Abstract
We cloned and characterized a full-length cDNA of mouse actin cross-linking family 7 (mACF7) by sequential rapid amplification of cDNA ends–PCR. The completed mACF7 cDNA is 17 kb and codes for a 608-kD protein. The closest relative of mACF7 is the Drosophila protein Kakapo, which shares similar architecture with mACF7. mACF7 contains a putative actin-binding domain and a plakin-like domain that are highly homologous to dystonin (BPAG1-n) at its NH2 terminus. However, unlike dystonin, mACF7 does not contain a coiled–coil rod domain; instead, the rod domain of mACF7 is made up of 23 dystrophin-like spectrin repeats. At its COOH terminus, mACF7 contains two putative EF-hand calcium-binding motifs and a segment homologous to the growth arrest–specific protein, Gas2. In this paper, we demonstrate that the NH2-terminal actin-binding domain of mACF7 is functional both in vivo and in vitro. More importantly, we found that the COOH-terminal domain of mACF7 interacts with and stabilizes microtubules. In transfected cells full-length mACF7 can associate not only with actin but also with microtubules. Hence, we suggest a modified name: MACF (microtubule actin cross-linking factor). The properties of MACF are consistent with the observation that mutations in kakapo cause disorganization of microtubules in epidermal muscle attachment cells and some sensory neurons.
Keywords: actin cross-linking family 7 (ACF7), plakin, actin, microtubule, cytoskeleton
The cytoskeleton of most eukaryotic cells consists of three types of filamentous networks: microfilaments (MFs), intermediate filaments (IFs), and microtubules (MTs). The cytoskeleton provides mechanical strength to the cell and controls many other cellular events, such as cell division, intracellular trafficking, and locomotion. The organization of these filaments is strongly dependent on their associated proteins. Thus, the identification of proteins that could associate with different types of filaments is of great importance. Several proteins belonging to the plakin family have been shown to connect cytoskeletal elements to each other and to the junctional complexes at the plasma membrane (Uitto et al. 1996; Ruhrberg and Watt 1997; Wiche 1998). So far, five plakin family members have been identified: desmoplakin, plectin, bullous pemphigoid antigen 1 (BPAG1), envoplakin, and periplakin (Green et al. 1992; Ruhrberg and Watt 1997; Ruhrberg et al. 1997). A typical plakin exhibits a three-domain structure, consisting of a central α-helical rod domain flanked by globular NH2-terminal head and COOH-terminal tail domains (Ruhrberg and Watt 1997). The rod domain contains stretches of heptad repeats that mediate coiled–coil dimer formation. The NH2-terminal head domain consists of six α-helical segments (NN, Z, Y, X, W, and V) that are conserved among plakins (Green et al. 1990; Ruhrberg et al. 1996; Ruhrberg and Watt 1997). These segments are organized into an antiparallel helical bundle and probably function as plaque to plate association domains. Except for periplakin, the COOH-terminal tail domains are made up of a variable number of homologous subdomains that interact directly with IFs (Green et al. 1990; Ruhrberg et al. 1996, Ruhrberg et al. 1997). As a result, plakins are believed to play an important role in anchoring IFs to the junctional complexes.
Among the fully characterized plakins, only plectin and two of the neuronal isoforms of BPAG1 (BPAG1-n, also known as dystonin) contain putative actin-binding domains (ABDs) at their NH2 termini (Wiche et al. 1991; Brown et al. 1995b). Plectin is an extremely large (>500 kD) cytoskeletal protein and was originally purified as a major component of IF extracts from a variety of cultured cells (Pytela and Wiche 1980). Aside from directly interacting with IFs, plectin also appears to associate with MFs and MTs, making it the most versatile cytoskeletal linker protein (for review see Wiche 1998). Dystonin/BPAG1-n was discovered from the analysis of BPAG1 knockout mice (Guo et al. 1995; Yang et al. 1996) and was identified as the mutated gene product of natural mouse mutants with the neurological disorder, dystonia musculorum (dt) (Brown et al. 1995a). Mice carrying mutations in the BPAG1 gene suffer from severe degeneration of primary sensory neurons and exhibit abnormal accumulations of IFs in axons (Duchen and Strich 1964; Janota 1972; Sotelo and Guenet 1988; al-Ali and al-Zuhair 1989; Guo et al. 1995). Recent studies revealed that the COOH-terminal tail domain of BPAG1-n interacts with neuronal IFs, whereas the NH2-terminal ABD associates with actin MFs (Yang et al. 1996; Leung et al. 1999). In addition, a truncated NH2-terminal protein of BPAG1-n was also shown to interact with MTs (Yang et al. 1999). Hence, like plectin, BPAG1-n could also function as a cytoskeletal cross-linking protein connecting neuronal IFs to MFs as well as MTs. Because of these functional features of plectin and BPAG1-n/dystonin, as well as their ability to connect IFs to proteins of junctional complexes, plakins are also called cytolinkers (Wiche 1998).
The ABDs found in plectin and dystonin/BPAG1-n are homologous to those found in members of the spectrin superfamily, which includes multiple isoforms of dystrophin, α-actinin, and spectrin itself (for review see Hartwig 1994). Spectrin superfamily members are all actin cross-linking proteins that are usually associated with membranes, where they maintain membrane integrity and modify membrane receptor functions. A major portion of each of these molecules is made up of repetitive homologous spectrin repeats that vary from 99–110 amino acid residues in length (Pascual et al. 1997). The spectrin repeats mediate dimerization of two molecules that are aligned in an antiparallel array. In addition, these repeats also confer flexibility to the molecule for adapting to the shape of cellular membranes. X-ray crystallography and secondary structure prediction based on sequence analysis revealed that the spectrin repeats conform to three-helix bundle structures composed of three left-handed, antiparallel α-helices joined by two loops (Speicher and Marchesi 1984; Yan et al. 1993). In addition to the spectrin repeats, most of the spectrin superfamily members also contain calmodulin-like, EF-hand calcium-binding motifs at their COOH termini, although it is not known if they are functional in vivo (Dubreuil et al. 1991).
A partial human actin cross-linking family 7 (ACF7) cDNA was originally isolated by screening for genes that encode proteins with ABDs homologous to that of dystrophin (Byers et al. 1995). Analysis of a longer mouse ACF7 (mACF7) cDNA revealed that it shares more sequence homology and isoform diversity with dystonin/BPAG1-n (Bernier et al. 1996), suggesting that mACF7 may represent a novel plakin/cytolinker. Three isoforms of ACF7 have been partially characterized in mice; two of them contain putative ABDs (Bernier et al. 1996). Here, we characterize a full-length mACF7 cDNA, and define its interaction partners. Sequence analysis shows that ACF7 is a hybrid of dystonin and dystrophin and is the mammalian homologue of Drosophila protein, Kakapo. Kakapo is a cytoskeletal protein that is essential for neuronal growth and adhesion between and within cell layers in Drosophila (Gregory and Brown 1998; Prokop et al. 1998; Strumpf and Volk 1998). Based on the morphological studies of the Drosophila kakapo mutants, Kakapo was proposed to be an MT actin organizer. In agreement with this notion, we show that the ABD of ACF7 is functional in interacting with actin filaments, whereas the COOH-terminal domain associates with and stabilizes MTs. Furthermore, transfected full-length ACF7 can associate with actin and MTs, suggesting that ACF7 may be a novel cytoskeletal linker protein.
Materials and Methods
cDNA Cloning
5′ and 3′ rapid amplification of cDNA ends (RACE)-PCR were performed on adapter-ligated mouse (BALB/c) brain Marathon-Ready™ cDNA (Clontech), and long-range PCR was done on regular mouse (BALB/c) brain QUICK-Clone™ cDNA (Clontech) using Advantage® cDNA PCR kit (Clontech). All procedures were carried out according to the manufacturer's protocols PT1156-1, PT1150-1, and PT-1580-1 (Clontech). In general, gene-specific 28-nucleotide primers were designed with high guanine cytosine contents (50–70%) and melting temperature >70°C. The following cycling parameters were employed for RACE-PCR: an initial denaturing step of 94°C for 30 s, followed by 5 cycles of 94°C (5 s) and 72°C (4 min), 5 cycles of 94°C (5 s) and 70°C (4 min), 25 cycles of 94°C (5 s) and 68°C (4 min), and a final extension step of 68°C for 5 min. PCR products were cloned into pGEM-T vector (Promega) or pCR2.1-TOPO vector (Invitrogen) for sequencing. Most of the cDNA clones were sequenced using the BigDye™ sequencing kit (Applied Biosystems) and processed by Perkin Elmer/Applied Biosystems Model ABI 377A Sequencers (DNA Facilities, Columbia University, New York). In some cases, cDNA clones were also sequenced manually with Sequenase 2.0 (U.S. Biochemical Corp.).
