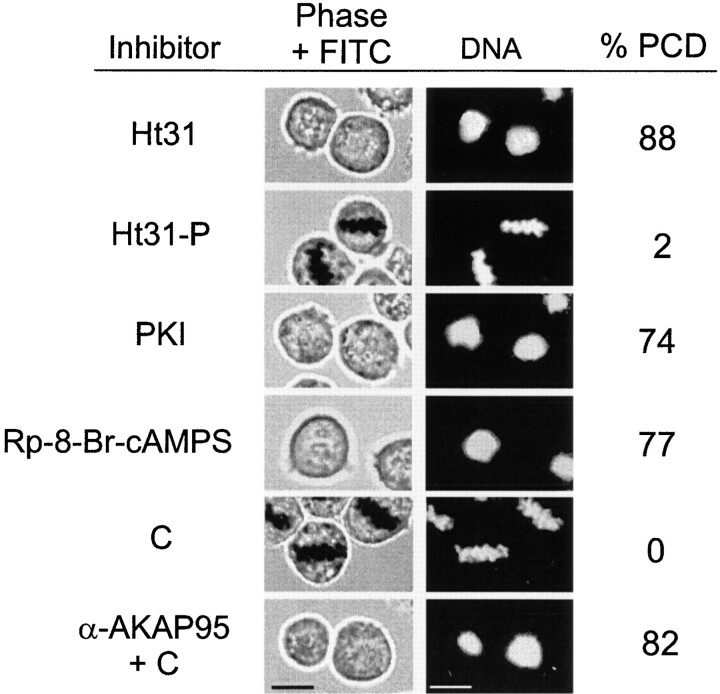
The requirements for AKAP–RII interaction and cAMP/PKA signaling events to maintain chromosomes condensed at mitosis were investigated by microinjecting M phase–arrested HeLa cells with either 50 nM Ht31 or Ht31-P, 10 nM PKI, 10 μM Rp-8-Br-cAMPS, ∼1 ng C, or 2–5 pg anti-AKAP95 antibodies together with ∼1 ng C. The cells remained exposed to nocodazole for 1 h after injections and were examined by phase-contrast microscopy and DNA staining. Data of Fig. 9 show that Ht31 (but not Ht31-P), PKI and Rp-8-Br-cAMPS induced PCD, indicating that PKA-AKAP anchoring, PKA activity, and cAMP signaling are required for maintenance of condensed chromatin throughout mitosis. Coinjection of anti–AKAP95 antibodies with C also promoted PCD (Fig. 9), arguing that functional inhibition of AKAP95 promotes chromatin decondensation even in the presence of unbound PKA.
Figure 9.
Disruption of AKAP95–RII interaction and downregulation of cAMP/PKA signaling promote PCD in mitotic cells. Mitotic HeLa cells were injected as in Fig. 7 with either 50 nM RII-anchoring inhibitor peptide Ht31, 50 nM control Ht31-P, 10 nM PKI, 10 μM cAMP antagonist Rp-8-Br-cAMPS, ∼1 ng C, or ∼1 ng C together with 2–5 pg anti–AKAP95 antibodies. Cells remained in nocodazole after injection and were examined as in Fig. 7. Percentage of PCD was calculated from 30–40 injected cells. Bars, 10 μm.
