How are the numbers of different cell types matched together during tissue development? The nervous system has provided an excellent model system for understanding this question as it contains two main classes of cell types, neurons and glia. Presynaptic neurons are believed to be precisely matched to their postsynaptic target cells, either muscle cells or other neurons, by a competition for limiting amounts of target-derived neurotrophic factors (Purves 1988). The number of some glial cells that survive also appears to depend on a competition for neuron-derived survival signals. In particular, the number of myelinating glial cells that survive appears to be precisely matched during development to the number and lengths of axons requiring myelination. Here we review recent evidence that axons are the master regulators of oligodendrocyte development.
Oligodendrocytes are postmitotic cells that develop from oligodendrocyte precursor cells (OPCs) that migrate into developing white matter from their germinal zones. The final number of oligodendrocytes in any part of the CNS could, in principle, depend upon the number of OPCs that migrate into it, the number of times the OPCs divide before they differentiate, and the number of oligodendrocytes and OPCs that undergo normal cell death in the region. This issue has been intensively studied in the rodent optic nerve, which contains two main glial cell types, astrocytes and oligodendrocytes, in addition to the axons of retinal ganglion cells.
Because the function of oligodendrocytes is to enhance nerve conduction along axons, it would make logical sense for axons to play an important part in controlling oligodendrocyte development. Surprisingly, a role for axons was not apparent initially as oligodendrocyte development appears to occur normally in axon-free cultures. OPCs in vitro and in vivo migrate, survive, and divide in response to astrocyte-derived PDGF (Noble et al. 1988; Raff et al. 1988; Richardson et al. 1988; Fruttiger et al. 1999). OPCs also differentiate on schedule into oligodendrocytes in the absence of axons (Abney et al. 1981; Raff et al. 1985). An important role for axons was revealed, however, when it was found that, after neonatal or postnatal optic nerves are transected behind the eye, few oligodendrocytes develop (Fulcrand and Privat 1977; Wender et al. 1980; Privat et al. 1981; David et al. 1984; Valat et al. 1988; Barres et al. 1993b). These observations revealed a powerful role of axons in controlling oligodendrocyte development in vivo. They also raised the questions of exactly how axons control oligodendrocyte development: do they effect migration, proliferation, differentiation, or survival of OPCs or oligodendrocytes, and by what molecular signals do they act? We summarize here the recent studies that have provided some answers to these questions.
Axons Stimulate OPC Proliferation and/or Survival but Do Not Regulate OPC Differentiation
To find out whether axons stimulate OPC proliferation, several groups have taken advantage of the fact that astrocytes, oligodendrocytes, and OPCs each have a characteristic antigenic phenotype (Raff et al. 1983a,Raff et al. 1983b, Raff et al. 1984). This allows the effects of transection on each cell type to be specifically investigated. Neonatal transection, for example, produces a large, more than a 10-fold decrease in the percentage of astrocytes that incorporate bromodeoxyuridine (BrdU; Burne and Raff 1997), suggesting that axons stimulate astrocyte proliferation, perhaps partly by releasing sonic hedgehog (Wallace and Raff 1999). In contrast, transection does not significantly alter the percentage of OPCs that incorporate BrdU (David et al. 1984; Ueda et al. 1999). On the face of it, these findings suggest that axons regulate astrocyte, but not OPC, proliferation.
The interpretation of these findings is not straightforward, however, because of an interesting difference in the behavior of astrocytes and OPCs when they withdraw from the cell cycle. Astrocytes do not alter their antigenic phenotype when they stop dividing, whereas OPCs do. When OPCs stop dividing, they quickly differentiate into oligodendrocytes, thus losing their OPC-specific markers. Thus, whereas the percentage of astrocytes that incorporate BrdU provides a meaningful index of their proliferation rate, the same may not be the case for OPCs. This point is vividly illustrated by measuring BrdU incorporation by purified OPCs in culture in response to different concentrations of their main mitogen PDGF (our unpublished observations). Regardless of PDGF concentration, the percentage of OPCs that incorporate BrdU does not vary. What varies, however, is the number of OPCs and oligodendrocytes. When PDGF concentration is high, most of the cells are OPCs and few are oligodendrocytes. When PDGF concentration is low, most of the cells become oligodendrocytes. Therefore, the failure of axotomy to influence the percentage of OPCs that incorporate BrdU does not necessarily mean that axons do not regulate their division.