Plasmid Construction
pTOPO-ACF-5A, pTOPO-ACF-5B, pTOPO-ACF-rod, and pTOPO-ACF-3A were generated by cloning the long-range PCR products into pCR2.1-TOPO vector or pCR-XL-TOPO vector (Invitrogen). To engineer pFLAG-ABD, the 0.8-kb PCR fragment of pTOPO-ACF-5A using sense primer 5′-AAGCCAGAATTCTGTGCTGGACCCTGC-3′ and antisense primer 5′-TAAAGTGCCTCGAGTTCAACAGGG-3′ was digested with EcoRI/XhoI and ligated to the EcoRI/XhoI-digested pcDNA-FLAG vector (Leung et al. 1999). Similarly, pGEM-mACF7-C was generated by cloning the PCR fragment of pTOPO-ACF-3A using sense primer 5′-CATGGAGAATTCCCGCAGTGGTAG-3′ and antisense primer 5′-TTATCGCTTGGGACCTGGAGTCCTGGGG-3′ into pGEM-T vector (Promega). The 1.3-kb EcoRI-NotI fragment of pGEM-mACF7-C was then ligated to the EcoRI/NotI-digested pcDNA-FLAG vector to make pFLAG-mACF7-C. pGEM-GARt was created by cloning the PCR fragment of pTOPO-ACF-3A using sense primer 5′-CAACAAGAATTCCTATCGGCCAAC-3′ and antisense primer 5′-TTATCGCTTGGGACCTGGAGTCCTGGGG-3′ into pGEM-T vector (Promega). The 0.85-kb EcoRI-NotI fragment of pGEM-GARt was then cloned into pcDNA-FLAG vector to generate pFLAG-GARt. To construct pFLAG-mACF7-mini, three-piece ligation was performed with 2.5-kb Not-NcoI fragment of pTOPO-ACF-5A, 0.85-kb NcoI-XhoI fragment of pFLAG-GARt, and 5.4-kb NotI/XhoI-digested pcDNA3 vector (Invitrogen). To create a FLAG-tagged ACF7-3A clone, the stop codon on ACF-3A was first removed by PCR and the resulting fragment was ligated inframe to a FLAG-epitope tag coding sequence. Clones ACF-5A, ACF-rod, and FLAG-tagged ACF-3A were sequentially ligated together using the unique restriction endonuclease recognition sites KpnI and SalI at cDNA position 3665 and 11728, respectively, to generate a full-length FLAG-tagged ACF7 cDNA, which was subcloned into a multiple cloning site–modified eukaryotic expression vector pCI (Promega) to construct pFLAG-mACF7-fl (Fig. 1 A).
Figure 1.
Mouse ACF7. (A) The composite full-length cDNA of mACF7 is 17.3 kb. Fine lines represent RACE-PCR clones and bold lines stand for long-range PCR products. Clone names are shown above each line. The putative start codon (ATG), stop codon (TAA), and unique restriction endonuclease recognition sites, Kpn I and Sal I, used for the construction of full-length mACF7 are indicated. (B) Deduced amino acid sequence of mACF7. The NH2-terminal ABD is shaded in gray and the globular plakin-like domain is shaded in black with white letters. The rod domain of mACF7 is made up of 23 spectrin repeats and the corresponding amino acid sequence is boxed.
In Situ Hybridization
All studies were conducted in accordance with the principles and procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. Timed pregnant mouse C57BL/6 embryonic day 14.5 embryos were isolated, freshly frozen, and embedded directly in the OCT compound (Sakura Finetek, Inc.). Both sagittal and transverse cryostat sections were prepared. 35S-labeled cRNA transcripts were synthesized in vitro from pGEM-GARt using Riboprobe Gemini Systems from Promega. Both sense and antisense [35S]UTP-labeled cRNA were prepared. cRNA probes were purified on Sephadex G-50 columns (Boehringer Mannheim). In situ hybridization was performed as described previously (Zheng et al. 1998). After the nuclear emulsion autoradiography was performed, slides were examined in Leitz microscopes under both bright field and dark field illuminations. Hybridization with control (sense) probes yielded only low background staining in all cases.
Transient Transfections and Indirect Immunofluorescence Microscopy
COS-7 cells were cultured at 37°C with 5% CO2 in DMEM (Life Technologies, Inc.) supplemented with 10% FBS. Transient transfections for immunofluorescence analysis were performed on 18-mm coverslips using GenePORTER™ transfection reagents (Gene Therapy System) or LipofectAMINE PLUS reagents (Life Technologies, Inc.). 48 h after transfection, coverslips with adherent cells were fixed in cold methanol at −20°C or 4% paraformaldehyde in PBS at room temperature. To optimize stress fiber staining, fixed cells were permeabilized with 0.1% Triton X-100 for 5 min before further processing. After rinsing several times with PBS, cells on coverslips were blocked with 5% normal goat serum and incubated with primary antibodies at room temperature for 1 h. The primary antibody–treated cells were then washed with PBS and incubated with appropriate secondary antibodies or rhodamine-conjugated phalloidin (Sigma Chemical Co.) for 30 min. Subsequently, the coverslips were washed with PBS and mounted onto slides with Aquamount (Lerner Laboratories) for indirect immunofluorescent microscopy. The following primary antibodies were used for immunostaining: mouse monoclonal anti-FLAG M2 antibody (IBI-Kodak); rabbit polyclonal anti–Glu- and anti–Tyr-tubulin antibodies; rat monoclonal anti–Tyr-tubulin YL1/2 antibodies (a gift from Dr. Gregg G. Gundersen, Columbia University, New York, NY).
In Vitro Binding Assays
The STP3 system (Novagen) was used to synthesize [35S]methionine-labeled proteins from the pcDNA-FLAG constructs that contain T7 RNA polymerase promoters. In vitro synthesized proteins were prespun before they were used for the binding assays. The actin-binding assays were performed with the Non-muscle Actin Binding Protein Spin-Down Biochem Kit (Cytoskeleton, Inc.). In each testing, 20 μl of crude 35S-labeled protein was incubated with 0.23 nmol of polymerized F-actin at room temperature for 30 min. After centrifugation for 90 min at 65,000 rpm, supernatants and pellets were separated and subjected to SDS-PAGE analysis. After SDS-PAGE, the gels were dried and exposed to X-ray film to visualize the 35S-labeled proteins. The MT-binding assays were performed with the Microtubule Associated Protein Spin-Down Assay Kit (Cytoskeleton, Inc.). In each case, 20 μl of crude 35S-labeled protein was incubated with 0.15 pmol of taxol stabilized MTs at room temperature for 20 min. After centrifugation for 40 min at 55,000 rpm through a 40% glycerol cushion buffer containing 20 μΜ taxol, supernatants and pellets were collected for SDS-PAGE analysis. The gels were dried and exposed to X-ray film to visualize 35S-labeled proteins.
Results
Isolation of mACF7 cDNA Clones
The mRNA transcripts of mACF7 were estimated to be 14–18 kb, of which only 6 kb had been characterized previously (Bernier et al. 1996). Using a RACE-PCR–based method, we successfully isolated consecutively overlapping clones of the remaining cDNA (Fig. 1 A). The 5′ RACE product contained a putative start codon (ATG) that matched the Kozak consensus (Kozak 1986). Moreover, an inframe stop codon was located 102 bp upstream of this ATG, making it the most likely translational start site. By sequentially performing 3′ RACE-PCR with primers specific for the end sequence of the previous RACE products, we obtained 10 overlapping cDNA clones that spanned ∼11 kb in length before reaching a polyadenylation signal followed by a polyA stretch. In addition, we also performed long-range PCR to obtain longer cDNA clones for more detailed sequence analysis. The composite cDNA was about 17.3 kb (sequence data available from EMBL/GenBank/DDBJ under accession no. AF 150755), and the longest open reading frame encoded a 5,327–amino acid polypeptide with a calculated molecular mass of 608 kD (Fig. 1 B).
Sequence Analysis of mACF7
The deduced amino acid sequence of mACF7 was used to search for homologous proteins in the GenBank database. The closest relative of mACF7 is the Drosophila protein Kakapo. mACF7 and Kakapo not only share homology in primary sequence but also the overall protein architecture, indicating that mACF7 is the mouse homologue of Kakapo. As described in the previous study (Bernier et al. 1996), mACF7 contains a dystonin/BPAG1-n homologous NH2-terminal region, which includes a calponin homology ABD and a globular plakin-like domain. Interestingly, following the NH2-terminal domains, the sequences of mACF7 and dystonin start to diverge. Sequence comparison of mACF7 with dystrophin revealed that the central region of mACF7 would fold into 23 spectrin repeats (Fig. 1 B). By analogy to β-spectrin, this collection of spectrin repeats would also constitute the rod domain (not a coiled–coil rod) of mACF7. Following the rod domain, two calmodulin-like, EF-hand calcium-binding motifs were identified (Fig. 2 A). Together, these features suggest that mACF7 is a new member of the spectrin superfamily with plakin-like features.
Figure 2.
Sequence analysis of mACF7 COOH-terminal domain. (A) The two putative EF-hand calcium binding motifs of mACF7 are compared with those of mouse calmodulin (CaM). The amino acids that contain side chains with oxygen atoms for Ca2+ binding are shaded in gray, whereas the highly conserved glycine and hydrophobic amino acid are shaded in black with white letters. (B) Sequence comparison of mACF7 GAR region, human KIAA 0465, Drosophila Kakapo, human GAR22, and human GAS2 protein. The identical amino acids are shaded. (C) Schematic representation of the domain structure of mACF7. The NH2-terminal head domain consists of an ABD and a plakin-like globular domain. The rod domain is composed of 23 dystrophin-like spectrin repeats. The COOH-terminal tail domain is composed of two EF-hand motifs (Ca2+) and a Gas2/GAR22 homology region (GAR). The number of the amino acids that marked the boundary of each domain is also indicated.