Immunoblocking of AKAP95 Inhibits Recruitment of Eg7 to Chromatin in Mitotic Extract
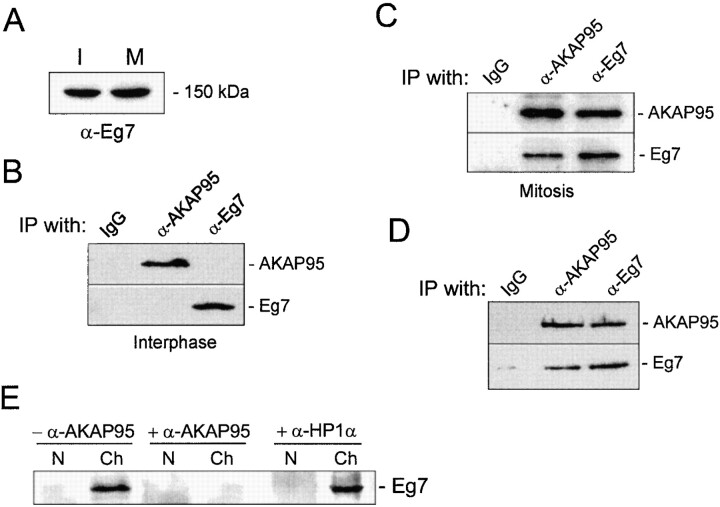
Several proteins have been shown to be required for mitotic chromosome condensation, including pEg7 (Cubizolles et al. 1998), a component of the 13S condensin complex (Hirano et al. 1997). To determine whether AKAP95 was part of the condensin complex or interacted with the complex, immunoprecipitations were carried out first from lysates of interphase or mitotic HeLa cells. Immunoblotting analysis of human Eg7 using a polyclonal antibody against the 15–carboxy-terminal amino acids of human Eg7 revealed that interphase and mitotic cells harbored similar levels of Eg7 (Fig. 10 A). Immunoprecipitation data showed that AKAP95 and Eg7 did not coprecipitate from interphase cell lysates, indicating that these proteins do not interact at this stage of the cell cycle (Fig. 10 B). In contrast, in mitotic cell lysates, AKAP95 and Eg7 coprecipitated regardless of the antibody used (Fig. 10 C), suggesting that AKAP95 and Eg7 interact at mitosis. Since AKAP95 was found to be associated with chromatin at mitosis (Fig. 1 C), we tested the possibility that the AKAP95–Eg7 interaction was mediated by DNA. To this end, mitotic chromatin was isolated, solubilized with micrococcal nuclease, and the DNA was digested with 400 μg/ml DNase I in the presence of protease inhibitors. Immunoprecipitation of AKAP95 or Eg7 from the DNase-treated fraction revealed that both proteins also coprecipitated (Fig. 10 D), suggesting that the mitotic AKAP95–Eg7 interaction is not mediated by DNA and is probably direct.
Figure 10.
Immunoblocking of AKAP95 inhibits the association of Eg7 to condensed chromatin in mitotic extract. (A) Immunoblot of interphase (I) and mitotic (M) HeLa cell lysates with antibodies against Eg7, a component of the human condensin complex. (B) AKAP95 and Eg7 were immunoprecipitated from interphase HeLa cell lysates and precipitates were immunoblotted with each precipitating antibody. (C) AKAP95 and Eg7 were immunoprecipitated from mitotic HeLa cell lysates and precipitates immunoblotted as in B. (D) AKAP95 and Eg7 were immunoprecipitated from mitotic chromatin after solubilization with micrococcal nuclease and digestion with DNase I. Precipitates were immunoblotted as in B. (B–D) Control immunoprecipitations were carried out with preimmune rabbit IgGs (IgG). (E) Interphase nuclei (N) loaded with preimmune IgGs (−α-AKAP95), anti–AKAP95 antibodies (+α-AKAP95) or anti–HP1α antibodies (+α-HP1α) were allowed to condense in mitotic extract. After 2 h, chromatin masses (Ch) were sedimented and immunoblotted using anti–Eg7 antibodies.
pEg7, the Xenopus homologue of human Eg7, has been previously shown to be recruited onto condensing chromosomes at mitosis (Cubizolles et al. 1998). Results from our laboratory have shown that chromatin condensation in the mitotic extract is accompanied by association of Eg7 with chromatin (Landsverk, H., K.L. Guellec, and P. Collas, unpublished observation). Since pEg7 is required for chromosome condensation, we determined whether immunoblocking nuclear AKAP95 would affect the recruitment of Eg7 to chromatin in vitro. Nuclei loaded with preimmune IgGs (−α-AKAP95) or anti–AKAP95 antibodies (+α-AKAP95) were allowed to condense for 2 h in mitotic extract. Chromatin masses were sedimented and immunoblotted using anti–Eg7 antibodies. As expected from our previous results anti–AKAP95 antibodies blocked chromatin condensation. Remarkably, the antibodies also prevented the recruitment of Eg7 to chromatin (Fig. 10 E, +α-AKAP95) that normally occurs in extract either in the absence of antibodies (not shown) or with nuclei loaded with preimmune IgGs (Fig. 10 D, −α-AKAP95). Loading nuclei with anti–HP1α antibodies did not impede association of Eg7 with chromatin (Fig. 10 E, +α-HP1α), indicating that inhibition of Eg7 recruitment by anti–AKAP95 antibodies is not a mere consequence of steric hindrance imposed by the antibody. Altogether, these results suggest that functional inhibition of AKAP95 prevents the association of Eg7 to chromatin required for condensation.
