Abstract
Bronchial smooth muscle (SM) mesenchymal cell precursors change their shape from round to spread/elongated while undergoing differentiation. Here we show that this change in cell shape induces the expression of laminin (LM) α2 chain not present in round mesenchymal cells. LM α2 expression is reversible and switched on and off by altering the cell's shape in culture. In comparison, the expression of LM β1 and γ1 remains unchanged. Functional studies showed that mesenchymal cell spreading and further differentiation into SM are inhibited by an antibody against LM α2. Dy/dy mice express very low levels of LM α2 and exhibit congenital muscular dystrophy. Lung SM cells isolated from adult dy/dy mice spread defectively and synthesized less SM α-actin, desmin, and SM-myosin than controls. These deficiencies were completely corrected by exogenous LM-2. On histological examination, dy/dy mouse airways and gastrointestinal tract had shorter SM cells, and lungs from dy/dy mice contained less SM-specific protein. The intestine, however, showed compensatory hyperplasia, perhaps related to its higher contractile activity. This study therefore demonstrated a novel role for the LM α2 chain in SM myogenesis and showed that its decrease in dy/dy mice results in abnormal SM.
Keywords: laminin, smooth muscle, myogenesis, dy/dy mice, lung
Laminins (LMs) are heterotrimeric basement membrane glycoproteins composed of α, β and γ chains linked together by disulfide bonds in a cruciform tertiary structure. The first LM identified, referred to as LM-1, is composed of α1, β1, and γ1 chains (Timpl et al. 1979; Burgeson et al. 1994). LM-2, also known as merosin, was isolated as a protein present in basement membranes of human placenta (Leivo and Engvall 1988) and is composed of α2, β1, and γ1 chains (Burgeson et al. 1994). In addition to forming part of LM-2, LM α2 chain binds covalently to LM β2 and γ1 chains to form LM-4 (Burgeson et al. 1994). LM-2 is the predominant isoform in striated muscle fiber basement membranes and is essential for skeletal muscle development and stability (Vachon et al. 1996).
The human LM α2 chain gene (Lama2) was localized to chromosome 6q22-23 (Vuolteenaho et al. 1994). Mutations in the LM α2 chain gene cause autosomal recessive congenital muscular dystrophies in humans and in dy/dy mice (Hillaire et al. 1994; Sunada et al. 1994; Xu et al. 1994a,Xu et al. 1994b). These mutations result in very low levels of normal LM α2 chain (Sunada et al. 1994; Xu et al. 1994b), or in synthesis of truncated chains (Xu et al. 1994a) which in turn lead to severely defective muscle basement membranes (Xu et al. 1994b). Knockout of the LM α2 chain gene in mice by homologous recombination has confirmed its importance in skeletal muscle development and function (Miyagoe et al. 1997).
The laminin α2 chain is expressed in the developing and adult human and mouse lung (Vuolteenaho et al. 1994; Bernier et al. 1995; Virtanen et al. 1996; Miner et al. 1997; Schuger et al. 1997; Flores-Delgado et al. 1998) and it is deposited in the bronchial epithelial basement membrane and adjacent to the peribronchial mesenchymal cells (Virtanen et al. 1996; Wu and Santoro 1996; N. Relan and L. Schuger, unpublished observations). During lung development, its expression lags that of the LM α1 chain and coincides with the period of active bronchial myogenesis (Virtanen et al. 1996). The functional role of LM α2 chain in the developing lung has not been elucidated, although it has been shown to facilitate attachment of lung myofibroblasts in culture (Flores-Delgado et al. 1998).
During development, embryonic cells undergo significant changes in shape, starting as round pluripotent cells and culminating in the multiple cellular configurations seen in mature tissues. Our studies (Schuger et al. 1997; Yang et al. 1998, Yang et al. 1999) as well as others (Leptin 1995; Loty et al. 1995; Anastasi et al. 1997; Fernandez-Valle et al. 1997; Martin-Blanco 1997, Martin-Blanco 1998; Bidwell et al. 1998) indicate that these changes in cell shape play an active role in the mechanistic pathways determining cell differentiation. Among the multiple factors potentially controlling cell shape, we identified LM-1 as relevant for bronchial myogenesis (Schuger et al. 1997; Yang et al. 1998). More specifically, we determined that cell spreading/elongation is essential for inducing SM differentiation (Yang et al. 1998, Yang et al. 1999) and that this change in cell shape is stimulated by LM α1 chain deposition and further polymerization at the airway basement membrane site (Schuger et al. 1997; Yang et al. 1998).
Here we show that cell spreading/elongation, whether in vivo or in vitro, activates expression of the LM α2 chain, which is absent in round cells. In addition, by blocking LM α2 with a specific antibody, we demonstrate that, once secreted, LM α2 promotes mesenchymal cell spreading/elongation and further SM myogenic differentiation. Our findings thus provide a potential explanation for how SM myogenesis could proceed after LM-1 in the epithelial basement membrane stimulates the first layer of mesenchymal cells to elongate and differentiate.
Since dy/dy mice express low levels of LM α2 chain, we used their cells to further study the role of this LM chain in myogenesis. Here we show that lung mesenchymal cells isolated from dy/dy mice spread defectively in culture and synthesize less SM α-actin, desmin and SM myosin than controls. These abnormalities were completely overcome by the addition of exogenous LM-2. Furthermore, histological examination of dy/dy mouse tissues revealed subtle but diffuse SM cell shortening that, in the absence of compensatory muscle hyperplasia, resulted in lower levels of SM-specific proteins in immunoblots. Our studies therefore demonstrate a novel role for LM α2 chain in visceral myogenesis. Its regulation by cell shape may contribute to a multilayered bronchial SM, and its absence may cause abnormal visceral muscle.
Materials and Methods
LMs and Antibodies
LM-1 from the mouse EHS tumor, type IV collagen, and fibronectin were purchased from Becton-Dickinson, and LM-2 was obtained from GIBCO BRL. A polyclonal rat antibody to EHS LM which recognizes LM α1, α2, and β1/γ1 chains (LM-1/LM-2) (Schuger et al. 1997), was a gift from Dr. Amy Skubitz (University of Minnesota, Minneapolis, MN). A rat monoclonal antibody to the NH2 terminus of mouse LM α2 chain (Schuler and Sorokin 1995) was obtained from Alexis Inc. A mouse monoclonal antibody to SM α-actin was obtained from Boehringer Mannheim, rabbit polyclonal antibodies to SM myosin were purchased from Biomedical Technologies, and a mouse monoclonal antibody to desmin was purchased from Dako (Carpinteria, CA). HRP-labeled secondary antibodies were purchased from Bio-Rad and normal rat IgG was purchased from Cappel.
Cells and Lung Fragments
To obtain embryonic mesenchymal cells, Crl: CD-1® (ICR) BR mice (Charles River) were mated and the day of finding a vaginal plug was designated as day zero of embryonic development. Lungs were removed at days 12–15 of gestation, minced, and then trypzinised into a single cell suspension, and the mesenchymal cells were isolated by differential plating as previously described (Schuger et al. 1993). The cells were directly cultured under conditions that promoted either cell rounding or cell spreading/elongation. The cell's shape was controlled by means of three different cell culture systems: (a) mesenchymal cells were plated on 10- or 20-μm-diam culture microsurfaces, the first to force cell rounding and the second to allow cell spreading/elongation (Yang et al. 1999). (b) Mesenchymal cells were plated on culture dishes pretreated with 0.05% poly-l-lysine or 0.05% poly-l-lysine was added to the cultures after cell spreading/elongation was completed, the first to force cell rounding and the second to allow cell spreading/elongation (Yang et al. 1998). (c) Mesenchymal cells were plated in untreated culture dishes at high density or at subconfluent densities without further treatment, the first to force cell rounding and the second to allow cell spreading (Yang et al. 1998). In some experiments, after 24 h in culture, the cells were switched from conditions that promoted cell rounding to conditions that promoted cell spreading/elongation and vice versa. In these cases all the cells were trypsinized after an additional 24 h in culture. Surface-anchored epithelial-mesenchymal cocultures (organotypic) were generated by plating at high density (2–4 × 106/ml) a mixture of epithelial and mesenchymal cells isolated from embryonic lungs (Schuger et al. 1995). Lung embryonic epithelial cells and intestine and kidney embryonic mesenchymal cells were used in some studies. These were also isolated by differential plating (Schuger et al. 1993) and cultured under conditions that promote cell rounding or cell elongation. To obtain adult mesenchymal cells, the lungs of adult CD-1 mice, C57BL/6J dy/dy mice, or C57BL/6J +/+ normal mice (both from Jackson ImmunoResearch Laboratories) were minced in 0.1% collagenase and incubated at 37°C for 60 min. Single cell suspensions were obtained and cultures of round or spread/elongated mesenchymal cells were generated as described above. All the cultures were incubated in MEM with nonessential amino acids, 0.29 μg/ml l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 0.25 μg/ml amphotericin B, and 10% fetal bovine serum.
