Abstract
The variant chicken kidney AE1 anion exchangers differ only at the NH2 terminus of their cytoplasmic domains. Transfection studies have indicated that the variant chicken AE1-4 anion exchanger accumulates in the basolateral membrane of polarized MDCK kidney epithelial cells, while the AE1-3 variant, which lacks the NH2-terminal 63 amino acids of AE1-4, primarily accumulates in the apical membrane. Mutagenesis studies have shown that the basolateral accumulation of AE1-4 is dependent upon two tyrosine residues at amino acids 44 and 47 of the polypeptide. Interestingly, either of these tyrosines is sufficient to direct efficient basolateral sorting of AE1-4. However, in the absence of both tyrosine residues, AE1-4 accumulates in the apical membrane of MDCK cells. Pulse–chase studies have shown that after delivery to the cell surface, newly synthesized AE1-4 is recycled to the Golgi where it acquires additional N-linked sugar modifications. This Golgi recycling activity is dependent upon the same cytoplasmic tyrosine residues that are required for the basolateral sorting of this variant transporter. Furthermore, mutants of AE1-4 that are defective in Golgi recycling are unable to associate with the detergent insoluble actin cytoskeleton and are rapidly turned over. These studies, which represent the first description of tyrosine-dependent cytoplasmic sorting signal for a type III membrane protein, have suggested a critical role for the actin cytoskeleton in regulating AE1 anion exchanger localization and stability in this epithelial cell type.
Keywords: epithelial, band 3 protein, Golgi, polarity, actin
The kidney plays an essential role in the regulation of pH equilibrium within the body. This regulatory function is accomplished by the wide variety of transporters and channels that mediate the vectorial transport of ions within the cells of the kidney. This selective transport of ions requires the presence of different complements of polypeptides in the apical and basolateral membrane domains of the polarized epithelial cells of the kidney. Although cytoplasmic (Casanova et al. 1991; Matter et al. 1992; Thomas et al. 1993; Hunziker and Fumey 1994; Honing and Hunziker 1995) and lumenal (Fiedler and Simons 1995; Scheiffele et al. 1995; Yeaman et al. 1997) signals have been shown to direct the basolateral and apical sorting, respectively, of type I membrane proteins in epithelial cells, the signals that direct the sorting of multi-membrane spanning transporters and channels to specific membrane domains in epithelial cells remain poorly defined.
A well-characterized example of vectorial transport by the cells of the kidney is the α-intercalated cell of the mammalian kidney collecting duct. In this acid-excreting cell type, basolateral chloride-bicarbonate exchangers and apical proton pumps function coordinately to dispose of bicarbonate and protons generated by intracellular carbonic anhydrase (Schwartz et al. 1985). Immunological studies (Drenckhahn et al. 1985; Alper et al. 1989) and studies with knockout mice (Peters et al. 1996) have indicated that the basolateral chloride-bicarbonate exchanger of α-intercalated cells is encoded by the AE1 anion exchanger gene. In contrast to the acid-excreting, α-intercalated cell of the collecting duct, the base-excreting, β-intercalated cell of the collecting duct possesses an apical chloride-bicarbonate exchanger and a basolateral proton pump (Schwartz et al. 1985). Although cell fractionation studies with an immortalized β-intercalated cell line have suggested that the apical anion exchanger of this base-secreting cell type is encoded by the AE1 gene (van Adelsberg et al. 1993), the molecular origin of this apical anion transporter has not yet been defined.
Molecular analyses have shown that mammalian kidney AE1 anion exchangers are identical to AE1 anion exchangers from erythroid cells except at the NH2 terminus of their cytoplasmic domains. In rats (Kudrycki and Shull 1989) and mice (Brosius et al. 1989), the kidney AE1 anion exchangers lack the 79 NH2-terminal amino acids of their erythroid AE1 counterparts, while the human kidney AE1 anion exchanger lacks the 65 NH2-terminal amino acids of the human erythroid AE1 anion exchanger (Kollert-Jons et al. 1993). Expression studies have indicated that the murine kidney AE1 anion exchanger can mediate anion transport (Brosius et al. 1989). However, in vitro binding studies have shown that the truncated NH2 termini of the mammalian kidney AE1 variants affect their ability to associate with specific elements of the cytoskeleton. The human (Wang et al. 1995) and murine (Ding et al. 1994) erythroid AE1 anion exchanger can associate with erythroid ankyrin in vitro, while the kidney AE1 variants from these species are unable to bind ankyrin.
Additional diversity has been detected among the anion exchangers encoded by the AE1 gene in chicken kidney. Three kidney AE1 transcripts, AE1-3, AE1-4, and AE1-5, are derived from a single promoter by alternative splicing (Cox et al. 1996). Similar to the kidney AE1 anion exchangers that have been characterized in mammalian species, AE1-3 and AE1-5 transcripts encode NH2-terminally truncated versions of the chicken erythroid AE1 anion exchangers (Cox and Cox 1995; Cox et al. 1995). However, the chicken kidney AE1-4 anion exchanger transcript contains an in-frame AUG in its unique 5′ sequence, resulting in an anion exchanger polypeptide with an alternative NH2-terminal cytoplasmic tail. To assess the functional significance of this NH2-terminal cytoplasmic diversity, the variant AE1-3 and AE1-4 anion exchangers have been expressed in MDCK epithelial cells. These studies have revealed that the alternative NH2 termini of these variant transporters target these polypeptides to opposite membrane domains in this epithelial cell type. Mutagenesis studies have shown the additional 63 amino acids at the NH2 terminus of AE1-4 that are absent in AE1-3 contain a tyrosine-dependent basolateral sorting signal. Interestingly, either one of two tyrosines in this region is sufficient to direct basolateral sorting. The sequences at the NH2 terminus of AE1-4 also direct the association of this variant transporter with the actin-based cytoskeleton, as well as recycling from the plasma membrane to the Golgi. This Golgi recycling activity is dependent upon the same tyrosine residues that are required for basolateral sorting. Furthermore, pulse–chase analyses with anion exchanger polypeptides that are defective in Golgi recycling suggest this activity is necessary for the stable accumulation of AE1 in MDCK cells.
Materials and Methods
Generation of Chicken Kidney AE1 Anion Exchanger Antibodies
Polyclonal antibodies have been generated in rabbit against the cytoplasmic domain of the chicken kidney AE1-4 anion exchanger that was expressed as a bacterial fusion protein in E. coli. In brief, the region of AE1-4 encompassing amino acids 1–359 was subcloned into the pFLAG-ATS prokaryotic expression vector (IBI) downstream of the eight–amino acid FLAG epitope sequence. The expression of this bacterial fusion protein is driven by the inducible tac promoter. After induction with IPTG, the fusion protein was purified from bacterial extracts by immunoaffinity chromatography using a monoclonal antibody to the FLAG epitope. The purified fusion protein was used as a source of antigen to inject rabbits.
Stable and Transient Expression of Variant AE1 Anion Exchangers in MDCK Cells
Madin-Darby canine kidney (MDCK) cells were maintained in DME supplemented with 5% fetal bovine serum, 2 mM l-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin at 37°C in 5% CO2. Cells were transfected with various AE1 anion exchanger cDNAs subcloned into the pcDNA3 eukaryotic expression vector (Invitrogen). Media was changed 24 h after transfection, and 48 h after transfection cells were analyzed for AE1 expression. Alternatively, at 48 h after transfection cells were split 1:10 in complete media containing 600 μM G418. Cells that could grow in the presence of G418 were selected and used for various assays.
Construction of AE1-4 Anion Exchanger Mutants
Sense primers complementary to nucleotides 89–103, 137–151, or 185–198 of the chicken AE1-4 anion exchanger cDNA (Cox and Cox 1995) were generated. Sense primers were flanked at their 5′ ends with a HindIII restriction site, which represents a unique site at the 5′ end of the AE1-4 cDNA in the pcDNA3 polylinker, followed by a Kozak sequence (Kozak 1986), and an AUG codon. Each sense primer was used for a polymerase chain reaction (PCR) in combination with an antisense primer corresponding to nucleotides 199–219 of the AE1-4 cDNA. This antisense primer encompassed a unique BamHI restriction site in the AE1-4 cDNA. PCR reactions using the full-length AE1-4 cDNA as a template were performed using Pfu polymerase (Stratagene). PCR products were digested with HindIII and BamHI restriction enzymes, and purified on low melting point agarose gels. The AE1-4Δ21, AE1-4Δ37, and AE1-4Δ53 constructs were generated by using these PCR products to replace the 5′ end of the AE1-4 cDNA in pcDNA3 that had been removed by digestion with HindIII and BamHI.
Point mutants were constructed using the Altered Sites II site-directed mutagenesis kit (Promega). In brief, the AE1-4 cDNA was subcloned into the pALTER-1 vector. After alkaline denaturation, a mutagenic oligonucleotide was annealed to the full-length AE1-4 cDNA template. The sequences of the mutagenic oligonucleotides are as follows: Y44A (5′-TCCACGTAGCCCTCAGCGGTGTCCCTGTGGGC-3′), Y47A (5′-TCG-TGCAGCTCCACGGCGCCCTCATAGGTGTC-3′), Y44A,Y47A (5′-TCGTGCAGCTCCACGGCGCCCTCAGCGGTGTCCCTGTGGGC-3′), and N638T (5′-CCGCGGGCGGTGCCGGTGGTCACCTCCAGCCC-3′). The mutant strand was elongated and ligated using T4 DNA polymerase and T4 DNA ligase, and cotransformed into a repair-minus E. coli strain (ES1301 mut S) along with helper phage DNA. All mutants were confirmed by sequencing using the dideoxy method. The mutant cDNAs were subcloned into pcDNA3 for transfection.