A partial human brain cDNA clone, KIAA0465 was found showing >80% sequence identity to mACF7 COOH terminus. According to the UniGene database, the gene coded for KIAA0465 is located on chromosome 1, between markers D1S2843 and D1S417. In mice, the mACF7 gene was mapped to chromosome 4, close to the marker D4mit11 (Bernier et al. 1996). Based on the chromosome synteny and the extensive sequence homology, the partial KIAA0465 cDNA should encode the human orthologue of mACF7. In addition, a short stretch of the mACF7 COOH terminus displays significant homology to a portion of the recently identified protein, GAR22 (Gas2 related on chromosome 22) (Fig. 2 B). This part of GAR22 is also closely related to the Gas2 protein (growth arrest–specific 2 protein) (Zucman-Rossi et al. 1996; Collavin et al. 1998). Because of this similarity, we designated this region of mACF7 as the GAR region. A schematic structure of mACF7 is depicted in Fig. 2 C. To study the expression pattern of mACF7, mouse embryos at embryonic day 14.5 were hybridized with a mACF7 ribonucleotide probe. As illustrated in Fig. 3, mACF7 was ubiquitously expressed in all tissues, with higher levels in the nervous system, muscle, lung, heart, and adrenal glands.
Figure 3.
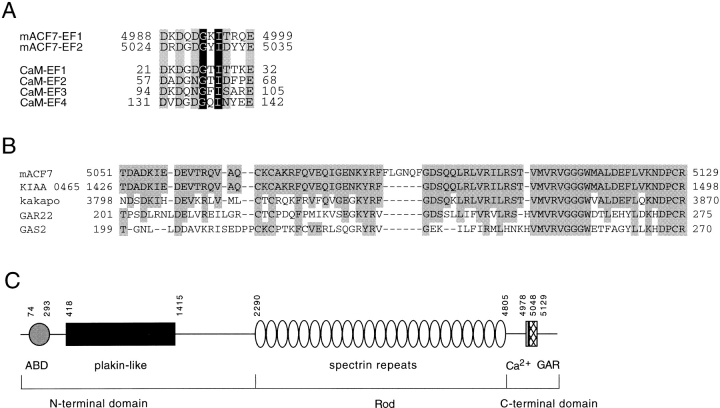
Expression of ACF7 mRNAs in the mouse embryo. Sagittal sections (A–C) and transverse sections (D–G) of an embryonic day 14.5 mouse embryo were hybridized with mACF7 antisense probe. Relative planes of sectioning for sections shown in D–G are illustrated on a schematic drawing of the embryo. In addition to ubiquitous expression of mACF7 mRNA throughout the embryo, high levels of expression are observed in the brain and spinal cord. In the brain, both ependymal layer (B, arrow) and mantle layer are heavily labeled. In the peripheral tissue, an intermediate to high level of ACF7 expression is observed in the dorsal root ganglia, olfactory epithelium (B, arrowhead), intrinsic muscle of the tongue, bronchial epithelium, and mesenchyme of the lung (B, arrows), skeletal muscle of the trunk (E, arrow), myocardium (F, arrow), and adrenal glands (G, arrow). Bars: 1 mm (A, B, D–G); and 0.1 mm (C).
Association of mACF7 with MFs In Vivo
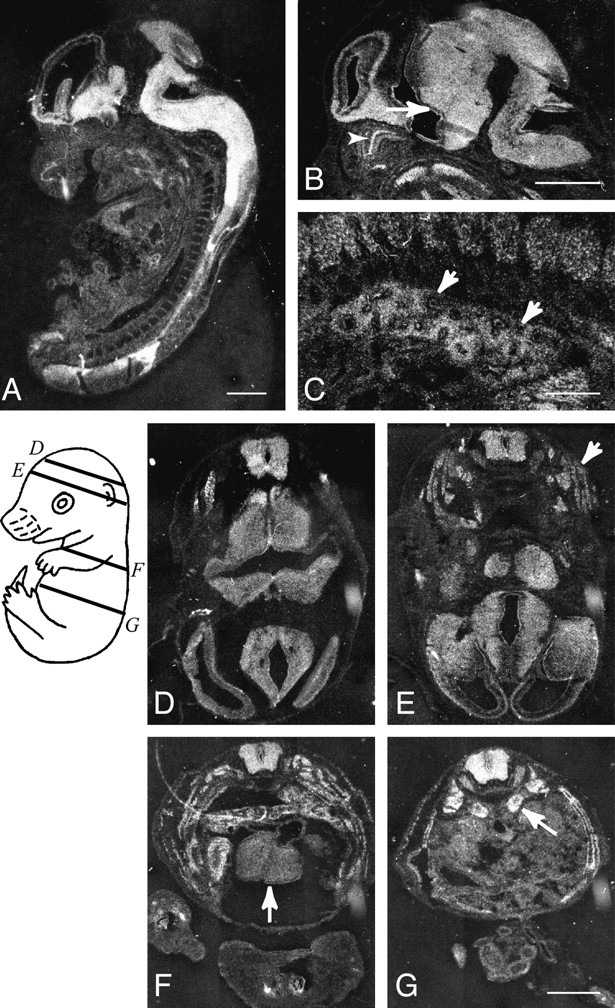
Full-length Gas2 was shown previously to be a component of the MF network, although the interaction domain had not been fully characterized (Brancolini et al. 1992; Collavin et al. 1998). Therefore, we examined possible interactions between the COOH-terminal domain of mACF7 (mACF7-C) and MFs by transient transfection assays. In addition, we also analyzed the actin-binding properties of the putative NH2-terminal ABD of mACF7. To facilitate the detection of the truncated proteins, the NH2 termini of mACF7-C and ABD were fused to FLAG epitope tags, which also provided the translational start codons. Transient transfections were performed on COS-7 cells. As shown in Fig. 4A and Fig. B, overexpressed ABD protein colocalized with filamentous actin in stress fibers and membrane ruffles of transfected cells, confirming the interaction of this highly conserved domain with MFs. In contrast, the mACF7-C protein, displayed a filamentous staining pattern that exhibited no significant correlation with the actin network (Fig. 4C and Fig. D). Therefore, we compared this staining pattern with that of the other two cytoskeletal networks, MTs and IFs, and found that mACF7-C proteins codistributed with MTs (Fig. 5), but not with vimentin (data not shown).
Figure 4.
Association of ACF7 ABD with MFs. COS-7 cells from transient transfections of pFLAG-ABD (A and B) or pFLAG-mACF7-C (C and D) were double-labeled with monoclonal anti-FLAG M2 antibody (A and C) and rhodamine-conjugated phalloidin (B and D). The FLAG-tagged ABD proteins colocalized perfectly with filamentous actin in stress fibers and membrane ruffles of the transfected cells. A filamentous staining pattern was obtained for FLAG-tagged mACF7-C proteins, yet it did not exhibit any correlation with actin structures. Bar, 20 μm.
Figure 5.
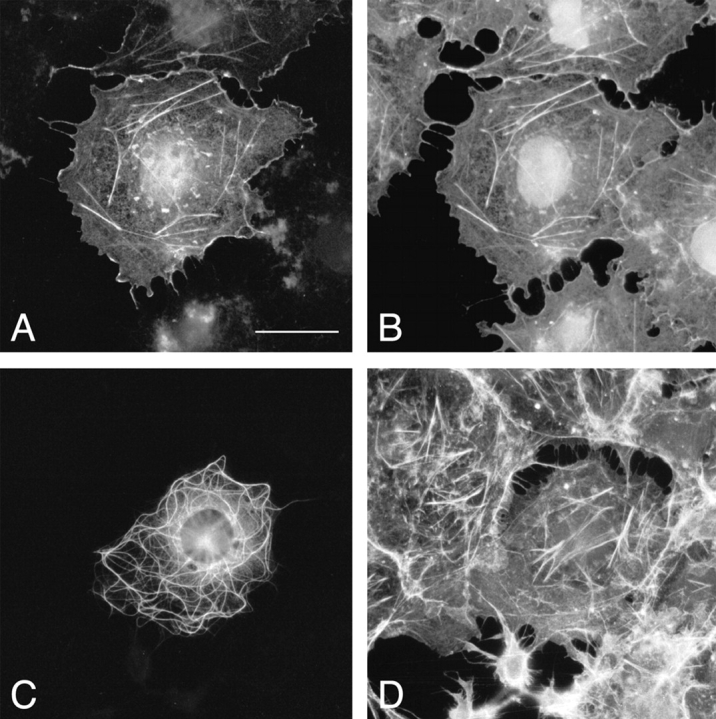
Association of ACF7 COOH-terminal domain with MTs. Transient transfections of pFLAG-mACF7-C (A–D) and pFLAG-ABD (E and F) were performed in COS-7 cells. Transfected cells were stained with monoclonal anti-FLAG M2 antibody (A, C, and E), polyclonal anti–Tyr-tubulin antibody (B), polyclonal anti–Glu-tubulin antibody (D), and polyclonal antitubulin antibody (F), and were examined by immunofluorescence microscopy. mACF7-C proteins colocalize with most of the Tyr-MTs (A and B) and all of the Glu-MTs (C and D). The noncolocalized Tyr-MTs are indicated by an arrow (B). Frequently, long MTs forming whorls were observed in pFLAG–mACF7-C transfected cells (B and D). The overexpressed ABD protein did not associate with the MT network (E and F). Bar, 20 μm.