In some studies, tissues were isolated from the embryo, immediately lysed, and used for immunoblot analysis. Whole lungs were microdissected from day 10–10.5 mouse embryos. Fragments of undifferentiated, round mesenchymal cells were microdissected from the periphery of day 12 mouse embryonic lungs, and fragments of elongated mesenchymal cells were microdissected, together with epithelial cells, from the proximal/central part of day 12 lungs.
Immunoblotting and Immunoprecipitation
Cells and tissues from CD-1, C57BL/6J dy/dy mice and C57BL/6J +/+ normal mice were lysed and resolved under reducing conditions by PAGE. Immunoblotting for LMs and SM proteins was then done as described (Schuger et al. 1997; Yang et al. 1998, Yang et al. 1999). For immunoprecipitation studies the cells were lysed and 50 μg of sample were cleared with protein A–conjugated sepharose (Sigma) and immunoprecipitated with 1 μg of monoclonal antibody against LM α2 chain for 16–24 h at 4°C in a rotary shaker. The immune complex was precipitated with protein A–conjugated Sepharose and resolved by 3.5% PAGE. The samples were transferred to nitrocellulose membranes and blotted with polyclonal antibody to LM-1/LM-2 (1:100 dilution) and bands were detected by chemiluminescence as previously described (Schuger et al. 1997).
RT-PCR and Ribonuclease Protection Assay
Mesenchymal cell cultures were briefly washed with PBS, lysed with Trizol (GIBCO BRL) and total RNA was extracted and used for RT-PCR and ribonuclease protection assay (RPA). RT-PCR was performed with the GeneAmp® RNA PCR kit (Perkin Elmer) following the manufacturer's instructions. 25 cycles were run for amplification of β1 and γ1 chains and 30 for amplification of α1, α2 chains, α4 and α5 chains. The following primers were used for PCR: LM α1 chain 5′ forward primer: 5′-tgggtgtgggatttcttagc-3′ and 3′ reverse primer: 5′-cctgaccgtctacccagtgt-3′, LM α2 chain 5′ forward primer: 5′-gcctgccaactctgagaaac-3′ and 3′ reverse primer: 5′-tcccaagagaatgatcccag-3′, LM α4 chain 5′ forward primer: 5′-gccatcgaagaagtagctgg-3′ and 3′-reverse primer: 5′-cccctgaggacactgtgttt-3′, LM α5 chain 5′ forward primer: 5′-tgaaggcagagggtagcagt-3′ and 3′ reverse primer 5′-gttgtctgcctggcttcttc-3′, LM β1 chain 5′ forward primer: 5′-gttcgagggaactgcttctg-3′ and 3′ reverse primer: 5′-tgccagtagccaggaagact-3′. LM γ1 chain 5′ forward primer: 5′-gagctttgaggacgaccttg-3′ and 3′ reverse primer 5′-ctggggtgtgtatgctgatg-3′.
For RPA a 356-bp LM α2 chain fragment which was cloned in the pCR II vector (Miner et al. 1997) was linearized and transcribed in vitro using the MAXIscript kit (Ambion Inc.) and UTP α-[32P] (400–900 Ci/mmol and 10 mCi/ml; NEN). The hybridization was carried out using an RPA kit (Ambion Inc.) and following the manufacturer's instructions. A 256-bp GAPDH linearized fragment obtained from Ambion Inc. was used as a control. The protected RNA fragments were resolved on a 5% denaturing polyacrylamide gel and autoradiographed.
Cell Attachment and Spreading Assays
Freshly isolated lung mesenchymal cells were cultured in the presence of anti-LM α2 chain antibody, anti-LM α2 chain antibody preincubated with 10-fold excess of LM-2 (molar:molar) or IgG control. The antibody and IgG were added at concentrations of 1, 2, 5, and 10 μg/ml to the cultures either at the time of cell plating to determine their effect on cell attachment or 40 min after plating, when attachment was completed, to determine their effect on cell spreading. The cultures were maintained for 2 and 4 h for cell attachment studies and for 4 and 24 h for cell spreading studies. At the end of the culture period the cells were either fixed or lysed. Fixed cells were counted and their diameters were determined on images projected on a computer screen with the aid of a digitized pad and the image analysis program Optimas 5.1. (Schuger et al. 1995). Statistical significance was determined by the Student's t test. Lysed cells were used for immunoblotting with anti-SM α actin, anti-desmin and anti-SM myosin antibodies as described above. In additional experiments mesenchymal cells were isolated from adult C57BL/6J dy/dy and C57BL/6J +/+ normal mice and plated on culture dishes uncoated or coated with 10 μg/ml of LM-2 as indicated in the manufacturer's instructions for cell attachment assays or with the same concentration of LM-1 or fibronectin. Cell attachment and spreading was then determined as just described.
Histological Studies
Lungs with trachea, stomach, and intestine from adult C57BL/6J dy/dy, C57BL/6J +/+ normal mice and CD-1 mice were formalin-fixed and paraffin-embedded. 5-μm sections were mounted on glass slides and stained with hematoxilin-eosin for light microscopic examination. Since the nuclei of SM cells are proportional to the cell's length and the cell boundaries are indistinct in vivo, nuclear length was determined as an indicator of cell elongation. Up to 460 nuclei (lung, 216; stomach, 120; intestine, 124 nuclei) were measured as described above in sections from lung, stomach, and intestine dissected from four dy/dy, four C57BL/6J +/+ and two CD-1 mice.
Immunohistochemistry
Mesenchymal cell cultures and organotypic epithelial-mesenchymal cocultures were immunostained with anti-LM α2 antibodies and frozen sections from dy/dy and normal lungs were immunostained with anti-SM α-actin following previous protocols (Schuger et al. 1997; Yang et al. 1998). Both antibodies were used at a concentration of 1 μg/ml. The corresponding secondary antibodies were used at a 1:50 dilution and the immunostaining was completed using a commercial peroxidase-anti-peroxidase kit (Dako), following the manufacturer's instructions. Fast red (red color) was used for detecting LM α2 and diaminobenzidine tetrahydrochloride (DAB) (brown color) was used for detecting SM α actin.
Results
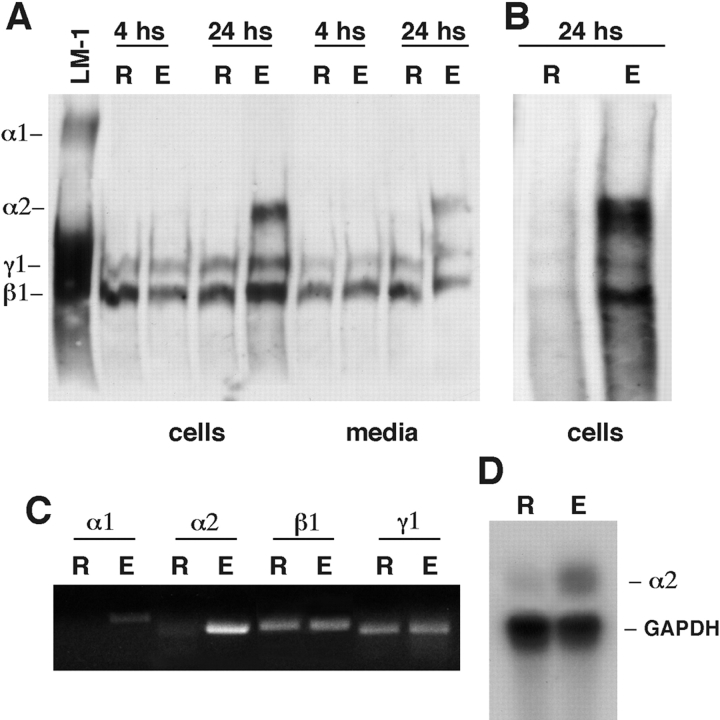
LM α2 chain expression is prevented by cell rounding and is induced by cell spreading/elongation. Regardless of the culture system used to facilitate cell rounding or cell spreading, we consistently observed that only spread cells expressed LM α2 chain. Immunoblots of round and elongated lung mesenchymal cells plated on culture microsurfaces showed LM α2 chain as a protein with a Mr of ∼350 kD present only in lysates from elongated cells (Fig. 1 A). The band was not detected during the first 4 h in culture, but progressively appeared within the next 20 h. Unlike the LM α2 chain, no modulations were found in expression of the LM β1 and γ1 chains, which was similar for both round and elongated cells. We used a monospecific antibody to confirm the identity of LM α2 chain. Since this antibody is not suited for immunoblotting, we used it for immunoprecipitation, followed by immunoblotting with the anti–LM-1/LM-2 antibody mentioned above. The lack of LM-2 in the round cell immunoprecipitates confirmed that only elongated cells synthesize LM α2 (Fig. 1 B).