Immunoblotting Analysis of AE1 Anion Exchanger Polypeptides
MDCK cells transiently or stably expressing wild-type or mutant AE1 anion exchangers were harvested and detergent lysed by incubation in 150 mM NaCl, 10 mM Tris (pH 7.5), 5 mM MgCl2, 2 mM EGTA, 6 mM β-mercaptoethanol, and 1% (vol/vol) Triton X-100 for 5 min on ice. In some instances, the cells were treated with 25 μg/ml of the actin depolymerizing drug, latrunculin B, for 1 h before lysis. Immunolocalization analyses have shown that phalloidin-stained microfilaments are absent in MDCK cells treated with this drug (data not shown). The lysate from treated or untreated cells was microcentrifuged for 5 min to separate the detergent soluble and insoluble fractions. The detergent insoluble pellet was resuspended in immunoprecipitation buffer containing 170 mM NaCl, 20 mM Tris (pH 7.5), 5 mM EGTA, 5 mM EDTA, 0.1% (wt/vol) SDS, 1% (vol/vol) Triton X-100, and 1% (wt/vol) sodium deoxycholate and sonicated three times. After sonication, the insoluble fraction was microcentrifuged for 5 min and the pellet discarded. The detergent soluble and insoluble fractions were then electrophoresed on a 6% SDS polyacrylamide gel, and the proteins were electrophoretically transferred to nitrocellulose. The filters were blocked with 5% powdered milk and incubated overnight with a 1:30,000 dilution of a chicken AE1-specific peptide antibody (Cox and Cox 1995). After washing, the filters were incubated with goat anti–rabbit (GAR) IgG conjugated to horseradish peroxidase, and immunoreactive species were detected on Kodak Biomax MR film using enhanced chemiluminescence.
Immunoprecipitation of AE1 Anion Exchanger Polypeptides
Cells expressing the various anion exchanger polypeptides were grown in 100-mm culture dishes or on 24-mm polycarbonate filters (Costar). The cells were washed once with methionine-free DME and incubated with this media for 15 min at 37°C. At this time, 65 μCi/ml 35S-Translabel™ (ICN) was added to the cells and they were incubated an additional 15 min at 37°C. After washing, the cells were incubated in DME containing 5% fetal calf serum for times ranging from 0 to 48 h. At each time point, the cells were detergent extracted as described above and separated into detergent soluble and insoluble fractions. In some instances, total cell lysates were prepared by directly lysing cells in immunoprecipitation buffer. Protein A–agarose beads that had been preloaded with rabbit polyclonal antibodies directed against amino acids 1–359 of chicken AE1-4 were added to these fractions and incubated at 4°C overnight. The protein A–agarose beads were then washed three times with immunoprecipitation buffer, and the immune complexes were released by incubation in SDS sample buffer. Immunoprecipitates were resolved on a 6% SDS polyacrylamide gel. The gels were treated with PPO in DMSO, dried, and exposed to Kodak Biomax MR film at −80°C.
Endoglycosidase H and N-Glycosidase F Digestion of AE1 Anion Exchanger Immunoprecipitates
MDCK cells stably expressing the various chicken AE1 anion exchanger polypeptides were pulsed with 35S-Translabel, chased for 0, 1, or 4 h, and AE1 immunoprecipitates were prepared as described above. After washing of the protein A–agarose beads with immunoprecipitation buffer, the beads were washed with 10 mM Tris (pH 7.4), and 150 mM NaCl (TBS). The beads were then resuspended and digested either with 2.5 mU endoglycosidase H (endo H), or 0.1 U N-glycosidase F as described previously (Ghosh et al. 1999). After digestion, the beads were washed one time in TBS and immune complexes were released by incubation in SDS sample buffer and analyzed on a 6% SDS polyacrylamide gel. The observed digestion patterns were the same when digests were carried out at 37°C.
Cell Surface Delivery of the AE1-4 Anion Exchanger
Three different labeling schemes were used to label MDCK cells stably expressing the chicken AE1-4 anion exchanger. (a) The cells were pulsed with 35S-Translabel™ in methionine-free DME for 15 min, followed by 15 min of chase in methionine-free DME. The cells were then chased an additional 45 min in methionine-free DME in the absence or presence of 1.5 mg/ml chymotrypsin. (b) The cells were incubated continuously with 1.5 mg/ml chymotrypsin during a 15-min preincubation in methionine-free DME, a 15-min pulse with 35S-Translabel™, and a 15-min chase (a total of 45 min). (c) The cells were pulsed for 15 min and chased for 1 h at 37°C. The cells were then shifted to 4°C and incubated an additional 45 min in the presence of 1.5 mg/ml chymotrypsin. For each labeling scheme, AE1 immunoprecipitates were prepared from total cell lysates as described above. A fraction of the total cell lysate was analyzed by immunoblotting analysis using a polyclonal antibody raised against chicken erythroid α-spectrin (Sigma) that recognizes α-fodrin or a chicken AE1-specific peptide antibody (Cox and Cox 1995).
Effects of Various Reagents on AE1-4 Anion Exchanger Processing
MDCK cells stably expressing the AE1-4 anion exchanger were pulsed with 35S-Translabel™ as described above. After a 45-min chase, either 10 μg/ml brefeldin A (BFA), 25 mM ammonium chloride, or 0.4 M sucrose was added to the media, and the cells were incubated until a total chase period of 4 h had elapsed. At each time point, AE1 immunoprecipitates were prepared from total cell lysates as described above. Immunoprecipitates were analyzed on a 6% SDS polyacrylamide gel, and labeled anion exchangers were detected by fluorography.
Quantitative Densitometry
X-ray films from the immunoblotting analyses and the immunoprecipitation experiments were scanned using DeskScan II 2.1 software, and quantitative densitometry was performed using NIH Image. Each value presented in the text represents the average from three independent experiments in which the exposures of individual bands were not yet saturating.
Immunolocalization Analyses
MDCK cells transiently or stably expressing the various chicken AE1 anion exchanger polypeptides were either grown on coverslips or on polycarbonate filters (Costar). The cells were washed in PBS, and fixed by incubating with 1% paraformaldehyde in PBS for 10 min at room temperature. The cells were then permeabilized in PBS containing 0.5% Triton X-100 or with acetone. After permeabilization, cells were incubated with a chicken AE1-specific peptide antibody, followed by incubation with donkey anti–rabbit (DAR) IgG conjugated to lissamine. After washing, immunoreactive polypeptides were visualized using a Zeiss Axiophot microscope or a BioRad laser scanning confocal microscope. Background levels of staining were observed in control experiments in which the peptide antigen used to generate the AE1-specific peptide antibody were included as a competitor. In some instances, actin microfilaments were stained by incubating cells with 1 μg/ml phalloidin conjugated to fluorescein isothiocyanate (FITC; Sigma). Additional assays also examined whether the localization of AE1 anion exchangers was affected by pre-extracting MDCK cells with PBS containing 0.5% Triton X-100 before fixation in paraformaldehyde.
The kidney from a 17-d-old chicken embryo was isolated and fixed by incubation for 30 min in 2% paraformaldehyde in PBS at 4°C. After fixation, the tissue was rinsed in PBS and incubated overnight at 4°C in 0.5 M sucrose in PBS. The tissue was then frozen in embedding media and 3-μm tissue sections were cut using a cryotome. The tissue sections were postfixed in 1% paraformaldehyde in PBS for 5 min, and extracted in PBS containing 0.5% Triton X-100. The sections were then incubated with a chicken AE1-specific peptide antibody and a monoclonal antibody directed against the H+-ATPase (the kind gift of Dr. S. Gluck). After washing, sections were incubated with donkey anti–rabbit (DAR) IgG conjugated to lissamine and donkey anti–mouse (DAM) IgG conjugated to fluorescein. Immunoreactive polypeptides were visualized using a BioRad laser scanning confocal microscope.
Results
Variant Chicken AE1 Anion Exchangers Accumulate in the Basolateral and Apical Membrane Domains of Kidney Epithelial Cells
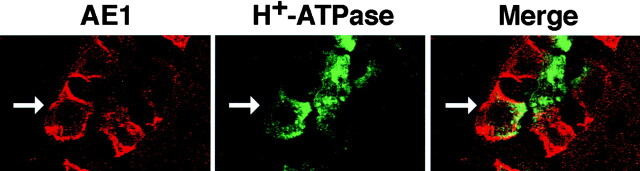
Previous studies have indicated that three variant AE1 anion exchanger transcripts, AE1-3, AE1-4, and AE1-5, are expressed in chicken kidney (Cox and Cox 1995). The AE1-3 and AE1-5 transcripts initiate translation at the same AUG and encode identical predicted polypeptides of ∼94 kD that lack the 78 NH2-terminal amino acids of the chicken erythroid AE1-1b anion exchanger (Cox et al. 1995). The AE1-4 transcript contains an in-frame AUG in its unique 5′ sequence, and encodes a predicted polypeptide of ∼101 kD that contains 63 amino acids at its NH2 terminus that are absent in AE1-3. To determine the localization of these variant anion exchangers in the cells of the kidney collecting duct, tissue sections prepared from a 17-d-old chicken embryo were probed with an AE1-specific peptide antibody, and with a monoclonal antibody directed against the H+-ATPase. Analysis of these tissue sections by confocal microscopy revealed that most (>95%) AE1 expressing cells in the collecting duct exhibited a basolateral pattern of localization for AE1, and an apical pattern of localization for the H+-ATPase (Fig. 1), typical of acid-secreting, α-intercalated cells (Alper et al. 1989; Drenckhahn et al. 1985). However, a small percentage of the AE1 anion exchanger expressing cells did not exhibit a polarized distribution for AE1. In the cell marked by the arrow in Fig. 1, AE1 accumulated in both the basolateral and apical membrane, while the H+-ATPase was restricted to the apical membrane. The cells that exhibited a nonpolarized distribution for AE1 typically lacked the highly elaborated apical membrane observed in cells where AE1 exclusively accumulated in the basolateral membrane. Whether these cells represent α-intercalated cells that have not yet fully differentiated is not known.
Figure 1.
Localization of AE1 anion exchangers and the H+-ATPase in the cells of the chicken kidney collecting duct. The kidney from a 17-d-old chicken embryo was fixed, and frozen in embedding media. 3-μm tissue sections were incubated with a polyclonal antibody specific for the chicken AE1 anion exchanger, and with a mouse monoclonal antibody specific for the H+-ATPase. Immunoreactive polypeptides were detected with DAR-IgG conjugated to lissamine and DAM-IgG conjugated to FITC, and visualized on a BioRad laser scanning confocal microscope. The 0.5-μm confocal images showing the distribution of AE1 (A) and the H+-ATPase (B) were merged (C) in Adobe Photoshop. The arrow denotes a cell with both basolateral and apical AE1 and apical H+-ATPase.