Association of mACF7 with MTs In Vivo
Within each cell there are dynamic and stable MTs. Stable MTs are a small subset of dynamic MTs and are thought to be selectively generated from dynamic MTs. These stable long-lived MTs accumulate a posttranslationally modified form of tubulins known as detyrosinated or Glu-tubulins. These MTs are distinct from their dynamic counterparts that contain predominantly tyrosinated tubulin (Tyr-tubulin). In transfected cells, the overexpressed mACF7-C proteins colocalized with many but not all Tyr-MTs (Fig. 5A and Fig. B). Interestingly, mACF7-C proteins (Fig. 5C and Fig. D) decorated all the Glu MTs. The dynamic Tyr MTs at the periphery of the cell did not appear to colocalize with mACF-7, although it is possible that the bound mACF-7 was present in low amounts too scarce to be detected. As expected, the ABD of mACF7 did not associate with MTs (Fig. 5E and Fig. F). Since long MT-forming whorls were frequently observed in the transfected cells, we considered the possibility that mACF7-C proteins might not only bind to, but also stabilize MTs. To explore this possibility, cells transfected with mACF7-C cDNA were treated with the MT depolymerization agent, nocodazole (10 μM) for 1.5 h before being fixed for immunofluorescence microscopy. These conditions have been shown to be sufficient to cause the complete depolymerization of the endogenous MTs (Khawaja et al. 1988). In contrast to cells without mACF7-C protein, the MT networks of the transfected cells remained intact and were decorated with mACF7-C proteins (Fig. 6, A–D), implying that the COOH-terminal domain of mACF7 can associate with and stabilize MTs. In similar assays, the ABD of mACF7 still only associated with the actin network (Fig. 6E and Fig. F).
Figure 6.
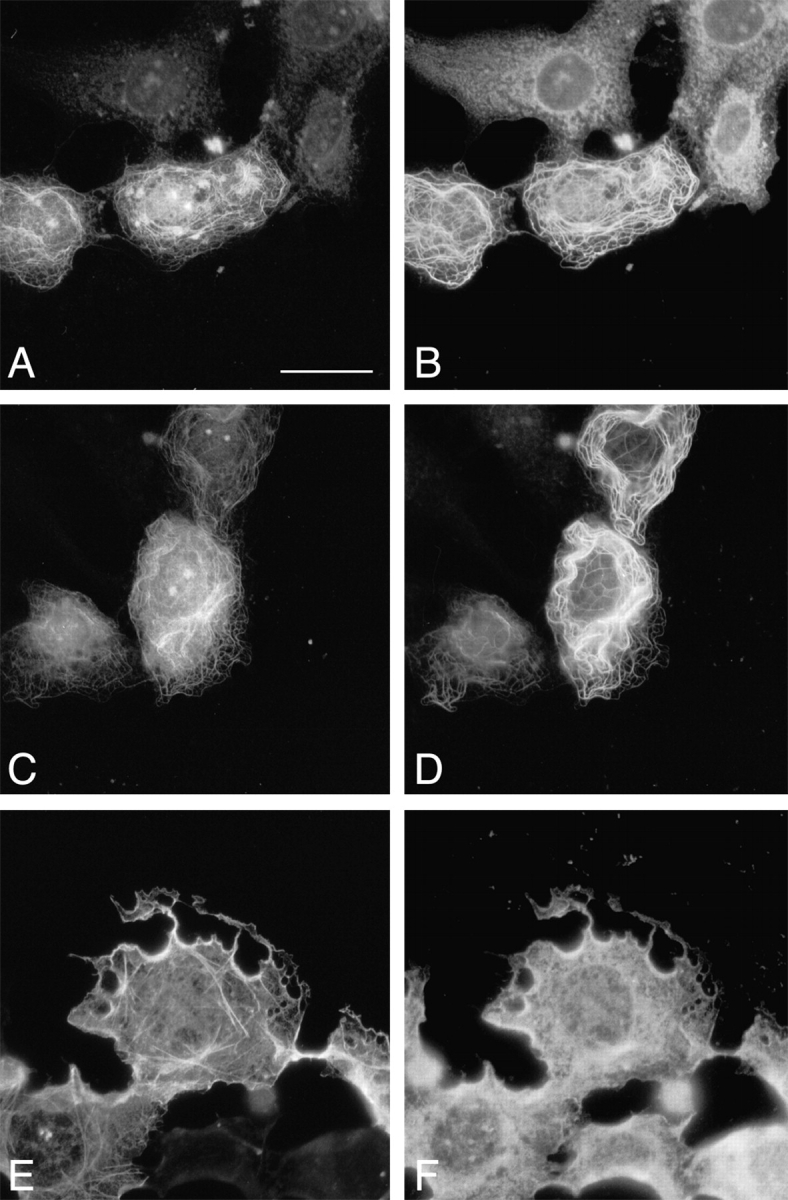
Overexpression of mACF7 COOH-terminal domain stabilizes cellular MTs. pFLAG-mACF7-C (A–D) and pFLAG-ABD (E and F) and transfected COS-7 cells were treated with nocodazole (10 μM) for 1.5 h before being fixed and double-labeled with monoclonal anti-FLAG M2 antibody (A, C, and E) polyclonal anti–Tyr-tubulin antibody (B), polyclonal anti–Glu-tubulin antibody (D), and polyclonal antitubulin antibody (F). The overexpressed mACF7-C protein stabilized MTs from depolymerization by nocodazole (compare positive FLAG-staining cell with its neighboring cells in A and B). Bar, 20 μm.
Interaction of mACF7 with MTs and MFs In Vitro
In vitro spin-down binding assays were carried out to ascertain interactions between ABD and actin filaments, and between mACF7-C and MTs. 35S-labeled proteins were synthesized in vitro and incubated with polymerized actin and tubulin before centrifugation. The bound proteins in the spin-down pellets were resolved on SDS-PAGE and visualized by autoradiography. As demonstrated in Fig. 7, the taxol stabilized MTs pull down mACF7-C protein but not ABD protein, whereas polymerized actin filament pulls down a significant amount of ABD protein and a small amount of mACF7-C protein. The interaction between mACF7-C and polymerized tubulins is most likely direct, because no MT-associated proteins (MAPs) are present in the taxol stabilized MTs, although proteins in the reticulocyte lysate system could enhance this binding. Since polymerized actin filaments were not able to pull down a partial BPAG1 COOH-terminal protein in similar assays (data not shown), the in vitro interaction between mACF7-C and actin filaments may also be specific. However, no obvious colocalization of mACF7-C and actin structures was observed in transfection studies; therefore, it is not clear that this interaction happens in vivo.
Figure 7.
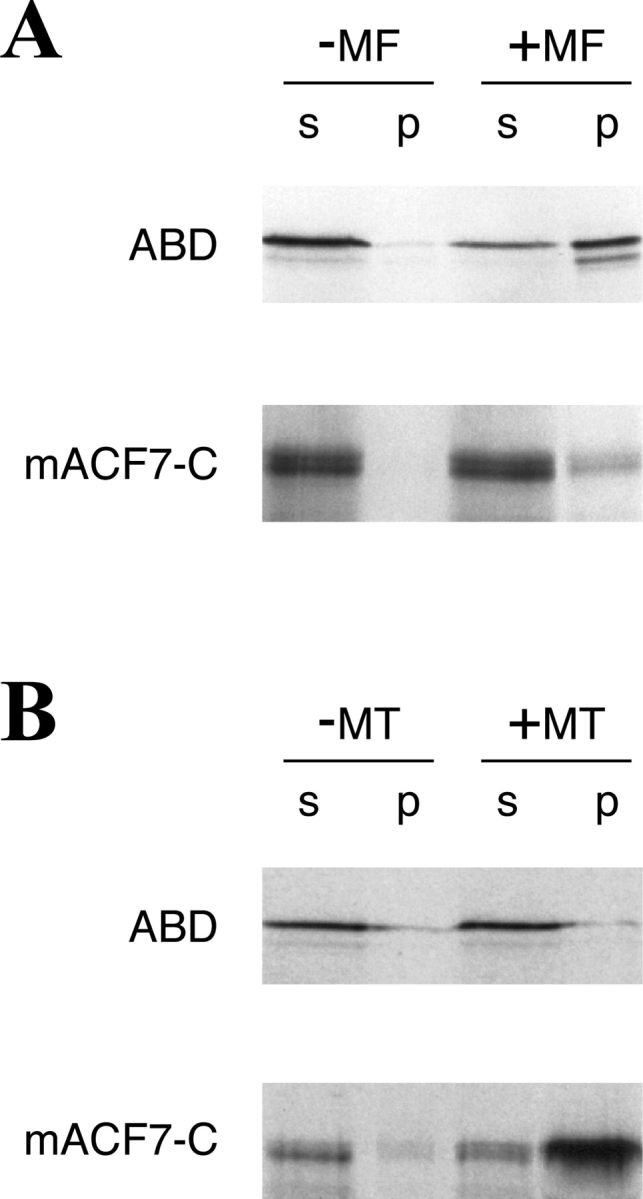
Interaction of mACF7 with polymerized actin and MTs in vitro. (A) Actin spin-down binding assays were performed with [35S]methionine ABD and mACF7-C proteins. In vitro translated mACF7-C protein appeared as a doublet in SDS-PAGE. These two different sized proteins might be the result of degradation or alternative initiation. In the presence of polymerized actin filaments (+MF), a significant portion of ABD was found in the pellet (p). Without actin (−MF), most of the ABD remained in the supernatant (s). A small portion of mACF7-C protein was also found in the pellet, indicating weak interaction between mACF7-C protein and actin filaments. (B) Microtubule spin-down binding assays were performed with [35S]methionine-labeled ABD and mACF7-C proteins. In the presence of polymerized MTs (+MT), mACF7-C but not ABD protein cosediment with the MT pellet (p). Without MTs (−MT), both of the proteins remained in the supernatant (s).