Figure 1.
LM α2 chain expression in round versus elongated lung mesenchymal cells. (A) Western blot using a polyclonal antibody to LM-1/LM-2 on cells cultured for 4 and 24 h on 10- (R, round) or 20-μm microsurfaces (E, elongated). Note the presence of LM α2 band in elongated cells (E) after 24 h of culture. LM α2 also appears in the culture media after 24 h of culture. The first lane is LM-1 from the EHS tumor, used as control. (B) Cell lysates from round (R) and elongated (E) mesenchymal cells were immunoprecipitated with a monoclonal antibody against LM α2 chain and blotted with a polyclonal antibody to LM-1/LM-2. The immunoblot shows that the elongated cells but not the round cells synthesize trimeric LM-2. (C) RT-PCR showing higher levels of LM α2 chain mRNA in elongated (E), compared with round cells (R) (30 PCR cycles for α1 and β1 chains, 25 cycles for β1 and γ1 chains). (D) RPA confirming the higher level of LM α2 chain mRNA in elongated (E) cells.
RT-PCR and RPAs demonstrated that the increment in LM α2 chain is, at least in part, the result of an increment in its steady-state mRNA levels (Fig. 1 C). RT-PCR demonstrated no changes in mRNA levels for LM α1, β1, and γ1 chains between round and elongated cells as well as no changes in LM α4 and α5 message levels (not shown). As expected, LM α1 polypeptide chain was not detected under any of these culture conditions. Our previous studies showed that LM α1 chain synthesis requires epithelial-mesenchymal cell–cell contact (Schuger et al. 1997). No differences were seen in cell spreading and LM α2 chain synthesis between cells plated on uncoated dishes or dishes coated with LM-1, LM-2, collagen IV, and fibronectin (not shown).
Immunohistochemical studies confirmed the absence of LM α2 chain in round mesenchymal cells and its presence in elongated cells (Fig. 2, A–C). In organotypic cultures epithelial cells rearrange into spheres and mesenchymal cells surround them forming also a surface-anchored monolayer (Schuger et al. 1993). A basement membrane is the novo assembled at the epithelial-mesenchymal interface (Schuger et al. 1998). The mesenchymal cells apposed to this new basement membrane elongate and become SM cells, while the rest remain round and undifferentiated (Yang et al. 1998). The elongated cells synthesized LM α2, whereas the rest remained negative (Fig. 2 D). Over time the mesenchymal cells formed cysts with central lumens and additional mesenchymal cells spread/elongated around them and synthesized LM α2 chain (Fig. 2, inset).
Figure 2.
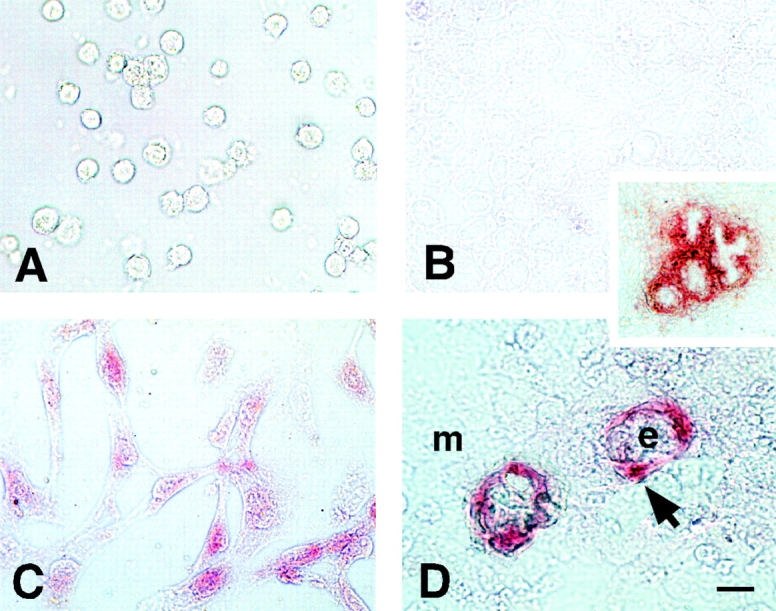
Immunohistochemical detection of LM α2 chain in round and elongated cells in monocultures and in organotypic epithelial-mesenchymal cocultures. (A) mouse mesenchymal cells cultured for 24 h in 0.05% poly-l-lysine coated dishes remain round and negative for LM α2 chain. (B) Mesenchymal cells plated at high cell density remain negative for LM α2 chain after 24 h in culture. (C) Mesenchymal cells allowed to spread for 24 h synthesize LM α2 chain (red). (D) Epithelial-mesenchymal organotypic cocultures. Mesenchymal cells spread around epithelial cell spheres (e) and express LM α2 (arrow). The rest of the mesenchymal cells (m) form a monolayer of round cells and remain negative for LM α2. (Inset) Organotypic cultures after three days have well developed epithelial cysts with central lumens. More mesenchymal cells spread around the cysts and become positive for LM α2 chain. Bars: (A–D) 15 μm; (inset) 60 μm.
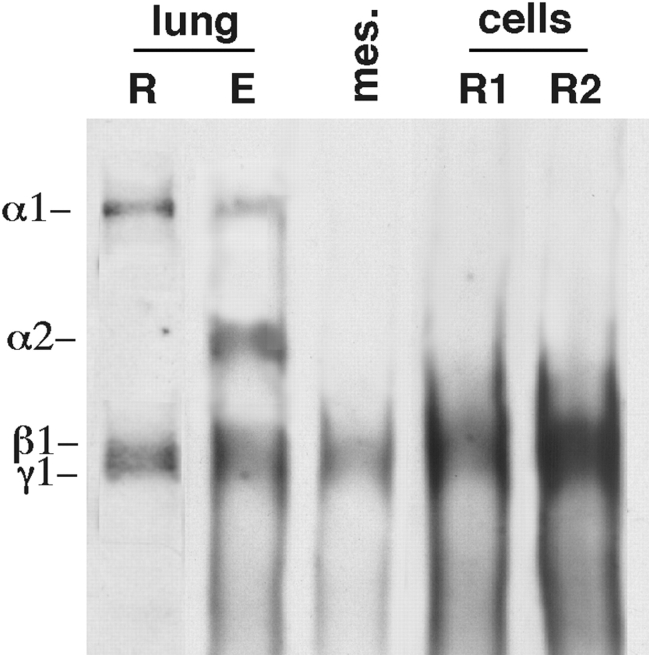
In vivo undifferentiated (round) mesenchymal cells do not express LM α2 chain, but elongated cells do. Here we determined whether the observations made in tissue cultures were relevant to the lung in vivo. At day 10.5 of gestation all the cells in the lung are round in shape. At this stage, the embryonic lung synthesizes LM α1 but not LM α2 (Fig. 3, first lane). At day 12 of gestation, most of the proximal peribronchial mesenchymal cells become spread/elongated and the distal epithelial cells become spread/flat. The undifferentiated mesenchymal cells, however, are still round. Western blots of microdissected airways and surrounding mesenchyme showed that the elongated cells express both LM α1 and α2 chains (Fig. 3, second lane), whereas Western blots of microdissected undifferentiated mesenchyme showed absence of LM α2 chains in round cells (Fig. 3, third lane).
Figure 3.