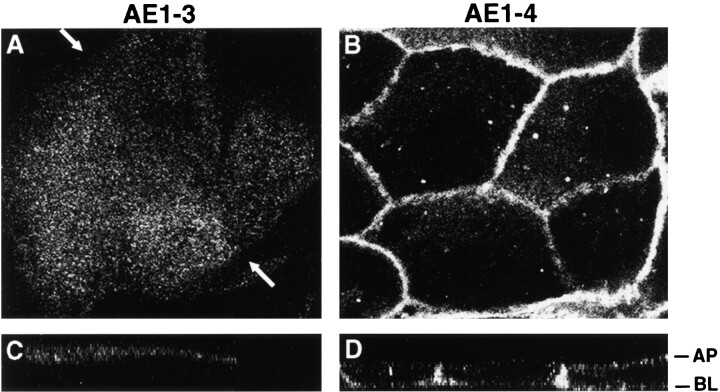
The AE1-specific peptide antibodies used in these immunolocalization studies recognized a sequence present in both AE1-3 and AE1-4. Therefore, we were unable to determine whether the alternative NH2 termini of the chicken kidney AE1 variants affect their localization in the cells of the kidney collecting duct. To investigate whether the variant cytoplasmic domains of these electroneutral transporters may be involved in directing their intracellular localization in kidney epithelial cells, the AE1-3 and AE1-4 anion exchangers were transiently expressed in MDCK cells. After the establishment of a polarized epithelial phenotype, transfected cells were fixed and stained with AE1-specific antibodies, and immunoreactive polypeptides were visualized by confocal microscopy. This analysis revealed that AE1-4 primarily accumulates in the basolateral membrane of transfected MDCK cells (Fig. 2B and Fig. D). In contrast, AE1-3 accumulates in or near the apical membrane where it exhibits a diffuse pattern of localization (Fig. 2A and Fig. C). Although AE1-3 is primarily apical in the cells shown in Fig. 2 A, this variant transporter also accumulated in an undefined intracellular compartment in some of the transfected cells (data not shown). These data suggest that the alternative NH2-terminal cytoplasmic domains of AE1-3 and AE1-4 serve as signals that direct these variant transporters to opposite membrane domains in this polarized epithelial cell type.
Figure 2.
Localization of the variant chicken AE1-3 and AE1-4 anion exchangers in transfected MDCK cells. MDCK cells transfected with the AE1-3 (A and C) or AE1-4 (B and D) anion exchangers were grown to confluency on polycarbonate filters. The cells were then fixed and incubated with an AE1-specific peptide antibody. Immunoreactive polypeptides were detected with DAR-IgG conjugated to lissamine and visualized on a BioRad laser scanning confocal microscope. The 0.5-μm x-y confocal images were collected at the apical surface (A) or near the center (B) of the cell monolayer. The corresponding x-z images are shown in C and D. The arrows in A indicate the boundary between adjacent cells.
Alternative NH2 Termini Affect the Posttranslational Modifications and Detergent Solubility Properties of the Kidney AE1 Variants
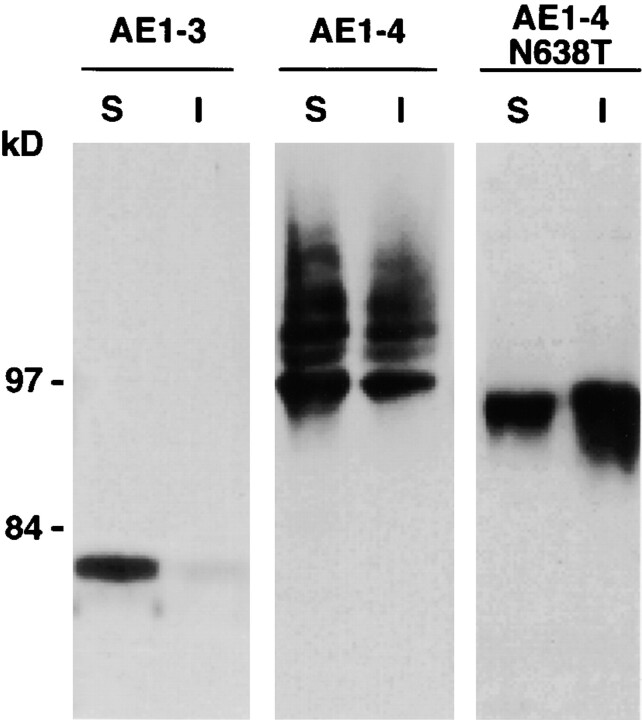
Fractionation studies have examined whether the differences in intracellular localization of the AE1-3 and AE1-4 anion exchangers correlated with differences in other properties of the variant transporters. Confluent MDCK cells transiently expressing AE1-3, AE1-4, or a point mutant of AE1-4 that eliminates its single N-linked glycosylation site, AE1-4N638T, were lysed with isotonic buffer containing 1% Triton X-100, and separated into soluble and insoluble fractions by centrifugation. Immunoblotting analysis of these fractions with AE1-specific antibodies detected a discrete polypeptide of ∼80 kD in AE1-3 transfected cells that was primarily detergent soluble (Fig. 3). In contrast to AE1-3 expressing cells, several polypeptides ranging in size from ∼97 to ∼115 kD were detected in both the detergent soluble and insoluble fractions of cells transfected with AE1-4 (Fig. 3). The polypeptides detected in AE1-4 expressing cells are similar in size to the array of AE1 anion exchangers detected in chicken kidney membrane preparations (Cox and Cox 1995). Quantitation of several fractionation experiments identical to those described above has indicated that ∼5% of AE1-3 is detergent insoluble, while ∼45% of AE1-4 is detergent insoluble. Although AE1-4N638T exhibited fractionation properties similar to AE1-4, a single species of ∼95 kD accumulated in cells transfected with this construct (Fig. 3). This suggests the complex array of polypeptides observed in AE1-4 transfected cells is due to heterogeneity in the N-linked modifications acquired by this AE1 variant. The size of the polypeptides detected for both AE1-3 and AE1-4 in this analysis is smaller than their predicted molecular masses. The basis for this discrepancy is currently unknown.
Figure 3.
Steady state fractionation properties of the transfected AE1-3 and AE1-4 anion exchangers. MDCK cells transiently expressing the AE1-3 and AE1-4 anion exchangers, or a point mutant of AE1-4 that eliminates the single N-linked glycosylation site, AE1-4N638T, were detergent lysed and separated into soluble (S) and insoluble (I) fractions by centrifugation. Equivalent amounts of the soluble and insoluble fractions were separated on a 6% SDS polyacrylamide gel, transferred to nitrocellulose, and probed with an AE1-specific antibody. After washing, the blot was incubated with GAR-IgG conjugated to horseradish peroxidase, and immunoreactive species were detected by enhanced chemiluminescence. Molecular mass markers to the left correspond to phosphorylase b (97 kD), and fructose-6-P-kinase (84 kD).
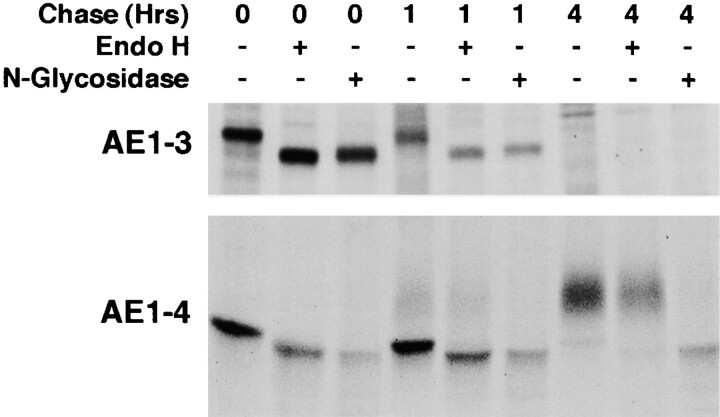
Pulse–chase analyses have investigated the mechanisms involved in generating the steady state profile of anion exchangers detected in AE1-3 and AE1-4 transfected cells. MDCK cells stably transfected with AE1-3 or AE1-4 were pulsed with 35S-Translabel™ for 15 min, and chased for 1 or 4 h. At each time point, the cells were lysed and immunoprecipitates prepared using AE1-specific peptide antibodies were either undigested, digested with endo H, or digested with N-glycosidase. These studies revealed an AE1-3 polypeptide of ∼80 kD accumulated in MDCK cells at the end of a 15-min pulse (Fig. 4). This species was susceptible to digestion with endo H and N-glycosidase yielding a polypeptide of ∼78 kD. The ∼80-kD AE1-3 polypeptide underwent no further modification, and was completely turned over by the end of a 4-h chase. Analysis of AE1-4 transfected cells revealed a discrete AE1-4 species of ∼97 kD accumulated in MDCK cells after the 15-min pulse (Fig. 4). This polypeptide was susceptible to digestion with endo H and N-glycosidase yielding a polypeptide of ∼95 kD. Quantitation of three independent experiments has shown that ∼50% of the newly synthesized AE1-4 polypeptides are turned over during a 4-h chase. The remaining AE1-4 polypeptides acquired additional modifications that eventually resulted in a diffuse array of polypeptides ranging in size from ∼105 to ∼112 kD (Fig. 4). The ∼105- to ∼112-kD polypeptides were still sensitive to digestion with N-glycosidase yielding a polypeptide of ∼95 kD. However, the N-linked sugar modifications of the ∼105- to ∼112-kD polypeptides were insensitive to digestion with endo H (Fig. 4). This suggests that AE1-4 initially receives high mannose or biantennary hybrid sugars on its single N-linked site. By 1 h after synthesis, ∼10% of these modifications are converted to endo H–resistant complex sugars, and by 4 h after synthesis the bulk of the newly synthesized AE1-4 polypeptides have received these more complex endo H-resistant sugar modifications. The addition of these complex sugars to AE1-4 requires the activity of α-mannosidase II and N-acetylglucosamine transferase, markers of the medial compartment of the Golgi. This suggests that AE1-4 passes through the medial compartment of the Golgi between 1 and 4 h after synthesis.
Figure 4.
Posttranslational modification and turnover of the chicken AE1-3 and AE1-4 anion exchangers. MDCK cells stably expressing the AE1-3 or AE1-4 anion exchanger were pulsed with 35S-Translabel™ in methionine-free DME for 15 min, and chased in DME containing 5% FCS for times ranging from 0–4 h. At each time point, immunoprecipitates were prepared from total cell lysates using polyclonal antibodies directed against the cytoplasmic domain of chicken AE1-4. Immunoprecipitates were either undigested, digested with endo H, or digested with N-glycosidase. Immune complexes were analyzed on a 6% SDS polyacrylamide gel, and labeled anion exchangers were detected by fluorography.