Connection of MFs and MTs by mACF7 In Vivo
To investigate whether mACF7 might be able to cross-link MFs and MTs in vivo, a construct (pFLAG-mACF7-mini) encoding for a chimeric protein, that contained the ABD and the COOH-terminal domain of mACF7 connected by a FLAG epitope tag, was used for transient transfection studies. Triple-labeling with phalloidin, anti-FLAG, and antitubulin was performed and the results are shown in Fig. 8. Similar to cells expressing mACF7-C protein, long MT-forming whorls and bundles were observed in cells transfected with pFLAG-mACF7-mini. In contrast to the straight stress fibers and membrane ruffles stained by phalloidin in nontransfected cells, many curly actin filaments were detected in cells expressing mACF7-mini. These unusual actin filaments were perfectly colocalized with MTs and decorated with mACF7-mini (Fig. 8). These results show that mACF7-mini could enhance the formation of actin filaments and efficiently cross-link them to MTs.
Figure 8.
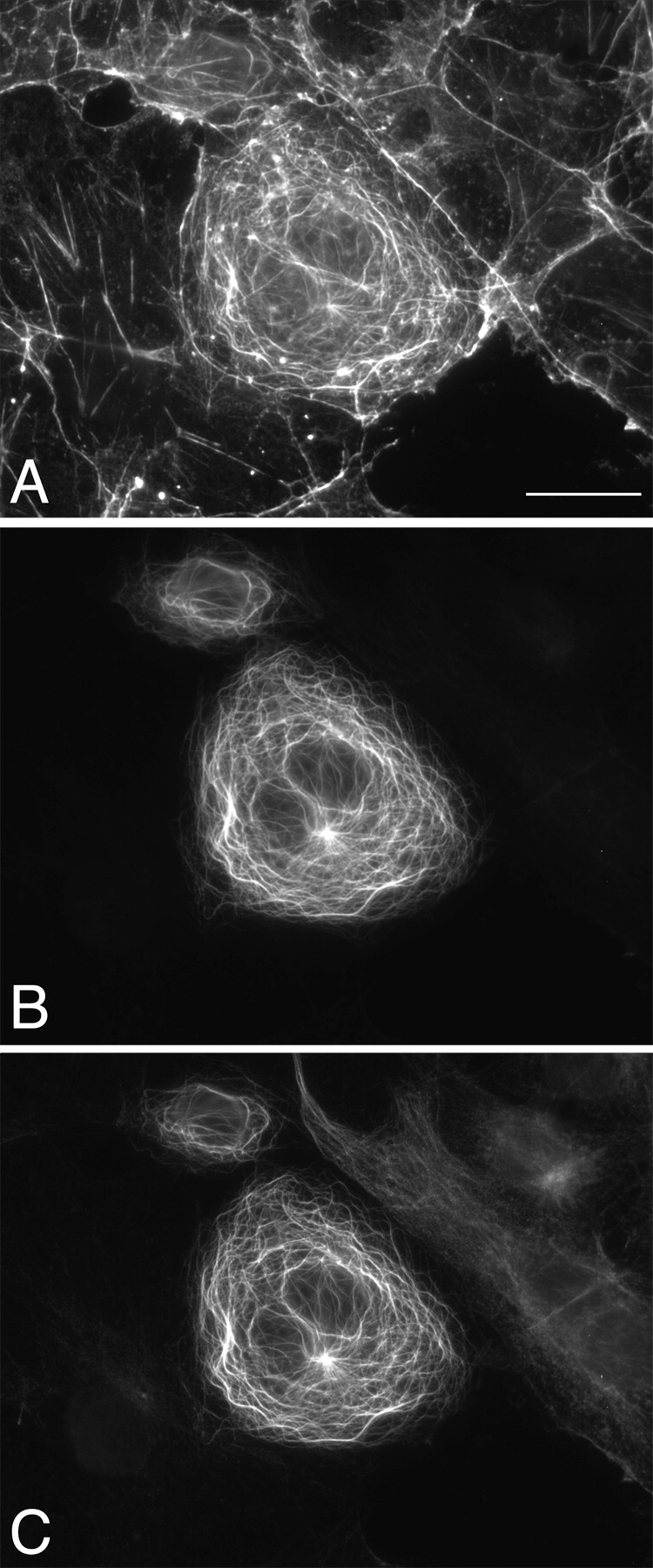
mACF7-mini cross-links MFs and MTs. pFLAG-mACF7-mini, encoding a chimeric protein of ABD and the COOH-terminal domain of mACF7, was transfected into COS-7 cells. Transfected cells were triple-labeled with rhodamine-conjugated phalloidin (A), mouse monoclonal anti-FLAG M2 antibody (B), and rat monoclonal anti–Tyr-tubulin antibody (C). Cells expressing mACF7-mini contain more actin filaments that are morphologically distinct from stress fibers. These actin filaments colocalized with mACF7-mini and MTs, demonstrating the MF-MT cross-linking property of mACF7-mini. Bar, 20 μm.
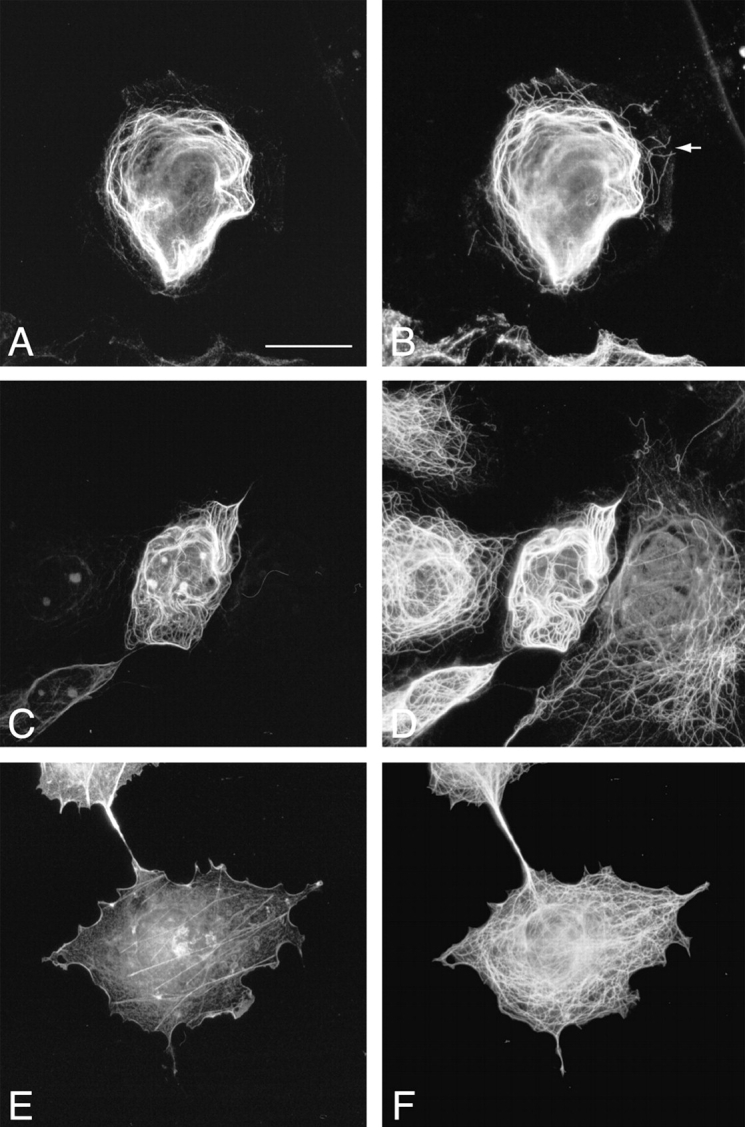
To further characterize the function of mACF7, a COOH-terminal FLAG-tagged full-length mACF7 construct was prepared for transient transfections. In some transfected cells, we observed partial coalignment of mACF7, MTs, and actin filaments (Fig. 9, A–C, arrows). However, in most cases, colocalization of mACF7, MTs, and actin was only observed in patches (Fig. 9, insets). These differences appear to be due to the differential expression levels of mACF7 in the transfected cells. Cells expressing lower levels of full-length mACF7 had more stress fibers that colocalized with mACF7. A subset of these stress fibers also colocalized with MTs (Fig. 9, A–C). In contrast, in most transfected cells there were few actin stress fibers (Fig. 9 D). In addition, mACF7 also colocalized with actin at membrane ruffles (Fig. 9D and Fig. E, arrows) and decorated all the MTs. These data show that mACF7 can bind to both actin filaments and MTs, and may have the ability to cross-link these cytoskeletal elements. Since vimentin has also been shown to associate with MTs (Gurland and Gundersen 1995), we studied the distribution of vimentin in mACF7-overexpressing cells, but found no obvious colocalization of IFs with mACF7 (data not shown).
Figure 9.