Discussion
This paper provides evidence that AKAP95 is a multivalent protein with a role in chromosome condensation at mitosis. AKAP95 acts as an anchor for a PKA-signaling complex onto mitotic chromosomes, which is required for maintenance of chromosomes in a condensed form throughout mitosis. AKAP95 also appears essential for the recruitment onto chromosomes of Eg7, a component of the condensin complex. The data also provide insights on the significance of the rise in cAMP level and PKA activity during mitosis.
Several lines of evidence illustrate a function of AKAP95 in chromosome condensation and maintenance of chromosomes in a condensed form during mitosis. First, immunoblocking intranuclear AKAP95 inhibits chromatin condensation in vitro and in vivo. Second, immunoblocking AKAP95 during mitosis or in vitro, or immunodepletion of soluble AKAP95 from the mitotic extract, induces PCD. Inhibition of chromatin condensation with anti–AKAP95 antibodies is unlikely to result from a nonspecific steric effect of the antibodies since antibodies against all variants of the heterochromatin protein HP1 (Minc et al. 1999) are ineffective. Clearly, AKAP95 is implicated in chromatin condensation before PKA associates with chromatin and interacts with AKAP95. This is consistent with the established downregulation of PKA required for mitotic nuclear envelope breakdown (Lamb et al. 1991) and with the absence of PKA in interphase nuclei (Eide et al. 1998). Thus, in principle, AKAP95 and RII cannot interact until the nuclear envelope has broken down. However, once nuclear envelope disassembly has occurred, the role of AKAP95 in maintaining condensed chromosomes requires cAMP/PKA signaling and AKAP95–PKA interaction.
A third observation supporting the involvement of AKAP95 in regulating chromosome structure at mitosis is the finding that a recombinant AKAP95 fragment (GST-AKAP95Δ1-386) prevents PCD in AKAP95-depleted extract, and restores condensation of prematurely decondensed chromatin, both in a dose-dependent manner. Thus, the domain(s) of AKAP95 required for this activity and for interaction of AKAP95 with chromatin reside(s) in the carboxy-terminal 306 amino acids (amino acids 387–692) of the protein. As expected from the RII binding requirement for maintenance of condensed chromatin, this AKAP95 fragment also contains the RII binding motif (Eide et al. 1998).
The consequences of immunoblocking AKAP95 may be accounted for by the hypothesis that AKAP95 function may be disrupted by antibodies only when they bind AKAP95 that is not associated with chromatin. Indeed, the antibodies block condensation when incorporated into interphase nuclei before association of AKAP95 with chromatin (Landsverk, H., and P. Collas, unpublished results). Thus, it is possible that anti–AKAP95 antibodies incubated in mitotic cytosol fail to inhibit chromatin condensation because by the time the nuclear envelope breaks down, nuclear AKAP95 has already associated with chromosomes. Similarly, induction of PCD in cytosol immunodepleted of AKAP95 suggests that the establishment of an equilibrium between chromosome-associated and soluble AKAP95 is critical for maintenance of condensation. By binding AKAP95 when it dissociates from chromosomes as well as soluble AKAP95, the antibodies may sharply decrease the on-rate of AKAP95 to chromosomes, causing decondensation of the chromatin.