Newly Synthesized AE1-4 Polypeptides Undergo Recycling from the Plasma Membrane to the Golgi
The acquisition of mature N-linked sugars by chicken erythroid AE1 anion exchangers occurs via recycling of newly synthesized polypeptides from the plasma membrane to the Golgi (Ghosh et al. 1999). To determine whether a similar mechanism is responsible for the slow acquisition of endo H-resistant sugars by AE1-4, pulse–chase studies have examined the time after synthesis that newly synthesized AE1-4 becomes susceptible to digestion with extracellular chymotrypsin. This analysis revealed that the immunoprecipitable AE1-4 species of ∼97 kD was not susceptible to digestion with extracellular chymotrypsin that was present in the media during the pulse and for the first 15 min of chase (Fig. 5 A, lane 2). However, when chymotrypsin was included in the media from 15 min to 60 min of chase, all of the immature AE1-4 species of ∼97 kD was susceptible to digestion (Fig. 5 A, lane 4). Immunoblotting analysis revealed that the steady state population of AE1-4 was digested to the same extent regardless of the time when chymotrypsin was added (Fig. 5 A). Additional controls revealed that α-fodrin was not susceptible to chymotrypsin digestion at either of the time points (Fig. 5 A) indicating the susceptibility of newly synthesized AE1-4 to chymotrypsin was not due to the cells becoming leaky during digestion. These results indicate that essentially all of the newly synthesized polypeptides arrive at the plasma membrane by 1 h after synthesis. Furthermore, these data suggest that the acquisition of endo H–resistant N-linked sugars by AE1-4 between 1 and 4 h of chase primarily occurs via recycling of cell surface polypeptides to the Golgi. Interestingly, when cells were shifted to 4°C after 1 h of chase to block vesicular trafficking and incubated with chymotrypsin for an additional 45 min at 4°C, newly synthesized AE1-4 was resistant to chymotrypsin digestion (Fig. 5 A, lane 5). This result together with the fact that newly synthesized polypeptides have undergone cell surface delivery by 1 h of chase suggests that newly synthesized AE1-4 is rapidly internalized after surface delivery.
Figure 5.
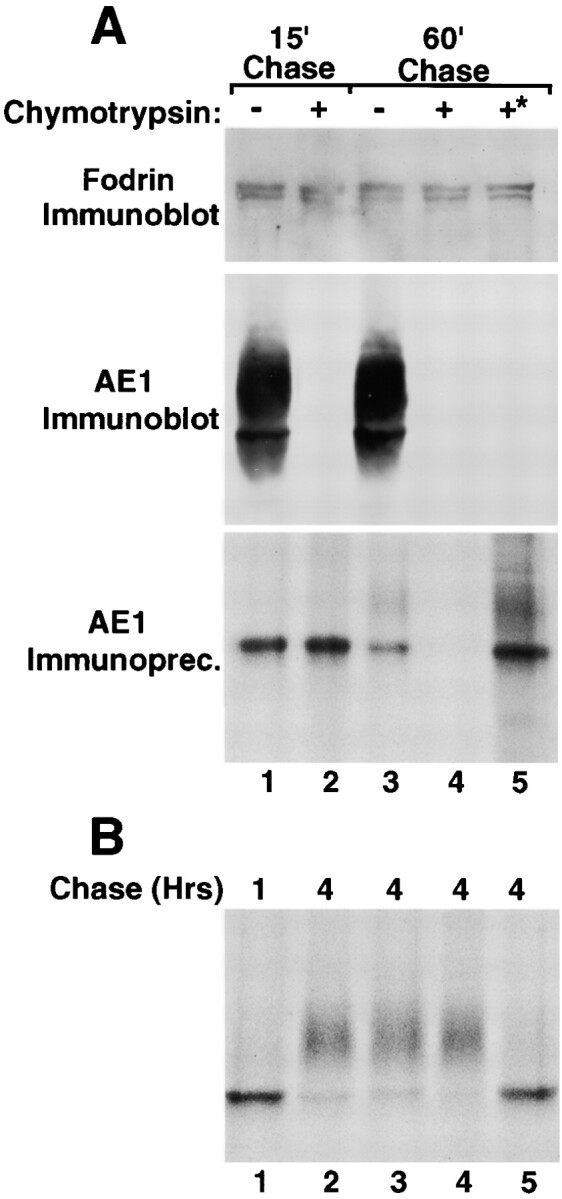
Surface delivery and recycling of newly synthesized AE1-4 anion exchangers. MDCK cells stably expressing AE1-4 were pulsed with 35S-Translabel™ for 15 min, followed by 15 min of chase (A, lane 1). After 15 min of chase, the cells were chased an additional 45 min in the absence (A, lane 3) or presence (A, lane 4) of 1.5 mg/ml chymotrypsin. Cells were also incubated continuously with 1.5 mg/ml chymotrypsin during a 15-min preincubation in methionine-free DME, a 15-min pulse with 35S-Translabel™, and a 15-min chase (A, lane 2). Alternatively, cells were pulsed for 15 min and chased for 1 h at 37°C. The cells were then shifted to 4°C, and incubated an additional 45 min in the presence of 1.5 mg/ml chymotrypsin (A, lane 5, marked with asterisk). For each labeling scheme, AE1 immunoprecipitates were prepared from total cell lysates using a polyclonal antibody directed against the cytoplasmic domain of AE1-4. A fraction of the total cell lysate was also analyzed by immunoblotting using a polyclonal antibody that recognizes α-fodrin (A) or a chicken AE1-specific peptide antibody (A). Additional studies examined the effect of various reagents on the posttranslational processing of AE1-4. MDCK cells stably expressing AE1-4 were pulsed with 35S-Translabel™ for 15 min, and chased for 1 h (B, lane 1), or 4 h (B, lanes 2–5). For some of the cells, 25 mM ammonium chloride (A, lane 3), 10 μg/ml BFA (B, lane 4), or 0.4 M sucrose (B, lane 5) was added to media after 45 min of chase, and was present for the remainder of the chase period. At each time point, AE1 immunoprecipitates were prepared from total cell lysates. Immunoprecipitates in A and B were analyzed on a 6% SDS polyacrylamide gel, and labeled anion exchangers were detected by fluorography.
Previous studies (Ghosh et al. 1999) have shown that recycling of chicken erythroid AE1 anion exchangers from the cell surface to the Golgi is sensitive to ammonium chloride (Chapman and Munro 1994), BFA (Stoorgovel et al. 1996), and 0.4 M sucrose (Hansen et al. 1993), reagents known to affect different steps in the endocytic pathway. Pulse–chase analyses have investigated whether the recycling of AE1-4 from the cell surface to the Golgi in MDCK cells exhibits a similar sensitivity to these reagents. Each reagent was added to MDCK cells expressing AE1-4 after a 15-min pulse and a 45-min chase, and the effect of the reagent on the acquisition of complex N-linked sugars by AE1-4 was monitored during a subsequent chase. These studies revealed that 0.4 M sucrose, which can inhibit clathrin-dependent endocytosis by dissociating clathrin from the plasma membrane (Hansen et al. 1993), completely blocked the acquisition of mature N-linked sugars by newly synthesized AE1-4 (Fig. 5 B, compare lanes 2 and 5). This result is consistent with the hypothesis that after delivery to the plasma membrane, the rapid internalization of AE1-4 occurs in a clathrin-dependent fashion. In contrast to 0.4 M sucrose, the posttranslational processing of AE1-4 in the presence of ammonium chloride or BFA (Fig. 5 B, lanes 3 and 4, respectively) was similar to that observed in control cells (Fig. 5 B, lane 2). The observation that AE1-4 recycling is insensitive to ammonium chloride and BFA suggests that the Golgi recycling pathway for AE1 in MDCK cells is distinct from the recycling pathway previously described for AE1 in chicken embryonic erythroid cells (Ghosh et al. 1999).
The Variant AE1-4 Anion Exchanger Colocalizes with the Actin Cytoskeleton in MDCK Cells
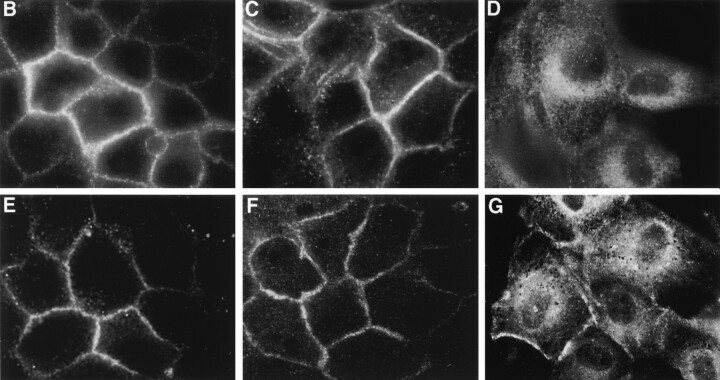
During the establishment of epithelial polarity, the actin cytoskeleton of MDCK cells undergoes a dramatic reorganization. Cells grown in subconfluent monolayers possess discrete actin stress fibers in the basal membrane. During polarization, these stress fibers become much less prevalent as actin is redistributed for the most part to sites of cell–cell contact. To assess the potential role of the actin cytoskeleton in directing the intracellular localization of AE1-3 and AE1-4 in MDCK cells, subconfluent monolayers of cells transfected with these variant transporters were double stained with AE1-specific antibodies and FITC-phalloidin. These studies revealed that the localization of AE1-4 in transfected cells substantially overlapped the distribution of both phalloidin-stained stress fibers in the basal membrane and cortical actin at sites of cell–cell contact (Fig. 6A, Fig. C, and Fig. E). In contrast, there was minimal if any overlap of AE1-3 with phalloidin-stained microfilaments in transfected cells (data not shown).
Figure 6.