LM α2 chain in round and elongated cells of the embryonic lung. Western blot using a polyclonal antibody to LM-1/LM-2. Lane 1 (lung R), day 10.5 lungs, containing only round cells. Lane 2 (lung E), airways and surrounding mesenchyme microdissected from day 12 lungs, containing mainly elongated cells. mes. (lane 3), peripheral mesenchyme isolated from E12 lungs, containing only round cells (R). Lane 4 (R1), round undifferentiated mesenchymal cells before culture. Lane 5 (R2), round mesenchymal cells plated at high density to prevent cell spreading. LM α2 chain is seen only in elongated cells (lane 2).
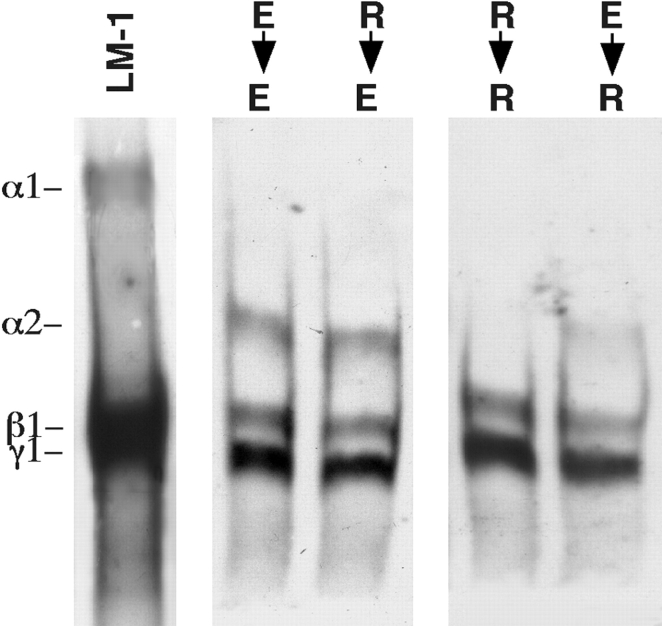
LM α2 chain expression is turned on and off by changes in cell shape. Switching cells from culture conditions that promote cell rounding to culture conditions that promote cell elongation and vice versa demonstrated that synthesis of LM α2 chain can be switched on and off by changes in cell shape (Fig. 4). Replating efficiency was similar for round and elongated cells (48 ± 12 and 50 ± 10% of replated cells attached, respectively).
Figure 4.
Switching cells from culture conditions promoting rounding or elongation. Mesenchymal cells cultured on poly-l-lysine (R) or with poly-l-lysine added to the culture medium after cell spreading is completed (E). After 24 h the cells were replated and cultured for an additional 24 h under similar or opposite conditions. Then immunoblotting was performed using a polyclonal antibody to LM-1/LM-2. LM α2 band appears in elongated cells (second and third lane) and is absent in the round cells (fourth and fifth lane). The first lane is LM-1 from EHS tumor, used as control.
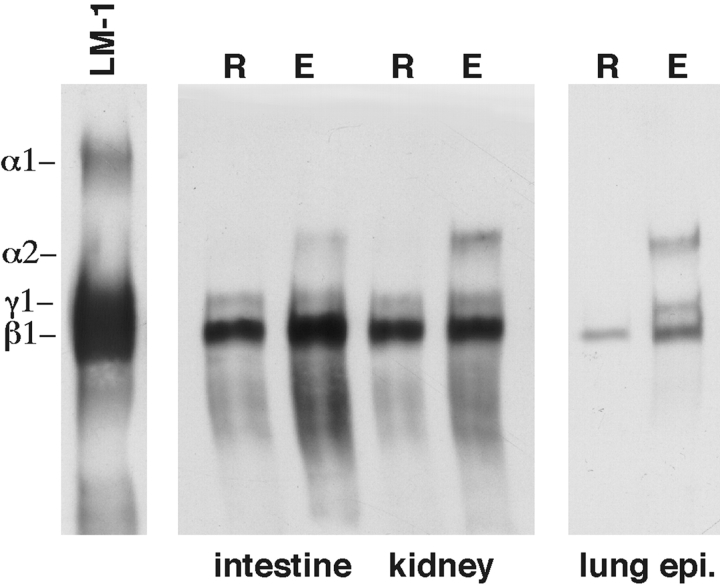
The control of LM α2 chain expression by the cell shape is not restricted to lung embryonic mesenchymal cells. During lung development, both epithelial and mesenchymal cells undergo changes in shape. The most dramatic changes take place on distal epithelial cells that become totally flat and spread by day 14–15 of gestation. Here we determined the role of cell shape on lung embryonic epithelial cells as well as on embryonic mesenchymal cells from kidney and intestine. Immunoblots showed that cell shape also regulates LM α2 chain expression in these cells in a similar manner as observed in mesenchymal cells. Like in embryonic mesenchymal cells, rounding prevented and spreading induced LM α2 chain synthesis (Fig. 5).
Figure 5.
LM α2 chain expression in mesenchymal cells from other organs and in lung epithelial cells. Western blot using a polyclonal antibody to LM-1/LM-2 on mesenchymal cells from day 15 intestine and day 12 kidney, and epithelial cells from day 15 lungs cultured on poly-l-lysine (R) or with poly-l-lysine added to the culture medium after cell spreading is completed (E). Note the presence of LM α2 band in elongated cells (E) from all three sources. The other lanes show β1 and γ1 bands only. The first lane is LM-1 from the EHS tumor, used as control.
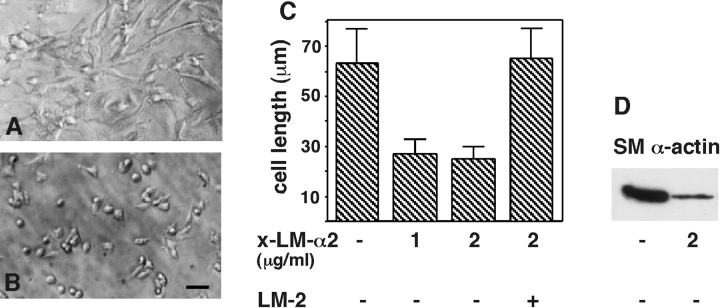
Cell spreading is blocked by monoclonal antibodies to LM α2 chain. Effect on SM differentiation. In functional studies, 1 and 2 μg /ml of monoclonal antibody against LM α2 chain were sufficient to block mesenchymal cell spreading in a statistically significant manner (Fig. 6, A–C). Higher concentrations of antibody did not further reduce spreading. Therefore, these studies indicated a reciprocal interaction between cell shape and LM α2 by showing that not only is the LM α2 chain induced by cell spreading, but also that cell spreading is induced by the LM α2 chain. Mesenchymal cells exposed to the anti-LM α2 chain antibodies showed a decrease in synthesis of SM-specific proteins, including SM α-actin, desmin and myosin when compared with cells exposed to control immunoglobulin (Fig. 6 D, and data not shown). Attachment assays in the presence of anti-LM α2 chain antibody or IgG control showed that the antibody also inhibited cell adhesion (64% less cells attached in the presence of 20 μg/ml of anti-LM α2 chain antibodies than in the presence of same concentration of control IgG; not shown).
Figure 6.
Cell spreading perturbation assays using antibodies to LM α2 chain. Lung mesenchymal cells were cultured in the presence of 1 and 2 μg/ml of LM α2 antibody, IgG control, or 2 μg/ml of LM α2 antibody preincubated with LM-2. Cell spreading was measured after 24 h in culture. (A) Cells in the presence of 2 μg/ml IgG. (B) Cells in the presence of 2 μg/ml LM α2 antibody. (C) Histogram obtained by measuring the maximal cell diameters under the various treatments. The inhibition in cell spreading was statistically significant, P < 0.0001 for 2 μg/ml LM α2 antibody. (D) Immunoblot showing less SM α actin in cells exposed to the anti-LM α2 chain antibody. Bar, 40 μm.