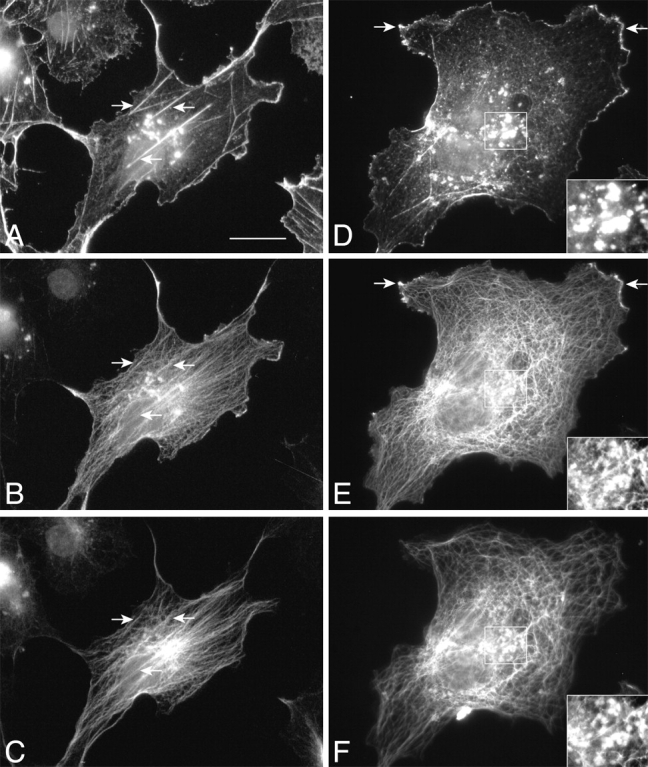
Full-length mACF7 associates with actin and MTs. COS-7 cells were transiently transfected with pFLAG-mACF7-fl and triple-labeled with rhodamine-conjugated phalloidin (A and D), mouse monoclonal anti-FLAG M2 antibody (B and E), and rat monoclonal anti–Tyr-tubulin antibody (C and F). In a small population of transfected cells, overexpressed mACF7 and endogenous MTs coaligned with some of the actin stress fibers (A–C, arrows). In most cases, colocalization of mACF7, actin, and tubulin was only observed in patches. Insets in D–F show high magnifications of squared areas that contain patches. In addition, full-length mACF7 also coaligned with MTs and partially colocalized with actin at the membrane ruffles (D and E, arrows). Bar, 20 μm.
Discussion
The plakin family is a group of sequence-related proteins that associate with IFs and localize to junctional complexes at the plasma membrane. Current interest in plakins has been spurred by the discoveries of their roles in cross-linking cytoskeletal elements, as implied by their other name, cytolinkers (Wiche 1998). This functional feature is particularly prominent for plectin and dystonin (BPAG1-n) as they contain both actin- and IF-binding domains. Although the precise functions played by each plakin are not fully understood, hereditary diseases and gene targeting experiments vividly demonstrate the detrimental results caused by their loss-of-functions. Defects of the plectin gene in humans cause the skin blistering disease epidermolysis bullosa simplex combined with muscular dystrophy (Gache et al. 1996; Smith et al. 1996; Mellerio et al. 1997). Mutations in desmoplakin cause striate palmoplantar keratoderma in humans (Armstrong et al. 1999). The loss of mouse dystonin/BPAG1 results in dystonia musculorum (Brown et al. 1995a; Yang et al. 1996), whereas desmoplakin-null embryos cannot survive beyond the egg cylinder stage (Gallicano et al. 1998). Plectin knockout mice die shortly after birth and exhibit severe defects in skin, skeletal muscle, and heart (Andra et al. 1997). Therefore, identification of new family members might help us gain further insights into the functions of this family of proteins and unexplained human genetic diseases. In fact, more potential members of the plakin family had been identified, such as ACF7 (Bernier et al. 1996) and a 450-kD epidermal protein (Fujiwara et al. 1996). Unfortunately, characterization of these new family members is very time consuming, because all plakins are large proteins encoded by tremendously long mRNAs. Therefore, rather than using the standard hybridization approach, we attempted to isolate the full-length mACF7 cDNA by an efficient PCR-based method. By sequentially performing RACE-PCR, we were able to obtain overlapping clones spanning 11 kb in a relatively short period of time. The success of this approach not only accelerates the characterization of plakins, but can also be applied to other large-sized proteins.
Because the partially characterized cDNA of mACF7 displayed 38–76% identity to that of dystonin/BPAG1-n (Bernier et al. 1996), mACF7 was initially speculated to be a new plakin. However, after careful examination of its completed primary sequence, we found that mACF7 also represents a novel member of the spectrin superfamily. mACF7 can be structurally divided into three domains: NH2-terminal head, central rod, and COOH-terminal tail domains. The head domain consists of an ABD followed by a plakin-like globular domain. This portion of mACF7 displays the strongest homology to dystonin/BPAG1-n as described previously (Bernier et al. 1996). However, unlike plakins, the rod domain of mACF7 does not conform to a coiled–coil; instead it is composed of 23 repetitive motifs that are more closely related to dystrophin's spectrin repeats. The tail domain of mACF7 contains two putative EF-hand calcium-binding motifs and a Gas2-like GAR region. Because mACF7 is structurally related to spectrin, bearing the highly homologous ABD, spectrin repeats, and EF-hand motifs, mACF7 also belongs to the spectrin superfamily.
As illustrated by in situ hybridization, mACF7 is ubiquitously expressed in mouse embryos, with the highest expression level in the nervous system. In transfected cells, the transiently expressed mACF7 COOH-terminal protein associated with the endogenous MT networks, and this interaction stabilized the MTs from disassembly by nocodazole. Interaction of the COOH-terminal domain of mACF7 and MTs was also suggested by in vitro binding assays. The putative ABD at the NH2 terminus of mACF7 was proven to be functional. In vivo, colocalization of overexpressed ABD and actin structures was observed. In vitro, polymerized actin filaments were able to pull down ABD, and to a lesser extent the COOH-terminal domain of mACF7 in spin-down assays. To study the actin-MT cross-linking properties of mACF7, we generated mACF7-mini and COOH-terminal FLAG-tagged full-length mACF7 constructs. Overexpression of mACF7-mini caused coalignment of MTs and actin filaments. In contrast, colocalization of full-length mACF7, MTs, and actin was observed mostly in patches. Most of the stress fibers were disrupted in the transfected cells. Colocalization of actin, MTs, and mACF7 were only occasionally observed in the remaining stress fibers. These differences in the transient transfection results between mACF7-mini and full-length mACF7 are most likely due to the presence of the plakin-like domain and spectrin repeats in full-length mACF7. The ABD and the MT-associated domains are separated by the flexible spectrin repeats in the rod domain, making full-length mACF7 less likely to form a directly observable link between MFs and MTs. In addition, the plakin-like domain and the spectrin repeats may mediate association of mACF7 with membrane proteins and induce dimerization of mACF7. Transient transfections with full-length plectin, another known cytoskeletal linker protein with a homologous ABD as mACF7, also showed that the actin stress fibers appeared considerably reduced in number and complexity. Full-length plectin led to the collapse of the vimentin network in transfected cells into perinuclear aggregates, which were immunoreactive with antivimentin and antiplectin, but colocalization of vimentin, plectin, and actin stress fibers (or MTs) was not readily evident (Andra et al. 1998). The transfection results that we obtained with full-length mACF7 are therefore consistent with those described for plectin.
The plakin-like domain is highly conserved among the plakin family members. In desmoplakin, this globular domain clusters desmosomal cadherin–plakoglobin complexes and binds directly to plakoglobin (Kowalczyk et al. 1997). A similar domain in plectin binds integrin β4 both in vitro and in vivo, although the plectin–integrin β4 interaction is probably more complex, involving multiple interfaces of the proteins and even the ABD of plectin (Rezniczek et al. 1998; Geerts et al. 1999). Hence, mACF7 might also interact with membrane proteins. Indeed, some of the Drosophila kakapo alleles were identified from a screen of mutations affecting processes requiring integrin adhesion, suggesting that the mACF7 homologue Kakapo is closely associated with integrin functions (Gregory and Brown 1998; Walsh and Brown 1998). By analogy to that of the other spectrin superfamily members, the spectrin repeats of mACF7 could confer flexibility to the molecule for adjusting to the curvature of the cell membrane and may also mediate dimerization of two antiparallel mACF7 molecules. Accordingly, mACF7 could also cross-link individual MF fibers and/or MT fibers in its dimerized form. Collectively, mACF7 represents a potential cytoskeletal cross-linking protein; the NH2-terminal ABD binds to MFs, whereas the COOH-terminal tail domain associates with MTs. Because mACF7 binds to MTs as well as actin, we suggest a modification of its name ACF7 (actin cross-linking family 7) to MACF (microtubule actin cross-linking factor).
So far, very few proteins have been reported to be able to bind both MFs and MTs. The yeast protein coronin promotes the rapid assembly and cross-linking of actin filaments and contains sequences homologous to the MT-binding region of MAP1B (Goode et al. 1999). However, the actin- and MT-binding domains are contiguous, and the region homologous to MAP1B is unique to yeast coronin. None of the mammalian homologues of coronin was found to possess this putative MT-binding domain (Goode et al. 1999). MAP1B is a 320-kD MAP that was originally copurified with MTs from mammalian brain. Microtubule-binding domains were found in both of the polypeptides, heavy chain and light chain, that constitute MAP1B (Noble et al. 1989; Zauner et al. 1992). Recently, transient transfection studies illustrated that a COOH-terminal fragment of the light chain was also able to associate with actin stress fibers (Togel et al. 1998). However, the overexpressed native light chain colocalized only with MTs in transfected cells, raising the possibility that the ABD of MAP1B is functional only under certain unknown circumstances. The other protein that has been reported to associate with MFs and MTs is plectin. Plectin contains a functional ABD near its NH2 terminus and a defined IF-binding domain at the COOH-terminal tail domain (Nikolic et al. 1996; Andra et al. 1997). Although plectin has been reported to bind MTs, the association appears to be indirectly through other MAPs in neurons (Herrmann and Wiche 1987). Nevertheless, plectin can directly associate with MTs in nonneuronal cells as observed by EM (Svitkina et al. 1996), although no specific MT-binding region on plectin has been defined. We have been able to show that the COOH terminus of MACF binds directly to MTs. The MT-binding region of MACF has no obvious sequence similarities to the MT-binding domains of other MAPs, such as the MT-binding repeats of tau, MAP2, and MAP4, or the MT-binding domains of MAP1B.