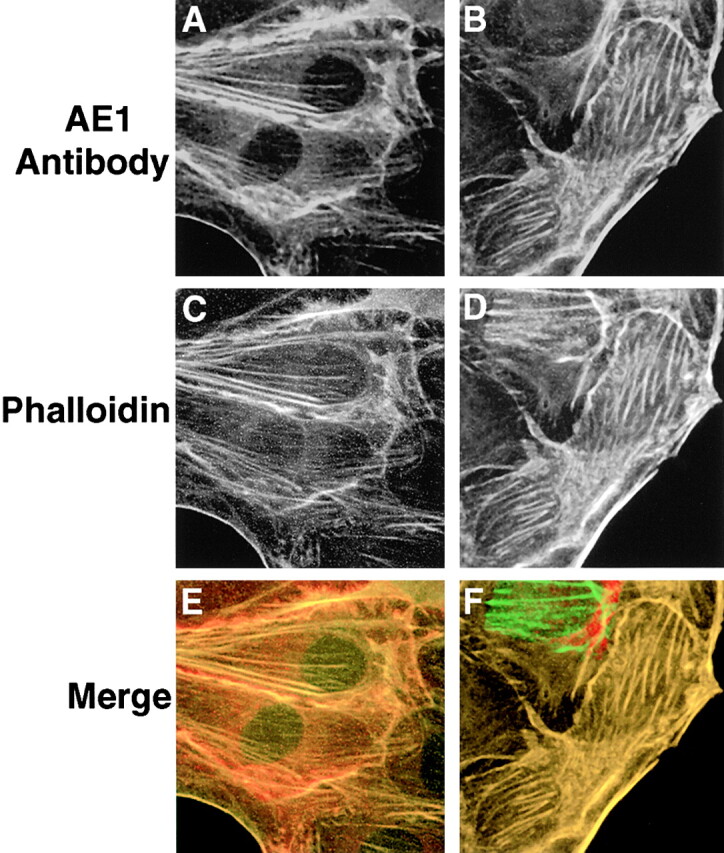
AE1-4 colocalizes with phalloidin-stained microfilaments in transfected MDCK cells. MDCK cells stably expressing the AE1-4 anion exchanger were grown in subconfluent monolayers. Cells were either untreated (A, C, and E) or extracted with 0.5% Triton X-100 in PBS (B, D, and F) before fixation in 1% paraformaldehyde in PBS. The cells were then incubated with an AE1-specific peptide antibody, followed by incubation with DAR-IgG conjugated to lissamine and FITC-conjugated phalloidin. Images showing the distribution of AE1-4 (A and B) and phalloidin-stained microfilaments (C and D) were collected on a Zeiss Axiophot microscope. The merged images (E and F) were generated in Adobe Photoshop.
To determine whether association with filamentous actin could account for the detergent solubility properties of AE1-4 (Fig. 3), transfected cells were extracted in situ with 0.5% Triton X-100 before fixation and staining with AE1-specific antibodies and phalloidin. This analysis revealed that AE1-4 still colocalized with phalloidin-stained stress fibers and filamentous actin at cell–cell junctions in these preextracted cells (Fig. 6B, Fig. D, and Fig. F) suggesting that AE1-4 is associated with the actin cytoskeleton. In contrast to AE1-4, there was essentially no detectable fluorescence in AE1-3 expressing cells that were detergent extracted before fixation and staining with AE1 antibodies (data not shown).
The Acquisition of Detergent Insolubility by Newly Synthesized AE1-4
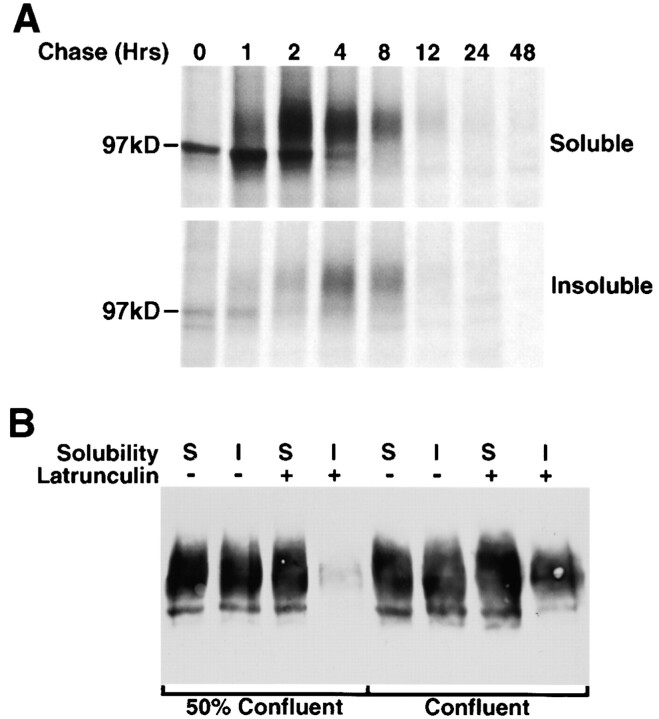
Pulse–chase studies have investigated the kinetics with which newly synthesized AE1-4 associates with the detergent insoluble fraction of MDCK cells. Subconfluent MDCK cells stably transfected with AE1-4 were pulsed for 15 min and chased for times ranging from 1 to 48 h (Fig. 7 A). At each time point, the cells were detergent fractionated and immunoprecipitates were prepared from the detergent soluble and insoluble fractions using AE1-specific antibodies. Quantitation of three independent experiments identical to the one shown in Fig. 7 A has revealed that newly synthesized AE1-4 was almost entirely (>95%) detergent soluble after 1 h of chase. By 2 h of chase, ∼50% of the AE1-4 polypeptides have acquired complex N-linked sugars, and ∼15% of the polypeptides have become detergent insoluble. The pool of polypeptides with complex N-linked sugars does not increase between 2 and 4 h of chase. However, there is a substantial reduction in the amount of the ∼97-kD polypeptide during this time period suggesting that most of the polypeptides that have not acquired complex N-linked sugars by 2 h of chase are turned over. In addition, there is a gradual shift of the remaining polypeptides from the detergent soluble to the insoluble pool such that by 8 h of chase ∼45% of the polypeptides are detergent insoluble. By 12 h of chase, ∼90% of the newly synthesized polypeptides have turned over, and immunoprecipitable AE1-4 was almost undetectable after 48 h of chase. These pulse–chase studies suggest that association of newly synthesized AE1-4 with the detergent insoluble fraction does not provide a mechanism for preferentially stabilizing AE1-4 in MDCK cells, since detergent soluble and insoluble AE1-4 turned over at a similar rate at the later time points of the analysis. Similar studies with MDCK cells that had been grown on permeable supports 5 d after cell–cell contact yielded identical results (data not shown) indicating the detergent fractionation properties and turnover rate of AE1-4 are not affected by the state of polarity of these epithelial cells.
Figure 7.
Acquisition and maintenance of detergent insolubility by AE1-4. MDCK cells stably expressing the AE1-4 anion exchanger were pulsed with 35S-Translabel™ for 15 min, and chased for times ranging from 0–48 h. At each time point, cells were detergent lysed and separated into soluble and insoluble fractions by centrifugation. Immunoprecipitates were prepared from each fraction using antibodies generated against the cytoplasmic domain of AE1-4. Immune complexes were analyzed on a 6% SDS polyacrylamide gel, and labeled anion exchangers were detected by fluorography (A). Confluent or 50% confluent monolayers of MDCK cells expressing AE1-4 were incubated in the absence or presence of 25 μg/ml latrunculin B for 1 h. Cells were then detergent lysed, and the detergent soluble and insoluble fractions were analyzed by immunoblotting analysis using AE1-specific peptide antibodies (B).
The Role of the Actin Cytoskeleton in the Maintenance of AE1-4 Detergent Insolubility Varies as a Function of MDCK Cell Polarity
The in situ extraction studies described above (Fig. 6) suggest that the acquisition of detergent insolubility by newly synthesized AE1-4 is at least in part due to its association with the insoluble actin cytoskeleton. To directly assess the role of the actin cytoskeleton in the maintenance of AE1-4 insolubility, transfected cells grown in subconfluent monolayers were treated with the actin-depolymerizing drug, latrunculin B, for 1 h before detergent lysis. Immunoblotting analysis revealed that the detergent insoluble pool of AE1-4 was dramatically reduced in cells treated with this reagent (Fig. 7 B). Interestingly, similar studies with cells grown in confluent monolayers revealed that latrunculin B treatment only had a small but reproducible effect on the detergent insolubility of AE1-4 (Fig. 7 B). These results indicate that the maintenance of AE1-4 insolubility is almost entirely dependent upon an intact actin cytoskeleton in subconfluent cells. However, as cells develop a more polarized phenotype, alternative mechanisms are primarily responsible for the insolubility of AE1-4.
Identification of Cytoplasmic Tyrosine Residues Required for the Basolateral Accumulation and Cytoskeletal Association of AE1-4
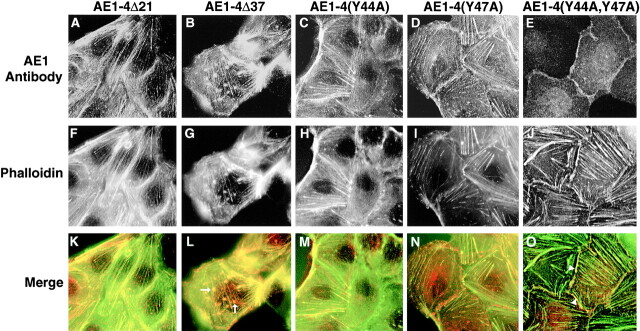
Other investigators have identified dominant cytoplasmic sorting signals that direct the basolateral sorting of integral membrane polypeptides in epithelial cells (Casanova et al. 1991; Matter et al. 1992; Thomas et al. 1993; Hunziker and Fumey 1994; Honing and Hunziker 1995). The studies described above have indicated that the alternative NH2-terminal cytoplasmic sequences of the chicken AE1-3 and AE1-4 anion exchangers direct these variant transporters to the apical and basolateral membrane, respectively, of polarized MDCK cells. To determine the sequences at the NH2 terminus of AE1-4 that direct its basolateral accumulation, a variety of mutants have been generated (Fig. 8 A). NH2-terminal truncation mutants that deleted the NH2-terminal 21 (AE1-4Δ21) or 37 (AE1-4Δ37) amino acids of AE1-4 primarily accumulated in the basolateral membrane of stably transfected MDCK cells (Fig. 8B and Fig. C). In contrast, AE1-4Δ53, which lacks the NH2-terminal 53 amino acids of AE1-4, exhibited a diffuse pattern of localization in or near the apical membrane, and accumulated in an undefined intracellular compartment of transfected cells (Fig. 8 D). This indicated that sequences between amino acids 37 and 53 of AE1-4 are required for its basolateral accumulation in MDCK cells.
Figure 8.
Localization of AE1-4 mutants in confluent MDCK monolayers. The amino acid sequence of the NH2-terminal truncation and point mutants of AE1-4 are illustrated in A. Dots indicate sequence that is identical among all of the constructs. Point mutants are underlined. MDCK cells stably expressing AE1-4Δ21 (B), AE1-4Δ37 (C), AE1-4Δ53 (D), AE1-4(Y44A) (E), AE1-4(Y47A) (F), or AE1-4(Y44A,Y47A) (G) were grown in confluent monolayers on coverslips. The cells were fixed and incubated with an AE1-specific peptide antibody, followed by incubation with DAR-IgG conjugated to lissamine. Immunoreactive polypeptides were visualized on a Zeiss Axiophot microscope. Images were collected near the center (B, C, E, and F) or at the apical surface (D and G) of the cells.