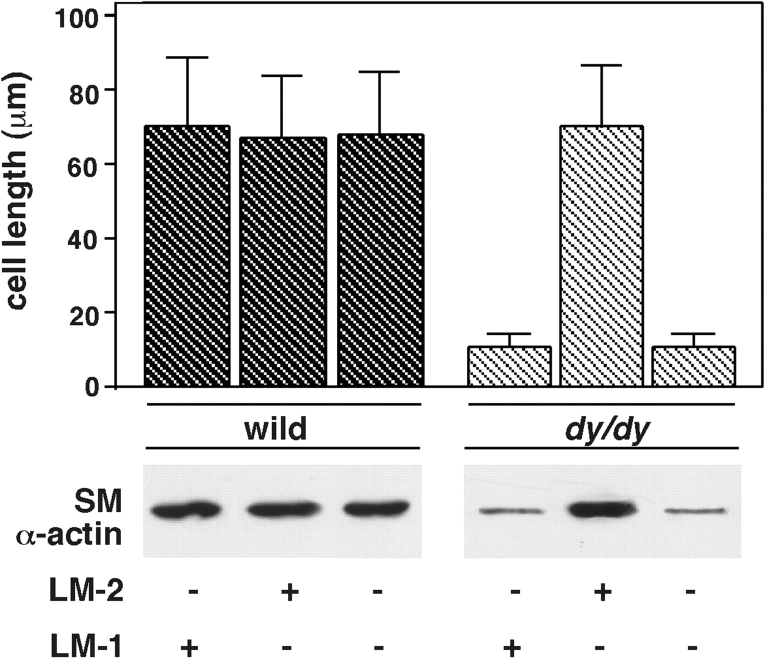
SM cells from adult dy/dy mouse lung spread poorly in culture and contain less SM-specific protein. Over 40% of the attached mesenchymal cells, whether isolated from normal or mutant mice, reacted with anti-desmin antibodies (not shown). All mesenchymal cell cultures isolated from adult dy/dy mouse lungs showed poor cell spreading and lower levels of SM-specific protein than controls. The deficiencies in cell spreading and SM protein synthesis disappeared by plating the cells on LM-2 (Fig. 7). LM-1 or fibronectin coating did not improve the abnormalities in cell spreading and SM protein synthesis seen in dy/dy mouse cells (Fig. 7 and data not shown).
Figure 7.
SM α actin production by lung mesenchymal cells isolated from adult dy/dy mice and controls. Cell spreading and SM α actin production were determined after 24 h in culture. The histogram shows the maximal cell diameters of wild-type and dy/dy mouse cells cultured on LM-1, LM-2, or uncoated dishes. The immunoblot shows SM α actin production by the cells. Notice the short diameter and low level of SM α actin in dy/dy mouse cells when cultured on LM-1 or uncoated dishes but not when cultured on LM-2.
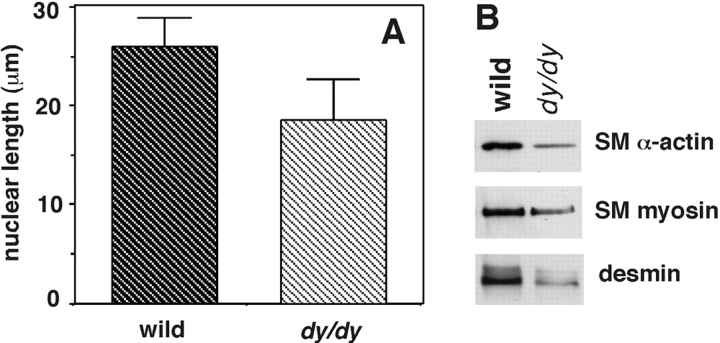
Dy/dy mice have SM abnormalities in vivo, including shorter cells and deficiencies in SM-specific protein production. Cell morphometry studies showed that bronchial SM cells in dy/dy mice were shorter than in control animals in a statistically significant manner (Fig. 8 A). No differences in size were found, however, in lung vascular SM cells or bronchial columnar epithelial cells (not shown). Immunoblots demonstrated that adult dy/dy mouse lungs contained less SM-specific proteins, including SM α-actin, desmin, and myosin (Fig. 8 B). On histological examination, the most abnormal SM was that of the trachea and main bronchi. In those sites, the visceral SM cells were shorter and underdeveloped (Fig. 9B and Fig. D). For comparison, A and C show tracheal and bronchial SM in normal animals of the same strain. Unlike the extraparenchymal airway, the changes in the intraparenchymal bronchial SM were subtle and detected mainly by cell morphometry (shorter cells) and immunoblotting as already shown in Fig. 8. On immunohistochemistry using an anti-SM α-actin antibody, the large and medium sized bronchial muscle exhibited slightly, but clear thinning and more discontinuity than the normal counterparts (Fig. 9E and Fig. F).
Figure 8.
SM cell size in intraparenchymal bronchi of dy/dy mice and controls. (A) Histogram showing maximal nuclear diameter as an indicator of cell size in hematoxylin eosin-stained histological sections of lungs (cell boundaries are not seen in organs). Dy/dy mice showed shorter nuclei compared with controls (P < 0.05). (B) Immunoblots demonstrating lower levels of SM proteins in dy/dy lungs compared with controls.
Figure 9.
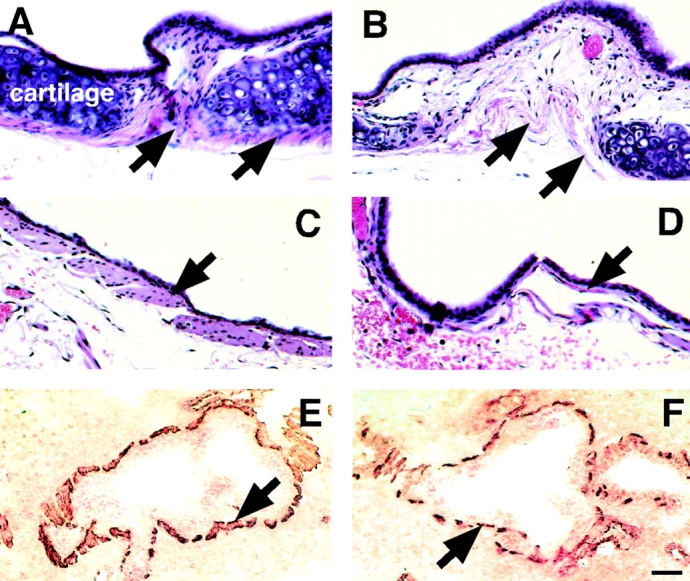
SM in trachea and bronchi of dy/dy mice and controls. (A–D) Hematoxylin eosin-stained histological sections of trachea and main bronchi. (A) Normal tracheal SM (arrows) surrounding tracheal cartilage; compare to the severely underdeveloped tracheal SM in dy/dy mouse, shown in B (arrows). (C) SM in normal main bronchus (arrow) is significantly thicker than in dy/dy mouse (D). (E and F) intraparenchymal bronchial SM immunostained with antibodies to SM α actin (brown). (E) Normal SM (arrow) surrounding medium sized bronchi; compare to thinner, more discontinuous bronchial muscle in dy/dy mice, shown in F (arrow). Bars: (A–D) 20 μm; (E and F) 60 μm.
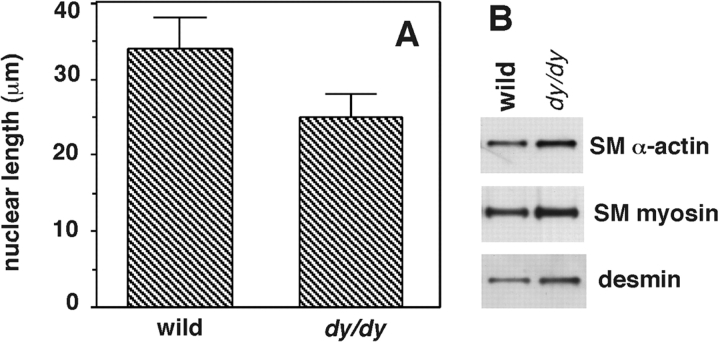
The SMs of stomach and intestine were also studied. Histological examination and morphometric studies of the stomach revealed no alterations in dy/dy mice when compared with controls. The intestine, however, exhibited shorter SM cells, similar to what was seen in the lung (Fig. 10 A). Unlike the trachea and bronchi, the SM cells in the intestinal wall were arranged in more layers (hyperplasia) resulting in a thicker muscularis wall. In the thinnest areas, the muscular circular layer was 2 ± 1-SM cell–thick in dy/dy mouse intestine and 1 ± 1-SM cell–thick in control animals. In the thickest sites, the circular layer was 15 ± 3-cell-thick in dydy intestine and 12 ± 2-cell-thick in controls. Reflecting this hyperplasia, immunoblotting showed a slight increase in SM-specific proteins in the intestine of dy/dy mice compared with controls (Fig. 10 B).
Figure 10.
SM cell size in circular layer of intestinal muscularis propria of dy/dy mice and controls. (A) Histogram showing maximal nuclear diameter as an indicator of cell size in hematoxylin eosin-stained histological sections (cell boundaries are not seen in organs). Dy/dy mice showed shorter nuclei compared with controls (P < 0.03). (B) Immunoblots demonstrating slightly higher levels of SM proteins in dy/dy intestine compared with controls, consistent with the hyperplasia described in Results.