Since it associates with chromatin early during mitotic nuclear disassembly, AKAP95 could conceivably either directly affect chromatin structure, or facilitate the recruitment of factors controlling mitotic chromosome condensation (Hirano and Mitchison 1994; Hirano et al. 1997). Sequence analysis of human AKAP95 (Eide et al. 1998) reveals that AKAP95 is unlikely to belong to the family of SMC proteins, factors shown to affect chromosome structure at mitosis and during development (Strunnikov 1998). Unlike SMC proteins, AKAP95 displays no coiled-coil domains, no amino-terminal ATP-binding sites and no DA box, a signature motif of SMCs (Strunnikov et al. 1993). Likewise, AKAP95 displays no consensus site for topoisomerase II (Adachi et al. 1991); thus, unlike SMCs or topoisomerase II, AKAP95 is probably unlikely to directly impose a conformational change on chromatin inducing condensation. Rather, our data suggest that a function of AKAP95 is to allow the recruitment of components of the condensin complex. First, immunoblocking of AKAP95 inhibits association of Eg7 with chromatin in vitro. Eg7 is the human homologue of Xenopus pEg7, a protein associated with the 13S condensin complex (Hirano and Mitchison 1994; Cubizolles et al. 1998), suggesting that anti–AKAP95 antibodies prevent binding of the condensin complex onto chromosomes. Second, AKAP95 can be coprecipitated with Eg7 from solubilized, DNase-digested mitotic chromatin, suggestive of an interaction between AKAP95 and Eg7 that is not mediated by DNA. Collectively, these findings raise the attractive possibility that one function of AKAP95 is to facilitate recruitment and/or anchoring of condensins to chromatin at mitosis.
The multivalent nature of AKAP95 is not only evidenced by its role in chromatin condensation, but also by the observation that AKAP95 maintains a crucial PKA-anchoring function during mitosis. Whereas AKAP95 and RII are found in a different compartment in interphase, a chromosome-associated AKAP95–PKA signaling complex is established at mitosis. Induction of PCD after immunodepletion of RIIα from mitotic cytosol reflects an equilibrium between chromatin-associated (via AKAP95) and soluble RIIα, and a requirement for a soluble pool of RIIα to maintain the chromatin fraction of the AKAP95–RIIα complex saturated and functional. Disruption of PKA anchoring with Ht31 peptides induces PCD in vitro and in vivo, indicating that the AKAP95–PKA interaction is essential for the maintenance of condensed chromatin. Several physiological functions implicating AKAP-anchored PKA have been reported using similar disruption approaches (Colledge and Scott 1999). Formation of the AKAP95–PKA complex early during chromosome condensation may be critical to mediate the increasing cAMP signal as mitosis progresses (Grieco et al. 1996). It is conceivable that maintenance of a condensed chromatin structure results from PKA-dependent phosphorylation of chromatin substrates such as histones (Van Hooser et al. 1998; Wei et al. 1999) or other DNA-related structural regulators such as topoisomerases (Roberge et al. 1990).
Our results provide some significance for the rising level of cAMP and increasing PKA activity during mitosis (Grieco et al. 1996). Implications of PKA downregulation in entry into mitosis have been addressed previously (Lamb et al. 1991). It has been reported that cAMP levels and PKA(−type II) activity are significant (Costa et al. 1976) or even rise (Grieco et al. 1996) during mitosis. We propose that rising cAMP levels during mitosis activate chromatin-associated PKA, which together with AKAP95, is required to ensure proper chromosome condensation throughout mitosis. This view is supported by the induction of PCD by cAMP antagonists or PKI. Moreover, anti–AKAP95 antibodies added to cytosol in vitro or in vivo together with free PKA C subunit also promote PCD. Since cytosolic anti–AKAP95 antibodies presumably inhibit binding of soluble AKAP95 to chromatin, the latter observation argues that only C bound to chromosomes is relevant for maintenance of condensed chromatin, whereas free cytosolic C is ineffective. Higher PKA activity at the end of mitosis facilitates chromatin decondensation and nuclear reassembly (Grieco et al. 1996). This scenario implies the induction of antagonistic effects of cAMP/PKA signaling on chromosome structure at mitosis that are temporally distinct. It also implicates the existence of a molecular switch upstream of the cAMP/PKA pathway, that controls the dual effect of cAMP/PKA activity at mitosis. A likely candidate might be M phase–promoting factor, as a threshold level of this activity has been shown to be required to initiate activation of the cAMP/PKA pathway during mitosis (Grieco et al. 1996). The dual effect of PKA during mitosis and at the exit of mitosis could also be due to the redistribution of AKAP95, which dissociates from PKA at mitosis exit.