The region between amino acids 37 and 53 of AE1-4 contains two tyrosine residues, at positions 44 and 47. Other investigators have shown that tyrosine residues are critical for the basolateral sorting of several type I integral membrane proteins in MDCK cells (Matter et al. 1992; Thomas et al. 1993; Honing and Hunziker 1995). To investigate the role of these residues in directing the intracellular localization of AE1-4, point mutants were generated in which an alanine was substituted for each of the tyrosine residues separately or together. Substituting alanine for either of these residues separately, AE1-4(Y44A) or AE1-4(Y47A), had no effect on the basolateral accumulation of AE1-4 (Fig. 8E and Fig. F). In contrast, the AE1-4(Y44A,Y47A) double mutant primarily accumulated in the apical membrane and in an undefined intracellular compartment of transfected MDCK cells (Fig. 8 G). In a small percentage of the cells, the AE1-4(Y44A,Y47A) mutant also accumulated in the basolateral membrane. These data suggest that efficient basolateral targeting of AE1-4 in MDCK cells is dependent upon these cytoplasmic tyrosine residues. Interestingly, either one of the tyrosines at amino acids 44 or 47 is sufficient to direct the basolateral accumulation of this variant transporter.
To determine whether the basolateral accumulation of the AE1-4 mutants correlated with their ability to colocalize with the actin cytoskeleton, subconfluent monolayers of MDCK cells expressing the mutant transporters were double stained with AE1-specific antibodies and FITC-phalloidin. This analysis revealed that only those mutants that were efficiently targeted to the basolateral membrane in confluent monolayers, including AE1-4Δ21, AE1-4Δ37, AE1-4(Y44A), and AE1-4(Y47A), colocalized both with stress fibers in the basal membrane of subconfluent MDCK cells, and with cortical actin at sites of cell-cell contact (Fig. 9). In addition to colocalizing with stress fibers in the basal membrane of cells, AE1-4Δ37 often accumulated in clusters in the basal membrane that were also stained by FITC-phalloidin (arrows in Fig. 9 L). These actin-containing clusters were rarely observed with the other mutant constructs, and they were negative for staining with talin antibodies, a marker for focal adhesions (data not shown). Although AE1-4(Y44A,Y47A) colocalized with cortical actin at sites of cell-cell contact in a small percentage of transfected cells (arrowheads in Fig. 9 O), this mutant transporter was never observed to colocalize with stress fibers in the basal membrane of cells. These results suggest that association of AE1-4 with specific elements of the actin cytoskeleton, like efficient basolateral targeting, requires at least one of the tyrosine residues at amino acids 44 and 47 of the polypeptide. At this time, we can not distinguish whether the association of AE1-4 with the detergent insoluble actin cytoskeleton is a prerequisite for or a consequence of the basolateral sorting of this variant transporter in MDCK cells.
Figure 9.
Localization of AE1-4 mutants in subconfluent MDCK monolayers. MDCK cells expressing AE1-4Δ21 (A), AE1-4Δ37 (B), AE1-4(Y44A) (C), AE1-4(Y47A) (D), or AE1-4(Y44A,47A) (E) were grown on coverslips in subconfluent monolayers. The cells were fixed and incubated with an AE1-specific peptide antibody, followed by incubation with DAR-IgG conjugated to lissamine (A–E) and FITC-conjugated phalloidin (F–J). Immunoreactive polypeptides and phalloidin stained microfilaments were visualized on a Zeiss Axiophot microscope. Images were collected at the basal surface of the cells. Merged images were generated in Adobe Photoshop (K–O). The arrows in L mark two of the actin-containing clusters that colocalize with AE1-4Δ37. The arrowheads in O indicate where AE1-4(Y44A,47A) colocalizes with cortical actin at sites of cell-cell contact.
The Acquisition of Detergent Insolubility and the Stability of AE1-4 Is Linked to Efficient Golgi Recycling
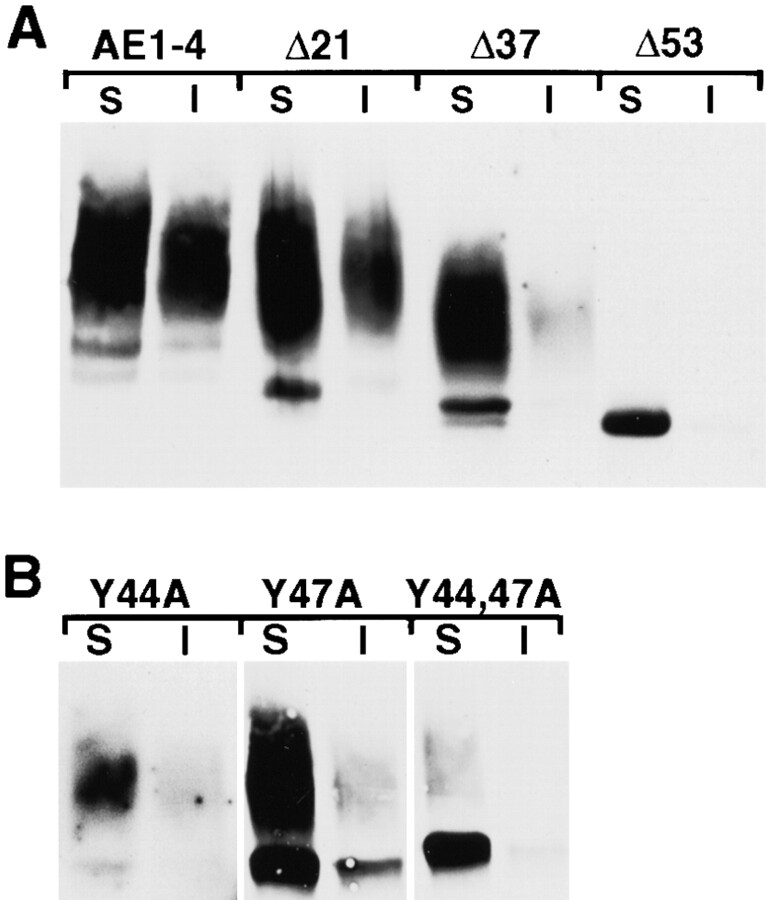
To determine whether the NH2-terminal truncation and point mutants of AE1-4 affected the detergent solubility properties and posttranslational modifications of this variant transporter, confluent MDCK cells stably transfected with each construct were detergent lysed, and separated into soluble and insoluble fractions by centrifugation. Immunoblotting analysis of these fractions with AE1-specific antibodies revealed that the AE1-4 mutants could be grouped into three classes. Quantitation of three independent experiments identical to the one shown in Fig. 10 has revealed that the first class of mutants, AE1-4Δ21 and AE1-4(Y44A), was similar to wild-type AE1-4 in terms of the percent of the steady state population that was detergent insoluble (∼40%), and the percent of the molecules that acquired complex N-linked sugars (∼95%). The second class of mutants, AE1-4Δ37 and AE1-4(Y47A), exhibited partial defects in their posttranslational processing (Fig. 10). The percentage of AE1-4(Y47A) polypeptides that acquired complex N-linked sugars (∼45%) was reduced relative to that observed with AE1-4, and both AE1-4Δ37 and AE1-4(Y47A) were substantially reduced in their ability to associate with the detergent insoluble fraction of MDCK cells (∼5% of total protein). Interestingly, unlike AE1-4, most of the AE1-4(Y47A) polypeptides that associated with the detergent insoluble fraction possessed only simple N-linked sugars suggesting that AE1-4(Y47A) may acquire detergent insolubility by a mechanism distinct from that used by AE1-4. Finally, AE1-4Δ53 failed to acquire complex N-linked sugars and was entirely detergent soluble (Fig. 10). A similar phenotype was observed for AE1-4(Y44A,Y47A) suggesting that Golgi recycling and the acquisition of detergent insolubility, like efficient basolateral targeting, requires at least one of the tyrosines at amino acids 44 and 47 of AE1-4.
Figure 10.
Steady state fractionation properties of the AE1-4 mutants. MDCK cells stably expressing the NH2-terminal truncation (A) or point mutants (B) of AE1-4 were detergent lysed and separated into soluble (S) and insoluble (I) fractions by centrifugation. Equivalent amounts of the resulting fractions were separated on a 6% SDS polyacrylamide gel, and transferred to nitrocellulose. The filter was incubated with an AE1-specific peptide antibody, followed by GAR-IgG conjugated to horseradish peroxidase. Immunoreactive species were detected by enhanced chemiluminescence.
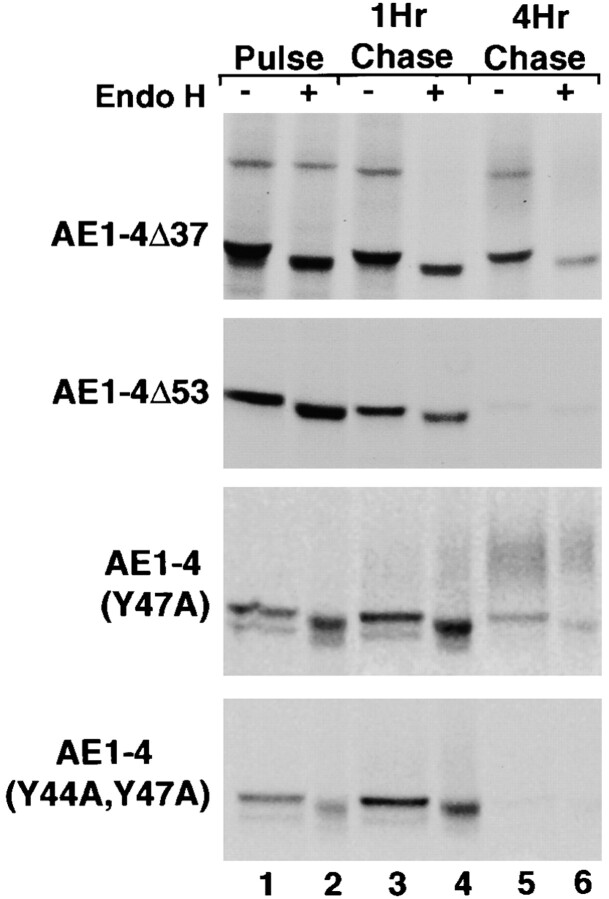
Pulse–chase analyses have examined whether mutations that alter the posttranslational processing of AE1-4 affect the kinetics with which these polypeptides acquire complex N-linked sugars as well as their stability. These studies revealed that each mutant acquired N-linked modifications that were sensitive to endo H during a 15-min pulse (Fig. 11). During the chase period, AE1-4Δ37 and AE1-4(Y47A) acquired additional sugar modifications that were resistant to endo H. However, the percentage of newly synthesized AE1-4Δ37 and AE1-4(Y47A) polypeptides that acquired complex N-linked sugars by 4 h of chase was substantially lower than that observed for AE1-4 (compare Fig. 4 and Fig. 11). These data indicate that tyrosine 47 and sequences in the NH2-terminal 37 amino acids of AE1-4 are both necessary for efficient Golgi recycling of AE1-4. The inefficient recycling of AE1-4Δ37 and AE1-4(Y47A) may account for the reduced ability of these mutant transporters to associate with the detergent insoluble fraction of MDCK cells. Similar analyses with AE1-4Δ53 and AE1-4(Y44A,Y47A) indicated that these mutants acquired no additional modifications after the pulse, and like AE1-3, they were almost completely turned over by 4 h of chase (Fig. 11). The pulse–chase and immunoblotting studies indicate that Golgi recycling requires at least one of the tyrosine residues at amino acids 44 and 47 of AE1-4. Furthermore, these data suggest that the stable accumulation of newly synthesized AE1 in this kidney epithelial cell type is completely dependent upon its ability to undergo recycling from the plasma membrane to the Golgi.