Recently, the Drosophila gene encoding for the MACF homologue, kakapo, was cloned and characterized in three studies by examinations of mutant flies with blistered wings or with a paralytic phenotype, and by library screening with an antibody that stained the epidermal muscle attachment (EMA) cells in a unique pattern (Gregory and Brown 1998; Prokop et al. 1998; Strumpf and Volk 1998). Similar to MACF, Kakapo also contains an ABD, a plakin-like domain, a rod domain composed of spectrin repeats, and a GAR-region containing COOH-terminal tail domain. These studies indicate that Kakapo expression is restricted to ectodermally derived cells and that mutations of the Kakapo gene cause defects in the muscle-dependent tendon cell differentiation and the local development of neuronal processes (Prokop et al. 1998). In EMA cells, Kakapo is localized to the termini of MT bundles (Strumpf and Volk 1998) and the absence of Kakapo causes detachment of these MT bundles from the basal membrane (Gregory and Brown 1998). In addition, disorganization of MTs was also observed in the scolopidial sensory neurons of kakapo mutants (Prokop et al. 1998). Therefore, Kakapo was deduced to mediate the connection between the actin network, MTs, and membrane-associated proteins. Since we have shown that the COOH-terminal region of MACF can associate with MTs, we can therefore infer that Kakapo could also associate with MTs. The ubiquitous expression of MACF implies that it could also function as a linker protein connecting MFs, MTs, and membrane-associated proteins in a variety of tissues. The high level of expression of MACF mRNA that we observe in the nervous system could also indicate that it may have an important function in neurons.
The architecture of MACF clearly demonstrates the intricacies of gene evolution. MACF is a union of different kinds of structural domains that have been conserved throughout evolution. Although some of these structural domains have been well-studied in other proteins, together they make MACF bear very unique properties that have not been described previously for other cytoskeletal linker proteins. It has a clearly identified ABD as well as an MT-binding domain that are spatially well-separated. These domains may connect both MFs and MTs. The importance of this kind of connection is indicated by some of the lethal alleles obtained in the kakapo locus of Drosophila. Although the functional significance of MACF in mice as well as in human remains to be studied, it is certain that the ABD of MACF is functional and that its COOH-terminal domain functions like a MAP, i.e., it interacts with and stabilizes MTs.
Acknowledgments
We thank Dr. Gregg Gundersen for helpful discussions and Ms. Beth Rosen for technical assistance.
This work was supported by National Institutes of Health grant NS15182. D. Sun and C.L. Leung were supported in part by training grant AG00189.
Footnotes
Abbreviations used in this paper: ABD, actin-binding domain; ACF7, actin cross-linking family 7; BPAG1, bullous pemphigoid antigen 1; GAR22, Gas2 related on chromosome 22; Gas2, growth arrest–specific 2 protein; IF, intermediate filament; MACF, microtubule actin cross-linking factor; mACF7, mouse ACF7; MAP, MT-associated protein; MF, microfilament; MT, microtubule; RACE, rapid amplification of cDNA ends.
References
- al-Ali S.Y., al-Zuhair A.G. Fine structural study of the spinal cord and spinal ganglia in mice afflicted with a hereditary sensory neuropathy, dystonia musculorum. J. Submicrosc. Cytol. Pathol. 1989;21:737–748. [PubMed] [Google Scholar]
- Andra K., Lassmann H., Bittner R., Shorny S., Fassler R., Propst F., Wiche G. Targeted inactivation of plectin reveals essential function in maintaining the integrity of skin, muscle, and heart cytoarchitecture. Genes Dev. 1997;11:3143–3156. doi: 10.1101/gad.11.23.3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andra K., Nikolic B., Stocher M., Drenckhahn D., Wiche G. Not just scaffoldingplectin regulates actin dynamics in cultured cells. Genes Dev. 1998;12:3442–3451. doi: 10.1101/gad.12.21.3442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong D.K.B., McKenna K.E., Purkis P.E., Green K.J., Eady R.A.J., Leigh I.M., Hughes A.E. Haploinsufficiency of desmoplakin causes a striate subtype of palmoplantar keratoderma. Hum. Mol. Genet. 1999;8:143–148. doi: 10.1093/hmg/8.1.143. [DOI] [PubMed] [Google Scholar]
- Bernier G., Mathieu M., De Repentigny Y., Vidal S.M., Kothary R. Cloning and characterization of mouse ACF7, a novel member of the dystonin subfamily of actin binding proteins. Genomics. 1996;38:19–29. doi: 10.1006/geno.1996.0587. [DOI] [PubMed] [Google Scholar]
- Brancolini C., Bottega S., Schneider C. Gas2, a growth arrest-specific protein, is a component of the microfilament network system. J. Cell Biol. 1992;117:1251–1261. doi: 10.1083/jcb.117.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A., Bernier G., Mathieu M., Rossant J., Kothary R. The mouse dystonia musculorum gene is a neural isoform of bullous pemphigoid antigen 1 Nat. Genet. 10 1995. 301 306a [DOI] [PubMed] [Google Scholar]
- Brown A., Dalpe G., Mathieu M., Kothary R. Cloning and characterization of the neural isoforms of human dystonin Genomics. 29 1995. 777 780b [DOI] [PubMed] [Google Scholar]
- Byers T.J., Beggs A.H., McNally E.M., Kunkel L.M. Novel actin crosslinker superfamily member identified by a two step degenerate PCR procedure. FEBS Lett. 1995;368:500–504. doi: 10.1016/0014-5793(95)00722-l. [DOI] [PubMed] [Google Scholar]
- Collavin L., Buzzai M., Saccone S., Bernard L., Federico C., DellaValle G., Brancolini C., Schneider C. cDNA characterization and chromosome mapping of the human GAS2 gene. Genomics. 1998;48:265–269. doi: 10.1006/geno.1997.5172. [DOI] [PubMed] [Google Scholar]
- Dubreuil R.R., Brandin E., Reisberg J.H., Goldstein L.S., Branton D. Structure, calmodulin-binding, and calcium-binding properties of recombinant alpha spectrin polypeptides. J. Biol. Chem. 1991;266:7189–7193. [PubMed] [Google Scholar]
- Duchen L.W., Strich S.J. Clinical and pathological studies of an hereditary neuropathy in mice (dystonia musculorum) Brain. 1964;87:367–378. doi: 10.1093/brain/87.2.367. [DOI] [PubMed] [Google Scholar]
- Fujiwara S., Kohno K., Iwamatsu A., Naito I., Shinkai H. Identification of a 450-kDa human epidermal autoantigen as a new member of the plectin family. J. Invest. Dermatol. 1996;106:1125–1130. doi: 10.1111/1523-1747.ep12340171. [DOI] [PubMed] [Google Scholar]
- Gache Y., Chavanas S., Lacour J.P., Wiche G., Owaribe K., Meneguzzi G., Ortonne J.P. Defective expression of plectin/HD1 in epidermolysis bullosa simplex with muscular dystrophy. J. Clin. Invest. 1996;97:2289–2298. doi: 10.1172/JCI118671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallicano G.I., Kouklis P., Bauer C., Yin M., Vasioukhin V., Degenstein L., Fuchs E. Desmoplakin is required early in development for assembly of desmosomes and cytoskeletal linkage. J. Cell Biol. 1998;143:2009–2022. doi: 10.1083/jcb.143.7.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerts D., Fontao L., Nievers M.G., Schaapveld R.Q.J., Purkis P.E., Wheeler G.N., Lane E.B., Leigh I.M., Sonnenberg A. Binding of integrin α6β4 to plectin prevents plectin association with F-actin but does not interfere with intermediate filament binding. J. Cell Biol. 1999;147:417–434. doi: 10.1083/jcb.147.2.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode B.L., Wong J.J., Butty A.C., Peter M., McCormack A.L., Yates J.R., Drubin D.G., Barnes G. Coronin promotes the rapid assembly and cross-linking of actin filaments and may link the actin and microtubule cytoskeletons in yeast. J. Cell Biol. 1999;144:83–98. doi: 10.1083/jcb.144.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K.J., Parry D.A., Steinert P.M., Virata M.L., Wagner R.M., Angst B.D., Nilles L.A. Structure of the human desmoplakins. Implications for function in the desmosomal plaque [published erratum appears in J. Biol. Chem. 265:11406–11407] J. Biol. Chem. 1990;265:2603–2612. [PubMed] [Google Scholar]
- Green K.J., Stappenbeck T.S., Parry D.A., Virata M.L. Structure of desmoplakin and its association with intermediate filaments. J. Dermatol. 1992;19:765–769. doi: 10.1111/j.1346-8138.1992.tb03777.x. [DOI] [PubMed] [Google Scholar]