Discussion
Reciprocal Interactions between LM α2 Chain and Embryonic Mesenchymal Cell Shape
During development, embryonic cells undergo significant changes in shape. In the early stages of embryogenesis, essentially all cells are round, but along with differentiation part of them become spindly whereas others adopt a columnar, cuboidal, or flat configuration. Our studies (Yang et al. 1998, Yang et al. 1999) and those of others (Leptin 1995; Loty et al. 1995; Anastasi et al. 1997; Fernandez-Valle et al. 1997; Martin-Blanco 1997, Martin-Blanco 1998; Bidwell et al. 1998) indicate that these changes in shape can be part of a mechanistic cascade directing precursor cells to specific differentiation lineages. Among the multiple factors controlling cell shape, we originally identified LM-1 as relevant for bronchial myogenesis (Schuger et al. 1997; Yang et al. 1998). We observed that during lung development, LM α1 chain synthesis is induced by epithelial-mesenchymal contact. LM α1 is deposited at the airway epithelial-mesenchymal interface, where it stimulates peribronchial mesenchymal cells to spread and to synthesize SM-specific proteins (Schuger et al. 1997).
Here we show that this change in cell shape from round to elongated results in the concomitant induction of LM α2 chain expression. In these studies we used different culture approaches to modulate cell shape and to determine its impact on LM α1, α2, β1, and γ1 chain expression. To confirm our in vitro findings, we analyzed tissue fragments microdissected from developing lungs containing either round cells or mostly elongated cells. Our studies demonstrated that, while LM β1 and γ1 chains are equally synthesized by round and elongated cells, LM α2 expression is under the control of cell shape in such a manner that round cells do not synthesize LM α2 chain, either in vivo or in vitro, but its expression is turned on by cell spreading/elongation. Furthermore, the induction of LM α2 chain is a reversible process that can be switched on and off in culture by cyclic changes in cell shape.
It has been shown that limiting the degree of cell spreading in culture may decrease protein secretion (Singhvi et al. 1994). In our study, however, LM α2 chain expression was not suppressed due to nonspecific inhibition in protein synthesis, as indicated by the fact that round and elongated cells synthesized the same amounts of LM β1/γ1 chains. Furthermore, we recently showed that round and elongated cells cultured on microsurfaces have similar metabolic rates, and round cells even synthesize higher levels of α-fetoprotein than their spread/elongated counterparts (Yang et al. 1999). Finally, LM α2 chain was not found in undifferentiated mesenchyme freshly isolated from the lungs, confirming its absence in round cells in vivo.
As previously stressed, among the several LM chains analyzed, the effect of cell shape on message and protein levels was specific to LM α2. Message RNA for LM α1, α4, α5, β1, and γ1 chains was not significantly altered by cyclic changes in cell shape, neither were LM β1 and γ1 polypeptide chain levels. In regard to LM α1 chain, its synthesis requires epithelial-mesenchymal cell contact (Schuger et al. 1997) and as expected it was not detected in fragments of mesenchyme alone or mesenchymal cell cultures. However, its presence in the early embryonic lung, where essentially all cells are round, and the lack of significant oscillations at the mRNA level (Fig. 1 B), indicated that LM α1 chain synthesis does not cycle with changes in cell shape.
Functional studies in which embryonic mesenchymal cell spreading/elongation was blocked by a monoclonal antibody against LM α2 chain demonstrated that, once secreted, LM α2 chain becomes a powerful stimulus for cell spreading. The antibody used in our functional studies reacts with the NH2-terminal of LM α2 chain, a region that has been shown to be involved in heparin binding, LM-2 polymerization (Cheng et al. 1997) and cell binding through integrins α1β1 and α2β1 (Collognato and Yurchenco, 1997). The functional role of the NH2 terminus is also underscored by an animal model of congenital muscular dystrophy, the dy2J/dy2Jmouse. This mutant animal has a truncated LM α2 chain which lacks only 57 amino acids at its NH2-terminal domain (Xu et al. 1994a; Sunada et al. 1995), and nevertheless this deletion is enough to cause muscular dystrophy. Integrins α1β1 and α2β1 are expressed by developing bronchial SM (Virtanen et al. 1996; Wu and Santoro 1996) and therefore may participate in LM α2 chain–mediated mesenchymal cell spreading. Supporting such a possibility, deletion of integrin α1 by homologous recombination permits normal murine development but gives rise to specific deficit in mesenchymal cell adhesion and spreading (Gardner et al. 1996). In addition, knockout of integrin β1 chain, which cause early embryonic death (Fassler and Meyer 1995), results in retardation of myogenic differentiation of embryonic stem cells in culture (Rohwedel et al. 1998).
Role of LM α2 Chain in Bronchial Myogenesis
We recently observed that most embryonic mesenchymal cells are potential SM precursors and if allowed to spread/elongate, they will differentiate into SM cells (Yang et al. 1998, Yang et al. 1999). Our functional studies demonstrated that LM α2 chain deposition in the extracellular matrix is per se a stimulus for cell spreading. This led us to determine the effect of inhibiting LM α2 chain–mediated cell spreading on SM differentiation. Mesenchymal cells exposed to the anti-LM α2 chain antibodies showed a decrease in synthesis of SM-specific proteins when compared with controls. Therefore, a feedback interaction seems to exist between the cells' shape, the levels of LM α2 chain and SM myogenesis.
The molecular pathways linking LM α2 chain, cell shape, and SM myogenesis are currently unknown. One possibility is that by promoting cell spreading/elongation, LM α2 chain stimulates specific cell receptors known to function as mechanotransductors, such as integrins (Wang et al. 1993). These then could activate gene expression by a signaling mechanism similar to that exerted by the breast extracellular matrix upon the mammary gland epithelium (Lelievre et al. 1996). An important player in cell shape signaling is the Rho family of G proteins (Bussey 1996). Interestingly, searching a subtracted cDNA library of undifferentiated, round mesenchymal cells, we found RhoA to be highly expressed in round mesenchymal cells with minimal expression in elongated cells (Liu, J., and L. Schuger, unpublished studies). It should be stressed, however, that cell shape controls gene expression through multiple mechanisms including signaling pathways not necessarily involving Rho (Martin-Blanco 1997, Martin-Blanco 1998), changing the cytoskeleton and nuclear matrix architecture (Loty et al. 1995; Fernandez-Valle et al. 1997; Shannon et al. 1998; Bidwell et al. 1998), inducing synthesis of auto/paracrine factors (Vijayakumar et al. 1999), etc. Therefore, multiple mechanisms may be involved in the process of LM α2 chain–induced SM myogenesis.
Visceral SM Abnormalities in dy/dy Mice
Dy/dy mice express lower levels of LM α2 chain, due to a spontaneous genetic mutation (Sunada et al. 1994; Xu et al. 1994a, Xu et al. 1994b). Therefore, we studied SM cells isolated from dy/dy lungs to determine how these cells behave in culture. These mutant mice have been used for many years as a model of skeletal muscular dystrophy, and although there are no previous reports describing SM defects in dy/dy mice, we predicted, based on our findings, that these animals should have abnormal SM cells. Indeed, we found that lung SM cells isolated from dy/dy mice spread defectively in culture and synthesize less SM proteins than controls. These deficiencies were overcome by the addition of exogenous LM-2 but not LM-1 or fibronectin.
On histological examination of lung sections, bronchial SM cells in dy/dy mice were shorter than in control animals and their lungs contained less SM-specific proteins. The morphological abnormalities diminished in severity from trachea, where the muscle was also underdeveloped, to the distal bronchi. Although the SM changes in the intraparenchymal bronchi were subtle, our current studies seem to indicate that these are sufficient to affect the electrical and contractile properties of dy/dy SM cells in culture (Yang, Y., and L. Schuger, ongoing studies). The SM cells of stomach and intestine were also examined. While no abnormalities were detected in the former, the intestine exhibited shorter SM cells, as seen in the bronchi. However, unlike the airways, the intestinal wall showed more SM cell layers, indicative of compensatory hyperplasia. The latter resulted in a net increase of SM-specific proteins in immunoblots.