Figure 11.
Posttranslational processing and stability of the AE1-4 mutants. MDCK cells stably expressing AE1-4Δ37, AE1-4Δ53, AE1-4(Y47A), or AE1-4(Y44A,Y47A) were pulsed with 35S-Translabel™ for 15 min (lanes 1 and 2), or chased for 1 h (lanes 3 and 4), or 4 h (lanes 5 and 6). At each time point, AE1 immunoprecipitates were prepared from total cell lysates using antibodies directed against the cytoplasmic domain of AE1-4. Immunoprecipitates were either undigested (lanes 1, 3, and 5) or digested (lanes 2, 4, and 6) with endo H before analysis on a 6% SDS polyacrylamide gel. Labeled anion exchangers were detected by fluorography.
Discussion
The alternative NH2-terminal sequences of the variant chicken kidney AE1 anion exchangers direct the intracellular trafficking of these membrane transporters in transfected MDCK cells. The AE1-4 variant accumulates in the basolateral membrane of transfected cells, while the AE1-3 variant, which lacks the 63 NH2-terminal cytoplasmic amino acids of AE1-4, primarily accumulates in the apical membrane. In addition to targeting AE1-4 to the basolateral membrane of transfected cells, the NH2-terminal 63 amino acids of AE1-4 are also required for recycling of this membrane transporter from the plasma membrane to the Golgi. Previous studies have shown that the association of chicken erythroid AE1 anion exchangers with cytoskeletal ankyrin occurs during recycling of newly synthesized polypeptides from the plasma membrane to the Golgi (Ghosh et al. 1999). The data described here have shown that association with the detergent insoluble cytoskeleton and the stable accumulation of the chicken kidney AE1 variants in MDCK cells is also linked to their ability to undergo Golgi recycling.
The sorting of type I membrane proteins to the basolateral membrane of kidney epithelial cells has been shown to be dependent upon dominant cytoplasmic sorting signals that direct the vectorial transport of newly synthesized proteins from the TGN to the basolateral membrane (Casanova et al. 1991; Matter et al. 1992; Thomas et al. 1993; Hunziker and Fumey 1994; Honing and Hunziker 1995). Many of these basolateral sorting signals are tyrosine-dependent (Matter et al. 1992; Thomas et al. 1993; Honing and Hunziker 1995). The accumulation of variant chicken AE1 anion exchangers in the basolateral membrane of transfected MDCK cells is also dependent upon cytoplasmic tyrosine residues. Furthermore, the NH2-terminal cytoplasmic sequence of AE1-4 contains two tyrosine residues at amino acids 44 and 47, either one of which is sufficient to direct the basolateral accumulation of this variant transporter. A similar result has previously been observed for the low density lipoprotein receptor, which contains two tyrosine-dependent cytoplasmic sorting signals, either one of which is sufficient to direct the basolateral sorting of this type I membrane protein (Matter et al. 1992). Interestingly, the same tyrosine residues that are involved in the basolateral accumulation of variant AE1 anion exchangers in MDCK cells are also required for the recycling of AE1 from the plasma membrane to the Golgi. Substitution of alanine for tyrosine 44 and 47 of AE1-4 results in a polypeptide that is almost completely deficient in Golgi recycling, and like AE1-3, the AE1-4(Y44A,Y47A) mutant is rapidly degraded.
Either of the tyrosine residues at amino acids 44 and 47 of AE1-4 is sufficient to direct the basolateral accumulation of this type III membrane protein. However, there are differential requirements for these tyrosine residues for Golgi recycling. Substitution of an alanine for tyrosine 47 reduces the efficiency of Golgi recycling of AE1-4 by ∼50%, while a similar substitution at tyrosine 44 has no significant effect on recycling. Tyrosine 47 resides within the sequence, YVEL, that is conserved among all characterized AE1 anion exchangers except for chicken AE1-3 (Cox and Cox 1995), and the mammalian kidney AE1 variants (Brosius et al. 1989; Kudrycki and Shull 1989; Kollert-Jons et al. 1993). This tetrapeptide matches the sequence motif, YXXΦ, where X is any amino acid and Φ is a hydrophobic residue. This sequence motif has been shown to associate with the μ subunits of the AP1, AP2, and AP3 adaptor complexes (Boll et al. 1996; Ohno et al. 1996, Ohno et al. 1998; Aguilar et al. 1997; Dell'Angelica et al. 1997), and function as an endocytic (Collawn et al. 1990; Eberle et al. 1991; Naim and Roth 1994; Schafer et al. 1995) and trans Golgi network recycling (Wong and Hong 1993) signal for several type I membrane proteins. The hydrophobic residue in the YXXΦ motif is critical for the ability of this sequence to serve as a sorting signal (Wong and Hong 1993; Naim and Roth 1994; Schafer et al. 1995), and for its ability to associate with adaptins (Ohno et al. 1996; Ohno et al. 1998). Future studies will address whether additional residues in the YVEL tetrapeptide of AE1-4 are involved in directing the intracellular trafficking of this variant transporter in MDCK cells.
The molecular basis of detergent insolubility of AE1 in MDCK cells varies as a function of polarity. Immunolocalization analyses coupled with studies using the actin-depolymerizing drug, latrunculin B, have indicated that the detergent insolubility of AE1-4 in subconfluent, nonpolarized MDCK cells is almost entirely dependent upon an intact actin cytoskeleton. Although the exact role of the actin cytoskeleton in directing the intracellular trafficking of AE1-4 is unclear, the ability of mutant AE1 constructs to accumulate in the basolateral membrane of this cell type correlated with their ability to colocalize with phalloidin-stained stress fibers. Immunolocalization studies with AE1-4Δ37 have further suggested that the organization of the actin cytoskeleton is modified as a consequence of associating with this AE1-4 mutant. Filamentous actin colocalizes with AE1-4Δ37 in large clusters in the basal membrane of subconfluent MDCK cells. This reorganization of actin, which was not observed with wild-type AE1-4 or the other mutant constructs, may contribute to the slow rate at which AE1-4Δ37 recycles to the Golgi and acquires mature N-linked sugars.
As cells establish a more polarized epithelial phenotype, the maintenance of detergent insolubility of AE1-4 is only partially dependent upon the actin cytoskeleton. The alternative interactions responsible for the maintenance of AE1-4 insolubility in polarized MDCK cells are not known. However, the fact that chicken erythroid AE1 anion exchangers acquire detergent insolubility in part through their association with cytoskeletal ankyrin (Ghosh et al. 1999) suggests that the detergent insolubility of AE1-4 in confluent MDCK cells could arise as a result of their association with the epithelial ankyrin 3 isoform (Peters et al. 1995). Previous investigators have postulated that the stable accumulation of the Na+,K+-ATPase in the basolateral membrane of polarized MDCK cells is dependent upon its association with the detergent insoluble cytoskeleton via its interaction with ankyrin and fodrin (Nelson and Hammerton 1989). Regardless of the specific interaction(s) that accounts for the detergent insolubility of AE1-4 in MDCK cells, the stability of this variant transporter is not dependent upon its continued association with the detergent insoluble fraction, since detergent soluble and insoluble AE1-4 turn over at a similar rate.
Other investigators have hypothesized that phosphorylation of the tyrosine residue in the YVEL tetrapeptide in the cytoplasmic domain of skate erythroid AE1 may be involved in regulating its association with elements of the cytoskeleton, such as ankyrin (Musch et al. 1999). Along these lines it is interesting to note that substitution of an alanine for the tyrosine in the Y47VEL sequence of AE1-4 only partially blocks Golgi recycling, while it almost entirely inhibits the ability of this polypeptide to associate with the detergent insoluble fraction of confluent MDCK cells. Since AE1-4(Y47A) appears to be unimpaired in its ability to associate with the actin cytoskeleton, these results suggest the possibility that tyrosine 47 may be directly involved in mediating the association of AE1-4 with alternative cytoskeletal receptors, such as ankyrin 3.
Our analyses have suggested that a subset of the newly synthesized AE1-3 anion exchanger is directed to the apical membrane of transfected MDCK cells. However, the rapid turnover of this polypeptide in this epithelial cell type prevents high levels of this variant transporter from accumulating in the apical membrane domain. This rapid rate of turnover may account for the fact that polypeptides with the predicted molecular mass of AE1-3 are not detected in chicken kidney membrane preparations by immunoblotting analysis (Cox and Cox 1995). Although it is possible that AE1-3 accumulates in the apical membrane of a subset of cells in the kidney collecting duct, AE1-3 may also function by modulating AE1-4 activity through heterodimer formation. Future studies will determine whether AE1-3 and AE1-4 have the capacity to heterodimerize in vivo and the consequences of heterodimerization on AE1-4 localization and stability in MDCK kidney epithelial cells.
Acknowledgments
This research was supported by a grant from the National Chapter of the American Heart Association (96-008610). We thank S. Ghosh and F.C. Dorsey for their critical evaluation of the manuscript. We thank Dr. S. Gluck for generously providing monoclonal antibodies directed against the H+-ATPase.
Footnotes
Abbreviations used in this paper: BFA, brefeldin A; endo H, endoglycosidase H.