Although the structural determinants mediating AKAP–RII interactions in general are being characterized (Colledge and Scott 1999), whether additional processes modulate these associations is unknown. Circumstantial evidence suggests the involvement of posttranslational modification of RII in such regulation. First, RIIα is primarily localized in the centrosome–Golgi area (Keryer et al. 1998) in interphase HeLa cells, presumably via a centrosomal AKAP (Witczak et al. 1999). Release of RIIα from centrosomes at mitosis correlates with CDK1-mediated phosphorylation of RIIα (Keryer et al. 1998). Second, CDK1-mediated phosphorylation of RIIα in vitro is sufficient to induce partial solubilization of RIIα from a Triton X-100 insoluble pool (Keryer et al. 1998). Third, association of AKAP95 with RIIα is mitosis-specific. Finally, decondensation of in vitro–condensed chromatin in an interphase extract is associated with release of RIIα from chromatin-associated AKAP95 (Collas, P., data not shown). This argues that binding of RIIα to AKAP95 at mitosis is not a mere consequence of both proteins being in the same subcellular compartment; rather, a modification of either protein seems to affect their interaction. Together with the data of Keryer et al. 1998, our results argue for a cell cycle–regulated redistribution of RIIα, suggesting an additional mechanism regulating PKA type II subcellular localization.
An emerging feature of AKAPs is their ability to anchor entire signaling complexes other than PKA to specific substrates. To date, AKAP79, AKAP220, gravin, yotiao/AKAP450/CG-NAP, and ezrin have been shown to act as polyvalent anchoring proteins for signaling units involving PKA or protein kinase C together with protein phosphatases (Colledge and Scott 1999). Additional binding partners of AKAP95 besides PKA at mitosis have yet to be identified. Nevertheless, our results extend the concept that AKAP95 is likely to be a multivalent targeting molecule anchoring signaling complexes as well as chromatin remodeling factors in a cell cycle–regulated manner. It will be exciting to determine whether additional AKAPs also harbor specific intrinsic cellular functions.
Acknowledgments
We are thankful to J.-C. Courvalin for gifts of antilamin B, anti–LBR, and anti–HP1 antibodies, T. Lømo (Institute of Neurophysiology, University of Oslo) for use of his photography facility, and K.A. Tasken for critical reading of the manuscript and discussions.
This work was supported by grants from the Norwegian Cancer Society, the Norwegian Research Council, the Anders Jahre Foundation, the Novo Nordisk Foundation Committee (to P. Collas and K. Tasken), and Association pour la Recherche contre le Cancer (No. B09808; to K. Le Guellec). P. Collas and K. Taskén are fellows of the Norwegian Cancer Society.
Footnotes
Abbreviations used in this paper: AKAP, A-kinase–anchoring protein; CDK1, cyclin-dependent kinase 1; LBR, lamin B receptor; PCD, premature chromatin decondensation; PKA, protein kinase A; R and C, regulatory and catalytic subunit of PKA, respectively; SMC, structural maintenance of chromosome.