It is unclear why some SMs were hyperplastic and others not. One reason could be related to the mechanical challenge sustained by the muscle. Muscles with peristalsis, such as the intestine, are rhythmically stimulated to contract. Since SM cells are able to proliferate, the dy/dy intestinal muscle may overcome the functional deficit caused by low LM α2 chain with an increment in cell number, thus, becoming hyperplastic. In contrast, muscles with minimal mechanical activity, such as the trachea and main bronchi, experience no mechanical challenge and therefore have no need to compensate. Between these two extremes, SMs with moderate contractile activity, such as the intraparenchymal bronchi, may require only intermediate grades of compensation. The gastric and vascular musculature may also fall under this category.
The Reciprocal Interaction between LM α2 Chain and Cell Shape May Play a Role in the Differentiation of Other Tissues
The effect of cell shape on LM α2 chain expression was not restricted to lung mesenchymal cells, but it was seen in other cells as well. Therefore, our findings suggested that cell shape–controlled LM α2 chain expression may play a broader role in development. In this regard, Virtanen et al. 1996 found that during the preglandular stage of human lung development, LM α2 chain is deposited in the distal airway basement membranes, where epithelial cells are undergoing a change in shape from round/cuboidal to flat/spread (Burry 1997). Similarly, Lefebvre et al. 1999 showed that during intestinal development, LM α2 chain is synthesized by elongating mesenchymal cells, but not by epithelial cells that are round/cuboidal in shape. Although neither of these papers intended to draw correlations between the cell shape and LM α2 chain expression, both support the possibility that epithelial cell shape and LM α2 chain may be functionally connected in vivo.
Proposed Roles for LM α1 and α2 in Bronchial Myogenesis
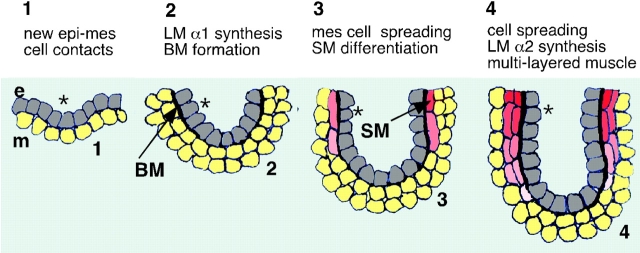
Fig. 11 depicts the proposed roles of LM α1 and α2 chains in bronchial myogenesis. Our studies suggest that the development of new epithelial-mesenchymal contacts during lung organogenesis results in the induction of LM α1 chain expression and deposition of LM-1 at the epithelial/mesenchymal interface (Schuger et al. 1997). LM-1 molecules polymerize, contributing to the formation of a basement membrane (Schuger et al. 1995, Schuger et al. 1998). The mesenchymal cells apposed to the newly formed basement membrane utilize this meshwork to spread/elongate through binding to LM α1 (Schuger et al. 1997). This change in cell shape induces them to synthesize LM α2 chain and concomitantly to differentiate into SM. Once secreted as part of LM-2, LM α2 chain stimulates spreading and SM differentiation of nearby mesenchymal cells, eventually leading to the establishment of a multilayered visceral muscle. The process of myogenesis could stop when LM α2 chain expression drops dramatically at the end of the pseudo glandular period (Virtanen et al. 1996) whereby preventing excessive SM formation.
Figure 11.
Proposed role of LM-1 and LM-2 in bronchial myogenesis. e, Epithelial cells (gray); m, mesenchymal cells (yellow); SM, smooth muscle cells (from pink to red); BM, basement membrane. The asterisk tags the relative position adopted by an epithelial cell from the moment it is born and establishes a new contact with the mesenchyme.
Acknowledgments
This work has been supported by NHLBI grant HL-48730 (L. Schuger) and George M. O'Brien Research Center Grant P50 DK45181 (J.M. Miner).
Footnotes
Abbreviations used in this paper: LM, laminin; RPA, ribonuclease protection assay; SM, smooth muscle.
References
- Anastasi S., Giordano S., Sthandier O., Gambarotta G., Maione R., Comoglio P., Amati P. A natural hepatocyte growth factor/scatter factor autocrine loop in myoblast cells and the effect of the constitutive Met kinase activation on myogenic differentiation. J. Cell Biol. 1997;137:1057–1068. doi: 10.1083/jcb.137.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernier S.M., Utani A., Sugiyama S., Doi T., Polistina C., Yamada Y. Cloning and expression of laminin α2 chain (m-chain) in the mouse. Matrix Biol. 1995;14:447–455. doi: 10.1016/0945-053x(95)90002-0. [DOI] [PubMed] [Google Scholar]
- Bidwell J.P., Alvarex M., Feister H., Onyia J., Hock J. Nuclear matrix proteins and osteoblast gene expression. J. Bone Miner. Res. 1998;2:155–167. doi: 10.1359/jbmr.1998.13.2.155. [DOI] [PubMed] [Google Scholar]
- Burgeson R.E., Chiquet M., Deutzmann R., Ekblom P., Engel J., Kleinman H., Martin G.R., Meneguzzi M., Sanes J. A new nomenclature for the laminins. Matrix Biol. 1994;263:16536–16544. doi: 10.1016/0945-053x(94)90184-8. [DOI] [PubMed] [Google Scholar]
- Burry P.H. Structural aspects of prenatal and postnatal development and growth of the lung. In: McDonald J.A., editor. In Lung Growth and Development. Marcel Dekker, Inc.; N.Y: 1997. pp. 1–36. [Google Scholar]
- Bussey H. Cell shape determinationa pivotal role for Rho. Science. 1996;272:224–225. doi: 10.1126/science.272.5259.224. [DOI] [PubMed] [Google Scholar]
- Cheng Y.-S., Champliaud M.-F., Burgeson R.E., Marinkovich M.P., Yurchenco P.D. Self-assembly of laminin isoforms. J. Biol. Chem. 1997;272:3125–3132. doi: 10.1074/jbc.272.50.31525. [DOI] [PubMed] [Google Scholar]
- Colognato H., Yurchenco P.D. The laminin α2-chain short arm mediates cell adhesion through both the α1β1 and α2β1 integrins. J. Biol. Chem. 1997;272:29330–29336. doi: 10.1074/jbc.272.46.29330. [DOI] [PubMed] [Google Scholar]
- Fassler R., Meyer M. Consequences of lack of β1 integrin gene expression in mice. Genes Dev. 1995;9:1896–1908. doi: 10.1101/gad.9.15.1896. [DOI] [PubMed] [Google Scholar]
- Fernandez-Valle C., Gorman D., Gomez A.M., Bunge M.B. Actin plays a role in both changes in cell shape and gene-expression associated with Schwann cell myelination. J. Neurosci. 1997;17:241–250. doi: 10.1523/JNEUROSCI.17-01-00241.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores-Delgado G., Bringas P., Warburton D. Laminin 2 attachment selects myofibroblasts from fetal mouse lung. Am. J. Physiol. 1998;19:L622–L630. doi: 10.1152/ajplung.1998.275.3.L622. [DOI] [PubMed] [Google Scholar]
- Gardner H., Kreidberg J., Koteliansky V., Jaenisch R. Deletion of integrin α1 by homologous recombination permits normal murine development but gives rise to a specific deficit in cell adhesion. Dev. Biol. 1996;175:301–313. doi: 10.1006/dbio.1996.0116. [DOI] [PubMed] [Google Scholar]
- Hillaire D., Leclerc A., Faure S., Topaloglu H., Chiannikulchai N., Guicheney P., Grinas L., Legos P., Philpot J., Evangelista T. Localization of merosin-negative congenital muscular dystrophy to chromosome 6q2 by homozygosity mapping. Hum. Mol. Genet. 1994;3:1657–1661. doi: 10.1093/hmg/3.9.1657. [DOI] [PubMed] [Google Scholar]
- Lefebvre O., Sorokin L., Kedinger M., Simon-Assmann P. Developmental expression and cellular origin of the laminin α2, α4, and α5 chains in the intestine. Dev. Biol. 1999;210:135–150. doi: 10.1006/dbio.1999.9270. [DOI] [PubMed] [Google Scholar]
- Leivo I., Engvall E. Merosin, a protein specific for basement membrane of Schwann cells, striated muscle and trophoblast, is expressed late in nerve and muscle development. Proc. Natl. Acad. Sci. USA. 1988;85:1544–1548. doi: 10.1073/pnas.85.5.1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelievre S., Weaver V.M., Bissell M.J. Extracellular matrix signaling from the cellular membrane skeleton to the nuclear skeletona model of gene regulation. Recent. Prog. Horm. Res. 1996;51:417–432. [PMC free article] [PubMed] [Google Scholar]