References
- Aguilar R.C., Ohno H., Roche K.W., Bonifacino J.S. Functional domain mapping of the clathrin-associated adaptor medium chains mu1 and mu2. J. Biol. Chem. 1997;272:27160–27166. doi: 10.1074/jbc.272.43.27160. [DOI] [PubMed] [Google Scholar]
- Alper S.L., Natale J., Gluck S., Lodish H.F., Brown D. Subtypes of intercalated cells in rat kidney collecting duct defined by antibodies against erythroid band 3 and renal vacuolar H+-ATPase. Proc. Natl. Acad. Sci. USA. 1989;86:5429–5433. doi: 10.1073/pnas.86.14.5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boll W., Ohno H., Songyang Z., Rapoport I., Cantley L.C., Bonifacino J.S., Kirchhausen T. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO (Eur. Mol. Biol. Organ.) J. 1996;15:5789–5795. [PMC free article] [PubMed] [Google Scholar]
- Brosius F.C.D., Alper S.L., Garcia A.M., Lodish H.F. The major kidney band 3 gene transcript predicts an amino-terminal truncated band 3 polypeptide. J. Biol. Chem. 1989;264:7784–7787. [PubMed] [Google Scholar]
- Casanova J.E., Apodaca G., Mostov K.E. An autonomous signal for basolateral sorting in the cytoplasmic domain of the polymeric immunoglobulin receptor. Cell. 1991;66:65–75. doi: 10.1016/0092-8674(91)90139-p. [DOI] [PubMed] [Google Scholar]
- Chapman R.E., Munro S. Retrieval of TGN proteins from the cell surface requires endosomal acidification. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:2305–2312. doi: 10.1002/j.1460-2075.1994.tb06514.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collawn J.F., Stangel M., Kuhn L.A., Esekogwu V., Jing S.Q., Trowbridge I.S., Tainer J.A. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell. 1990;63:1061–1072. doi: 10.1016/0092-8674(90)90509-d. [DOI] [PubMed] [Google Scholar]
- Cox K.H., Adair-Kirk T.L., Cox J.V. Four variant chicken erythroid AE1 anion exchangers. Role of the alternative N-terminal sequences in intracellular targeting in transfected human erythroleukemia cells. J. Biol. Chem. 1995;270:19752–19760. doi: 10.1074/jbc.270.34.19752. [DOI] [PubMed] [Google Scholar]
- Cox K.H., Adair-Kirk T.L., Cox J.V. Variant chicken kidney AE1 anion exchanger transcripts are derived from a single promoter by alternative splicing. Gene. 1996;173:221–226. doi: 10.1016/0378-1119(96)00211-9. [DOI] [PubMed] [Google Scholar]
- Cox K.H., Cox J.V. Variant chicken AE1 anion exchangers possess divergent NH(2)-terminal cytoplasmic domains. Am. J. Physiol. 1995;268:F503–F513. doi: 10.1152/ajprenal.1995.268.3.F503. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica E.C., Ohno H., Ooi C.E., Rabinovich E., Roche K.W., Bonifacino J.S. AP-3an adaptor-like protein complex with ubiquitous expression. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:917–928. doi: 10.1093/emboj/16.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y., Casey J.R., Kopito R.R. The major kidney AE1 isoform does not bind ankyrin (ANK1) in vitro. J. Biol. Chem. 1994;269:32201–32208. [PubMed] [Google Scholar]
- Drenckhahn D., Schluter K., Allen D.P., Bennett V. Colocalization of band 3 with ankyrin and spectrin at the basal membrane of intercalated cells in the rat kidney. Science. 1985;230:1287–1289. doi: 10.1126/science.2933809. [DOI] [PubMed] [Google Scholar]
- Eberle W., Sander C., Klaus W., Schmidt B., von Figura K., Peters C. The essential tyrosine of the internalization signal in lysosomal acid phosphatase is part of a beta turn. Cell. 1991;67:1203–1209. doi: 10.1016/0092-8674(91)90296-b. [DOI] [PubMed] [Google Scholar]
- Fiedler K., Simons K. The role of N-glycans in the secretory pathway. Cell. 1995;81:309–312. doi: 10.1016/0092-8674(95)90380-1. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Cox K.H., Cox J.V. Chicken erythroid AE1 anion exchangers associate with the cytoskeleton during recycling to the Golgi. Mol. Biol. Cell. 1999;10:455–469. doi: 10.1091/mbc.10.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S.H., Sandvig K., van Deurs B. Clathrin and HA2 adaptorseffects of potassium depletion, hypertonic medium, and cytosol acidification. J. Cell Biol. 1993;121:61–72. doi: 10.1083/jcb.121.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honing S., Hunziker W. Cytoplasmic determinants involved in direct lysosomal sorting, endocytosis, and basolateral targeting of rat lgp120 (lamp-I) in MDCK cells. J. Cell Biol. 1995;128:321–332. doi: 10.1083/jcb.128.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunziker W., Fumey C. A di-leucine motif mediates endocytosis and basolateral sorting of macrophage IgG Fc receptors in MDCK cells. EMBO (Eur. Mol. Biol. Organ.) J. 1994;13:2963–2967. doi: 10.1002/j.1460-2075.1994.tb06594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollert-Jons A., Wagner S., Hubner S., Appelhans H., Drenckhahn D. Anion exchanger 1 in human kidney and oncocytoma differs from erythroid AE1 in its NH2 terminus. Am. J. Physiol. 1993;265:F813–F821. doi: 10.1152/ajprenal.1993.265.6.F813. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Kudrycki K.E., Shull G.E. Primary structure of the rat kidney band 3 anion exchange protein deduced from a cDNA. J. Biol. Chem. 1989;264:8185–8192. [PubMed] [Google Scholar]
- Matter K., Hunziker W., Mellman I. Basolateral sorting of LDL receptor in MDCK cellsthe cytoplasmic domain contains two tyrosine-dependent targeting determinants. Cell. 1992;71:741–753. doi: 10.1016/0092-8674(92)90551-m. [DOI] [PubMed] [Google Scholar]
- Musch M.W., Hubert E.M., Goldstein L. Volume expansion stimulates p72(syk) and p56(lyn) in skate erythrocytes. J. Biol. Chem. 1999;274:7923–7928. doi: 10.1074/jbc.274.12.7923. [DOI] [PubMed] [Google Scholar]
- Naim H.Y., Roth M.G. Characteristics of the internalization signal in the Y543 influenza virus hemagglutinin suggest a model for recognition of internalization signals containing tyrosine. J. Biol. Chem. 1994;269:3928–3933. [PubMed] [Google Scholar]
- Nelson W.J., Hammerton R.W. A membrane cytoskeletal complex containing Na+,K+-ATPase, ankyrin, and fodrin in MDCK cellsImplications for the biogenesis of epithelial cell polarity. J. Cell Biol. 1989;108:893–902. doi: 10.1083/jcb.108.3.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno H., Aguilar R.C., Yeh D., Taura D., Saito T., Bonifacino J.S. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J. Biol. Chem. 1998;273:25915–25921. doi: 10.1074/jbc.273.40.25915. [DOI] [PubMed] [Google Scholar]
- Ohno H., Fournier M.C., Poy G., Bonifacino J.S. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J. Biol. Chem. 1996;271:29009–29015. doi: 10.1074/jbc.271.46.29009. [DOI] [PubMed] [Google Scholar]
- Peters L.L., John K.M., Lu F.M., Eicher E.M., Higgins A., Yialamas M., Turtzo L.C., Otsuka A.J., Lux S.E. Ank3 (epithelial ankyrin), a widely distributed new member of the ankyrin gene family and the major ankyrin in kidney, is expressed in alternatively spliced forms, including forms that lack the repeat domain. J. Cell Biol. 1995;130:313–330. doi: 10.1083/jcb.130.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters L.L., Shivdasani R.A., Liu S.C., Hanspal M., John K.M., Gonzalez J.M., Brugnara C., Gwynn B., Mohandas N., Alper S.L., Orkin S.H., Lux S.E. Anion exchanger 1 (band 3) is required to prevent erythrocyte membrane surface loss but not to form the membrane skeleton. Cell. 1996;86:917–927. doi: 10.1016/s0092-8674(00)80167-1. [DOI] [PubMed] [Google Scholar]
- Schafer W., Stroh A., Berghofer S., Seiler J., Vey M., Kruse M.L., Kern H.F., Klenk H.D., Garten W. Two independent targeting signals in the cytoplasmic domain determine trans-Golgi network localization and endosomal trafficking of the proprotein convertase furin. EMBO (Eur. Mol. Biol. Organ.) J. 1995;14:2424–2435. doi: 10.1002/j.1460-2075.1995.tb07240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheiffele P., Peranen J., Simons K. N-glycans as apical sorting signals in epithelial cells. Nature. 1995;378:96–98. doi: 10.1038/378096a0. [DOI] [PubMed] [Google Scholar]
- Schwartz G.J., Barasch J., Al-Awqati Q. Plasticity of functional epithelial polarity. Nature. 1985;318:368–371. doi: 10.1038/318368a0. [DOI] [PubMed] [Google Scholar]
- Stoorgovel W., Ooschot V., Geuze H.J. A novel class of clathrin-coated vesicles budding from endosomes. J. Cell Biol. 1996;132:21–33. doi: 10.1083/jcb.132.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D.C., Brewer C.B., Roth M.G. Vesicular stomatitis virus glycoprotein contains a dominant cytoplasmic basolateral sorting signal critically dependent upon a tyrosine. J. Biol. Chem. 1993;268:3313–3320. [PubMed] [Google Scholar]
- van Adelsberg J.S., Edwards J.C., al-Awqati Q. The apical Cl/HCO3 exchanger of beta intercalated cells. J. Biol. Chem. 1993;268:11283–11289. [PubMed] [Google Scholar]
- Wang C.C., Moriyama R., Lombardo C.R., Low P.S. Partial characterization of the cytoplasmic domain of human kidney band 3. J. Biol. Chem. 1995;270:17892–17897. doi: 10.1074/jbc.270.30.17892. [DOI] [PubMed] [Google Scholar]
- Wong S.H., Hong W. The SXYQRL sequence in the cytoplasmic domain of TGN38 plays a major role in trans-Golgi network localization. J. Biol. Chem. 1993;268:22853–22862. [PubMed] [Google Scholar]
- Yeaman C., Le Gall A.H., Baldwin A.N., Monlauzeur L., Le Bivic A., Rodriguez-Boulan E. The O-glycosylated stalk domain is required for apical sorting of neurotrophin receptors in polarized MDCK cells. J. Cell Biol. 1997;139:929–940. doi: 10.1083/jcb.139.4.929. [DOI] [PMC free article] [PubMed] [Google Scholar]