References
- Adachi Y., Luke M., Laemmli U.K. Chromosome assembly in vitrotopoisomerase II is required for condensation. Cell. 1991;64:137–148. doi: 10.1016/0092-8674(91)90215-k. [DOI] [PubMed] [Google Scholar]
- Buendia B., Courvalin J.-C. Domain-specific disassembly and reassembly of nuclear membranes during mitosis. Exp. Cell Res. 1997;230:133–144. doi: 10.1006/excr.1996.3395. [DOI] [PubMed] [Google Scholar]
- Carr D.W., Stofko-Hahn R.E., Fraser I.D., Bishop S.M., Acott T.S., Brennan R.G., Scott J.D. Interaction of the regulatory subunit (RII) of cAMP-dependent protein kinase with RII-anchoring proteins occurs through an amphipathic helix binding motif. J. Biol. Chem. 1991;266:14188–14192. [PubMed] [Google Scholar]
- Chaudhary N., Courvalin J.-C. Stepwise reassembly of the nuclear envelope at the end of mitosis. J. Cell Biol. 1993;122:295–306. doi: 10.1083/jcb.122.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghlan V.M., Langeberg L.K., Fernandez A., Lamb N.J., Scott J.D. Cloning and characterization of AKAP 95, a nuclear protein that associates with the regulatory subunit of type II cAMP-dependent protein kinase. J. Biol. Chem. 1994;269:7658–7665. [PubMed] [Google Scholar]
- Collas P. Nuclear envelope disassembly in mitotic extract requires functional nuclear pores and a nuclear lamina. J. Cell Sci. 1998;111:1293–1303. doi: 10.1242/jcs.111.9.1293. [DOI] [PubMed] [Google Scholar]
- Collas P. Sequential PKC- and Cdc2-mediated phosphorylation events elicit zebrafish nuclear envelope disassembly. J. Cell. Sci. 1999;112:977–987. doi: 10.1242/jcs.112.6.977. [DOI] [PubMed] [Google Scholar]
- Collas P., Courvalin J.-C., Poccia D.L. Targeting of membranes to sea urchin sperm chromatin is mediated by a lamin B receptor-like integral membrane protein. J. Cell Biol. 1996;135:1715–1725. doi: 10.1083/jcb.135.6.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge M., Scott J.D. AKAPsfrom structure to function. Trends Cell Biol. 1999;9:216–221. doi: 10.1016/s0962-8924(99)01558-5. [DOI] [PubMed] [Google Scholar]
- Costa M., Gerner E.W., Russell D.H. Cell cycle-specific activity of type I and type II cyclic adenosine 3′,5′-monophosphate-dependent protein kinases in Chinese hamster ovary cells. J. Biol. Chem. 1976;251:3313–3319. [PubMed] [Google Scholar]
- Cubizolles F., Legagneux V., Le Guellec R., Chartrain I., Uzbekov R., Ford C., Le Guellec K. pEg7, a new Xenopus protein required for mitotic chromosome condensation in egg extracts. J. Cell Biol. 1998;143:1437–1446. doi: 10.1083/jcb.143.6.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duband-Goulet I., Courvalin J.C., Buendia B. LBR, a chromatin and lamin binding protein from the inner nuclear membrane, is proteolyzed at late stages of apoptosis. J. Cell Sci. 1998;111:1441–1451. doi: 10.1242/jcs.111.10.1441. [DOI] [PubMed] [Google Scholar]
- Eide T., Coghlan V., Orstavik S., Holsve C., Solberg R., Skalhegg B.S., Lamb N.J., Langeberg L., Fernandez A., Scott J.D., Jahnsen T., Tasken K. Molecular cloning, chromosomal localization, and cell cycle–dependent subcellular distribution of the A-kinase anchoring protein, AKAP95. Exp. Cell Res. 1998;238:305–316. doi: 10.1006/excr.1997.3855. [DOI] [PubMed] [Google Scholar]
- Grieco D., Avvedimento E.V., Gottesman M.E. A role for cAMP-dependent protein kinase in early embryonic divisions. Proc. Natl. Acad. Sci. USA. 1994;91:9896–9900. doi: 10.1073/pnas.91.21.9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grieco D., Porcellini A., Avvedimento E.V., Gottesman M.E. Requirement for cAMP-PKA pathway activation by M phase-promoting factor in the transition from mitosis to interphase. Science. 1996;271:1718–1723. doi: 10.1126/science.271.5256.1718. [DOI] [PubMed] [Google Scholar]
- Hirano T., Mitchison T.J. A heterodimeric coiled-coil protein required for mitotic chromosome condensation in vitro. Cell. 1994;79:449–458. doi: 10.1016/0092-8674(94)90254-2. [DOI] [PubMed] [Google Scholar]