- Gregory S.L., Brown N.H. kakapo, a gene required for adhesion between and within cell layers in Drosophila, encodes a large cytoskeletal linker protein related to plectin and dystrophin. J. Cell Biol. 1998;143:1271–1282. doi: 10.1083/jcb.143.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo L., Degenstein L., Dowling J., Yu Q.C., Wollmann R., Perman B., Fuchs E. Gene targeting of BPAG1abnormalities in mechanical strength and cell migration in stratified epithelia and neurologic degeneration. Cell. 1995;81:233–243. doi: 10.1016/0092-8674(95)90333-x. [DOI] [PubMed] [Google Scholar]
- Gurland G., Gundersen G.G. Stable, detyrosinated microtubules function to localize vimentin intermediate filaments in fibroblasts. J. Cell Biol. 1995;131:1275–1290. doi: 10.1083/jcb.131.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J.H. Actin-binding proteins 1spectrin superfamily. Protein Profile. 1994;1:706–778. [PubMed] [Google Scholar]
- Herrmann H., Wiche G. Plectin and IFAP-300K are homologous proteins binding to microtubule-associated proteins 1 and 2 and to the 240-kilodalton subunit of spectrin. J. Biol. Chem. 1987;262:1320–1325. [PubMed] [Google Scholar]
- Janota I. Ultrastructural studies of an hereditary sensory neuropathy in mice (dystonia musculorum) Brain. 1972;95:529–536. doi: 10.1093/brain/95.3.529. [DOI] [PubMed] [Google Scholar]
- Khawaja S., Gundersen G.G., Bulinski J.C. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J. Cell Biol. 1988;106:141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk A.P., Bornslaeger E.A., Borgwardt J.E., Palka H.L., Dhaliwal A.S., Corcoran C.M., Denning M.F., Green K.J. The amino-terminal domain of desmoplakin binds to plakoglobin and clusters desmosomal cadherin-plakoglobin complexes. J. Cell Biol. 1997;139:773–784. doi: 10.1083/jcb.139.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986;44:283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Leung C.L., Sun D., Liem R.K.H. The intermediate filament protein peripherin is the specific interaction partner of mouse BPAG1-n (dystonin) in neurons. J. Cell Biol. 1999;144:435–446. doi: 10.1083/jcb.144.3.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellerio J.E., Smith F.J., McMillan J.R., McLean W.H., McGrath J.A., Morrison G.A., Tierney P., Albert D.M., Wiche G., Leigh I.M. Recessive epidermolysis bullosa simplex associated with plectin mutationsinfantile respiratory complications in two unrelated cases. Br. J. Dermatol. 1997;137:898–906. [PubMed] [Google Scholar]
- Nikolic B., Mac Nulty E., Mir B., Wiche G. Basic amino acid residue cluster within nuclear targeting sequence motif is essential for cytoplasmic plectin-vimentin network junctions. J. Cell Biol. 1996;134:1455–1467. doi: 10.1083/jcb.134.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble M., Lewis S.A., Cowan N.J. The microtubule binding domain of microtubule-associated protein MAP1B contains a repeated sequence motif unrelated to that of MAP2 and tau. J. Cell Biol. 1989;109:3367–3376. doi: 10.1083/jcb.109.6.3367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pascual J., Castresana J., Saraste M. Evolution of the spectrin repeat. Bioessays. 1997;19:811–817. doi: 10.1002/bies.950190911. [DOI] [PubMed] [Google Scholar]
- Prokop A., Uhler J., Roote J., Bate M. The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons. J. Cell Biol. 1998;143:1283–1294. doi: 10.1083/jcb.143.5.1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pytela R., Wiche G. High molecular weight polypeptides (270,000-340,000) from cultured cells are related to hog brain microtubule-associated proteins but copurify with intermediate filaments. Proc. Natl. Acad. Sci. USA. 1980;77:4808–4812. doi: 10.1073/pnas.77.8.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezniczek G.A., de Pereda J.M., Reipert S., Wiche G. Linking integrin α6β4-based cell adhesion to the intermediate filament cytoskeletondirect interaction between the β4 subunit and plectin at multiple molecular sites. J. Cell Biol. 1998;141:209–225. doi: 10.1083/jcb.141.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C., Watt F.M. The plakin familyversatile organizers of cytoskeletal architecture. Curr. Opin. Genet. Dev. 1997;7:392–397. doi: 10.1016/s0959-437x(97)80154-2. [DOI] [PubMed] [Google Scholar]
- Ruhrberg C., Hajibagheri M.A., Simon M., Dooley T.P., Watt F.M. Envoplakin, a novel precursor of the cornified envelope that has homology to desmoplakin. J. Cell Biol. 1996;134:715–729. doi: 10.1083/jcb.134.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruhrberg C., Hajibagheri M.A., Parry D.A., Watt F.M. Periplakin, a novel component of cornified envelopes and desmosomes that belongs to the plakin family and forms complexes with envoplakin. J. Cell Biol. 1997;139:1835–1849. doi: 10.1083/jcb.139.7.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith F.J., Eady R.A., Leigh I.M., McMillan J.R., Rugg E.L., Kelsell D.P., Bryant S.P., Spurr N.K., Geddes J.F., Kirtschig G. Plectin deficiency results in muscular dystrophy with epidermolysis bullosa. Nat. Genet. 1996;13:450–457. doi: 10.1038/ng0896-450. [DOI] [PubMed] [Google Scholar]
- Sotelo C., Guenet J.L. Pathologic changes in the CNS of dystonia musculorum mutant mousean animal model for human spinocerebellar ataxia. Neuroscience. 1988;27:403–424. doi: 10.1016/0306-4522(88)90277-1. [DOI] [PubMed] [Google Scholar]
- Speicher D.W., Marchesi V.T. Erythrocyte spectrin is comprised of many homologous triple helical segments. Nature. 1984;311:177–180. doi: 10.1038/311177a0. [DOI] [PubMed] [Google Scholar]
- Strumpf D., Volk T. Kakapo, a novel cytoskeletal-associated protein is essential for the restricted localization of the neuregulin-like factor, vein, at the muscle-tendon junction site. J. Cell Biol. 1998;143:1259–1270. doi: 10.1083/jcb.143.5.1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina T.M., Verkhovsky A.B., Borisy G.G. Plectin sidearms mediate interaction of intermediate filaments with microtubules and other components of the cytoskeleton. J. Cell Biol. 1996;135:991–1007. doi: 10.1083/jcb.135.4.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Togel M., Wiche G., Propst F. Novel features of the light chain of microtubule-associated protein MAP1Bmicrotubule stabilization, self interaction, actin filament binding, and regulation by the heavy chain. J. Cell Biol. 1998;143:695–707. doi: 10.1083/jcb.143.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uitto J., Pulkkinen L., Smith F.J., McLean W.H. Plectin and human genetic disorders of the skin and muscle. The paradigm of epidermolysis bullosa with muscular dystrophy. Exp. Dermatol. 1996;5:237–246. doi: 10.1111/j.1600-0625.1996.tb00124.x. [DOI] [PubMed] [Google Scholar]
- Walsh E.P., Brown N.H. A screen to identify Drosophila genes required for integrin-mediated adhesion. Genetics. 1998;150:791–805. doi: 10.1093/genetics/150.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiche G. Role of plectin in cytoskeleton organization and dynamics. J. Cell Sci. 1998;111:2477–2486. doi: 10.1242/jcs.111.17.2477. [DOI] [PubMed] [Google Scholar]
- Wiche G., Becker B., Luber K., Weitzer G., Castanon M.J., Hauptmann R., Stratowa C., Stewart M. Cloning and sequencing of rat plectin indicates a 466-kD polypeptide chain with a three-domain structure based on a central alpha-helical coiled coil. J. Cell Biol. 1991;114:83–99. doi: 10.1083/jcb.114.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Y., Winograd E., Viel A., Cronin T., Harrison S.C., Branton D. Crystal structure of the repetitive segments of spectrin. Science. 1993;262:2027–2030. doi: 10.1126/science.8266097. [DOI] [PubMed] [Google Scholar]
- Yang Y., Dowling J., Yu Q.C., Kouklis P., Cleveland D.W., Fuchs E. An essential cytoskeletal linker protein connecting actin microfilaments to intermediate filaments. Cell. 1996;86:655–665. doi: 10.1016/s0092-8674(00)80138-5. [DOI] [PubMed] [Google Scholar]
- Yang Y., Bauer C., Strasser G., Wollman R., Julien J.-P., Fuchs E. Integrators of the cytoskeleton that stabilize microtubules. Cell. 1999;98:229–238. doi: 10.1016/s0092-8674(00)81017-x. [DOI] [PubMed] [Google Scholar]
- Zauner W., Kratz J., Staunton J., Feick P., Wiche G. Identification of two distinct microtubule binding domains on recombinant rat MAP 1B. Eur. J. Cell Biol. 1992;57:66–74. [PubMed] [Google Scholar]
- Zheng M., Leung C.L., Liem R.K. Region-specific expression of cyclin-dependent kinase 5 (cdk5) and its activators, p35 and p39, in the developing and adult rat central nervous system. J. Neurobiol. 1998;35:141–159. doi: 10.1002/(sici)1097-4695(199805)35:2<141::aid-neu2>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Zucman-Rossi J., Legoix P., Thomas G. Identification of new members of the Gas2 and Ras families in the 22q12 chromosome region. Genomics. 1996;38:247–254. doi: 10.1006/geno.1996.0625. [DOI] [PubMed] [Google Scholar]