- Leptin M. Drosophila gastrulationfrom pattern formation to morphogenesis. Annu. Rev. Cell Dev. Biol. 1995;11:189–212. doi: 10.1146/annurev.cb.11.110195.001201. [DOI] [PubMed] [Google Scholar]
- Loty S., Forest N., Boulekbache H., Sautier J.M. Cytochalasin D induces changes in cell shape and promotes in vitro chondrogenesisa morphological study. Biol. Cell. 1995;83:149–161. doi: 10.1016/0248-4900(96)81303-7. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E. Regulation of cell differentiation by the Drosophila Jun kinase cascade. Curr. Opin. Genet. Dev. 1997;5:666–671. doi: 10.1016/s0959-437x(97)80015-9. [DOI] [PubMed] [Google Scholar]
- Martin-Blanco E. Regulatory control of signal transduction during morphogenesis in Drosophila . Int. J. Dev. Biol. 1998;42:363–368. [PubMed] [Google Scholar]
- Miner J.H., Patton B.L., Lentz S.I., Gilbert D.J., Snider W.D., Jenkins N.A., Copland N.G., Sanes J.R. The laminin α chainsexpression, developmental transitions, and chromosomal locations of α1-5, identification of heterotrimeric laminins 8-11, and cloning of a novel α3 isoform. J. Cell Biol. 1997;137:685–701. doi: 10.1083/jcb.137.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagoe Y., Hanaoka K., Nonaka I., Hayasaka M., Nabeshima Y., Arahata K., Nabashima Y., Takeda S. Laminin α2 chain-null mutant mice by targeted disruption of the Lama2 genea new model of merosin (laminin 2)-deficient congenital muscular dystrophy. FEBS Lett. 1997;415:33–39. doi: 10.1016/s0014-5793(97)01007-7. [DOI] [PubMed] [Google Scholar]
- Patton B.L., Miner J.H., Chiu A.Y., Sanes J.R. Distribution and function of laminins in the neuromuscular system of developing, adult, and mutant mice. J. Cell Biol. 1997;139:1507–1521. doi: 10.1083/jcb.139.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohwedel J., Guan K., Zuschratter W., Jin S., Ahnert-Hilger G., Furst D., Fassler R., Wobus A.M. Loss of β1 integrin function results in a retardation of embryonic stem cells in vitro. Dev. Biol. 1998;201:167–184. doi: 10.1006/dbio.1998.9002. [DOI] [PubMed] [Google Scholar]
- Schuler F., Sorokin L.M. Expression of laminin isoforms in mouse myogenic cells in vitro and in vivo. J. Cell Sci. 1995;108:3795–3805. doi: 10.1242/jcs.108.12.3795. [DOI] [PubMed] [Google Scholar]
- Schuger L., Varani J., Mitra J., Gilbride K. Retinoic acid stimulates mouse lung development by a mechanism involving epithelial-mesenchymal interaction and regulation of epidermal growth factor receptors. Dev. Biol. 1993;159:462–473. doi: 10.1006/dbio.1993.1256. [DOI] [PubMed] [Google Scholar]
- Schuger L., Skubitz A.P.N., Morenas A., Gilbride K. Different laminin domains facilitate lung development by independent mechanisms of action. Dev. Biol. 1995;169:520–532. doi: 10.1006/dbio.1995.1166. [DOI] [PubMed] [Google Scholar]
- Schuger L., Yurchenco P.D., Relan N., Yang Y. Laminin fragment E4 inhibition studiesbasement membrane assembly and embryonic lung epithelial cell polarization requires laminin polymerization. Int. J. Dev. Biol. 1998;41:217–220. [PubMed] [Google Scholar]
- Schuger L., Skubitz A.P.N., Zhang J., Sorokin L., He L. Laminin α1 chain synthesis in mouse developing lungrequirement of epithelial mesenchymal contact and possible role in bronchial smooth muscle development. J. Cell Biol. 1997;139:553–562. doi: 10.1083/jcb.139.2.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon J.M., Pan T., Edeen K.E., Nielsen L.D. Influence of the cytoskeleton on surfactant protein gene expression in cultured rat alveolar type II cells. Am. J. Physiol. 1998;274:L87–L96. doi: 10.1152/ajplung.1998.274.1.L87. [DOI] [PubMed] [Google Scholar]
- Singhvi R., Kumar A., Lopez G.P., Stephanopoulos G.N., Wang D.I., Whitesides G.M., Ingber D.E. Engineering cell shape and function. Science. 1994;264:696–698. doi: 10.1126/science.8171320. [DOI] [PubMed] [Google Scholar]
- Sunada Y., Bernier S.M., Kozak C.A., Yamada Y., Campbell K.P. Deficiency of merosin in dystrophic dy mice and genetic linkage of laminin M chain gene to dy locus. J. Biol. Chem. 1994;269:13729–13732. [PubMed] [Google Scholar]
- Sunada Y., Bernier S.M., Utani A., Yamada Y., Campbell K.P. Identification of a novel mutant transcript of laminin α2 chain gene responsible for muscular dystrophy and dysmyelination in dy2J mice. Human Mol. Gen. 1995;4:1055–1061. doi: 10.1093/hmg/4.6.1055. [DOI] [PubMed] [Google Scholar]
- Timpl R., Rhode M., Gehron-Robey P., Rennard S.T., Foidart J.M., Martin G.R. Laminin-A glycoprotein from basement membranes. J. Biol. Chem. 1979;254:9933–9937. [PubMed] [Google Scholar]
- Vachon P.H., Loechel F., Xu H., Wewer U.M., Engvall E. Merosin and laminin in myogenesis; specific requirement for merosin in myotube stability and survival. J. Cell Biol. 1996;134:1483–1497. doi: 10.1083/jcb.134.6.1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar S., Takito J., Hikita C., Al-Awqati Q. Hensin remodels the apical cytoskeleton and induces columnarization of intercalated epithelial cellsprocesses that resemble terminal differentiation. J. Cell Biol. 1999;144:1057–1067. doi: 10.1083/jcb.144.5.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virtanen I., Laitinen A., Taneli T., Paakko P., Laitinen L.A., Burgeson R.E., Letho V.P. Differential expression of laminins and their integrin receptors in developing and adult human lung. Am. J. Respir. Cell Mol. Biol. 1996;15:184–196. doi: 10.1165/ajrcmb.15.2.8703474. [DOI] [PubMed] [Google Scholar]
- Vuolteenaho R., Nissinen M., Sainio K., Byers M., Eddy R., Hirvonen H., Shows T.B., Sariola H., Engvall E., Tryggvason K. Human laminin M chain (merosin)complete primary structure, chromosomal assignment, and expression of the M and A chain in human fetal lung. J. Cell Biol. 1994;124:381–394. doi: 10.1083/jcb.124.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Butler J.P., Ingber D.E. Mechanotransduction across the cell surface and through the cytoskeleton. Science. 1993;260:1124–1127. doi: 10.1126/science.7684161. [DOI] [PubMed] [Google Scholar]
- Wu J.E., Santoro S.A. Differential expression of integrin α subunits supports distinct roles during lung branching morphogenesis. Dev. Dyn. 1996;206:169–181. doi: 10.1002/(SICI)1097-0177(199606)206:2<169::AID-AJA6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Xu H., Wu X.R., Wewer U.M., Engvall E. Murine muscular dystrophy caused by a mutation in the laminin α2 (Lama2) gene Nat. Genet. 8 1994. 297 302a [DOI] [PubMed] [Google Scholar]
- Xu H., Christmas P., Wu X.R., Wewer U.M., Engvall E. Defective muscle basement membrane and lack of M-laminin in the dystrophic dy/dy mouse Proc. Natl. Acad. Sci. USA. 91 1994. 5572 5576b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Palmer K.C., Relan N.K., Diglio C., Schuger L. Role of laminin polymerization at the epithelial mesenchymal interface in bronchial myogenesis. Development. 1998;125:2621–2629. doi: 10.1242/dev.125.14.2621. [DOI] [PubMed] [Google Scholar]
- Yang Y., Relan N.K., Przywara D., Schuger L. Differentiation of smooth muscle cell precursors is determined by the cell's shape. Development. 1999;126:3027–3033. doi: 10.1242/dev.126.13.3027. [DOI] [PubMed] [Google Scholar]