- Hirano T., Kobayashi R., Hirano M. Condensins, chromosome condensation protein complexes containing XCAP-C, XCAP-E and a Xenopus homolog of the Drosophila Barren protein. Cell. 1997;89:511–521. doi: 10.1016/s0092-8674(00)80233-0. [DOI] [PubMed] [Google Scholar]
- Keryer G., Yassenko M., Labbe J.C., Castro A., Lohmann S.M., Evain-Brion D., Tasken K. Mitosis-specific phosphorylation and subcellular redistribution of the RIIalpha regulatory subunit of cAMP-dependent protein kinase. J. Biol. Chem. 1998;273:34594–34602. doi: 10.1074/jbc.273.51.34594. [DOI] [PubMed] [Google Scholar]
- Lamb N., Cavadore J.-C., Labbé J.-C., Maurer R.A., Fernandez A. Inhibition of cAMP-dependent protein kinase plays a key role in the induction of mitosis and nuclear envelope breakdown in mammalian cells. EMBO (Eur Mol. Biol. Organ.) J. 1991;10:1523–1533. doi: 10.1002/j.1460-2075.1991.tb07672.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazebnik Y.A., Cole S., Cooke C.A., Nelson W.G., Earnshaw W.C. Nuclear events of apoptosis in vitro in cell-free mitotic extractsa model system for analysis of the active phase of apoptosis. J. Cell Biol. 1993;123:7–22. doi: 10.1083/jcb.123.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minc E., Allory Y., Worman H.J., Courvalin J.C., Buendia B. Localization and phosphorylation of HP1 proteins during the cell cycle in mammalian cells. Chromosoma. 1999;108:220–234. doi: 10.1007/s004120050372. [DOI] [PubMed] [Google Scholar]
- Reyes J.C., Muchardt C., Yaniv M. Components of the human SWI/SNF complex are enriched in active chromatin and are associated with the nuclear matrix. J. Cell Biol. 1997;137:263–274. doi: 10.1083/jcb.137.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riabowol K.T., Fink J.S., Gilman M.Z., Walsh D.A., Goodman R.H., Feramisco J.R. The catalytic subunit of cAMP-dependent protein kinase induces expression of genes containing cAMP-responsive enhancer elements. Nature. 1988;336:83–86. doi: 10.1038/336083a0. [DOI] [PubMed] [Google Scholar]
- Roberge M., Th'ng J., Hamaguchi J., Bradbury E.M. The topoisomerase II inhibitor VM-26 induces marked changes in histone H1 kinase activity, histones H1 and H3 phosphorylation, and chromosome condensation in G2 phase and mitotic BHK cells. J. Cell Biol. 1990;111:1753–1762. doi: 10.1083/jcb.111.5.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh N., Goldberg I.G., Wood E.R., Earnshaw W.C. ScIIan abundant chromosome scaffold protein is a member of a family of putative ATPases with an unusual predicted tertiary structure. J. Cell Biol. 1994;127:303–318. doi: 10.1083/jcb.127.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J.D. Cyclic nucleotide-dependent protein kinases. Pharmacol. Ther. 1991;50:123–145. doi: 10.1016/0163-7258(91)90075-w. [DOI] [PubMed] [Google Scholar]
- Strunnikov A.V. SMC proteins and chromosome structure. Trends Cell Biol. 1998;8:454–459. doi: 10.1016/s0962-8924(98)01370-1. [DOI] [PubMed] [Google Scholar]
- Strunnikov A.V., Larionov V.L., Koshland D. SMC1an essential yeast gene encoding a putative head-rod-tail protein is required for nuclear division and defines a new ubiquitous protein family. J. Cell Biol. 1993;123:1635–1648. doi: 10.1083/jcb.123.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Hooser A., Goodrich D.W., Allis C.D., Brinkley B.R., Mancini M.A. Histone H3 phosphorylation is required for the initiation, but not maintenance, of mammalian chromosome condensation. J. Cell Sci. 1998;111:3497–3506. doi: 10.1242/jcs.111.23.3497. [DOI] [PubMed] [Google Scholar]
- Wei Y., Yu L., Bowen J., Gorovsky M.A., Allis C.D. Phosphorylation of histone H3 is required for proper chromosome condensation and segregation. Cell. 1999;97:99–109. doi: 10.1016/s0092-8674(00)80718-7. [DOI] [PubMed] [Google Scholar]
- Witczak O., Skalhegg B.S., Keryer G., Bornens M., Tasken K., Jahnsen T., Orstavik S. Cloning and characterization of a cDNA encoding an A-kinase anchoring protein located in the centrosome, AKAP450. EMBO (Eur Mol. Biol. Organ.) J. 1999;18:1858–1868. doi: 10.1093/emboj/18.7.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]