To reexamine the effects of axons on OPC proliferation, Barres and Raff 1993 measured the total number of OPCs and oligodendrocytes rather than the percentage that incorporated BrdU. When developing optic nerves are transected, the number of mitotic OPCs falls by 90% in 4 d. This same percentage reduction was obtained regardless of whether we measured the total number of mitotic OPCs per longitudinal section, the total number of BrdU1 OPCs per entire optic nerve, or the number of all OPCs per optic nerve. If the same experiment is performed in mutant mice whose axons do not degenerate after a transection (Brown et al. 1991), proliferation of oligodendrocyte precursor cells also decreases by 90%, raising the possibility that the proliferation depends on electrical activity in axons (Barres and Raff 1993). Consistent with this possibility, intraocular injection of tetrodotoxin (TTX), which silences the electrical activity of retinal ganglion cells and their axons, decreases the number of OPCs by 80%. The effect of TTX is prevented by experimentally increasing the concentration of PDGF in the developing optic nerve, suggesting that axonal electrical activity normally controls the production and/or release of the growth factors that are responsible for the proliferation and/or survival of OPCs (Barres and Raff 1993). Electrical activity could act indirectly by increasing glial production of mitogens such as PDGF or directly by increasing neuronal production or release of mitogens. Whatever the mechanism, these data provided strong evidence that axons control the rate of proliferation and/or survival of developing OPCs.
In contrast, there is no evidence as yet that axons are required for OPC migration or differentiation into oligodendrocytes. It has recently been reported that axons are not necessary for OPC migration (Ueda et al. 1999). Purified OPCs in culture differentiate constitutively into oligodendrocytes in serum-free medium lacking specific inducing signals (Raff et al. 1983b; Temple and Raff 1986; Barres et al. 1992). In the absence of axons, OPCs can differentiate into oligodendrocytes in vivo (Fulcrand and Privat 1977; David et al. 1984; Ueda et al. 1999). Axons in embryonic and neonatal rats may instead inhibit the ability of OPCs to differentiate into oligodendrocytes. OPCs express Notch1 receptors and activation of the Notch pathway in purified OPCs in culture prevents their differentiation into oligodendrocytes (Wang et al. 1998). As neonatal axons express the Notch ligand Jagged1, which is downregulated concurrently with the onset of rapid oligodendrocyte generation, it is possible that axons help to regulate the timing of myelination by preventing oligodendrocyte differentiation during the first neonatal week. Similarly, developing Schwann cells express little of the myelin protein P0 before myelination in vivo, yet in culture purified Schwann cells in the absence of axons express a higher level of P0 (Cheng and Mudge 1996). Thus, whereas axons are not required to stimulate differentiation of OPCs into oligodendrocytes, they may inhibit OPC differentiation before myelination.
Axons Promote Oligodendrocyte Survival
Oligodendrocytes require survival signals in vitro and during normal development in vivo to avoid programmed cell death (Barres et al. 1992, Barres et al. 1993b). At least 50% of the oligodendrocytes produced in the optic nerve normally die within 2 to 3 d after they are generated (Barres et al. 1992; Raff et al. 1993). Most of the oligodendrocytes that survive for 3 or more days appear to survive for the lifetime of the organism. Experimentally increasing the levels of PDGF, IGF-1, CNTF, or NT-3 in the developing optic nerve greatly decreases the death and increases the number of oligodendrocytes that develop (Barres et al. 1992, Barres et al. 1993a,Barres et al. 1993b, Barres et al. 1994). These findings indicate that all of these signaling molecules are normally present in subsaturating amounts in the developing nerve and suggest that normally occurring oligodendrocyte death may reflect a competition for survival signals that are limited in amount or availability. As PDGF, IGF-1, NT-3, and CNTF are all produced by optic nerve astrocytes and all promote the survival of oligodendrocyte lineage cells in vitro and in vivo, it seems likely that astrocytes play a part in supporting the survival of this lineage in the nerve, at least during development.
In addition, many studies have suggested that the survival of oligodendrocytes depends strongly on axons. There is general agreement that when the optic nerves are examined several weeks after a neonatal transection, very few oligodendrocytes and OPCs are found (Fulcrand and Privat 1977; Privat et al. 1981; David et al. 1984; Valat et al. 1988; Barres et al. 1993b). This could be explained largely by the effects of axons in stimulating OPC division and/or survival or there could be additional effects of axons in promoting oligodendrocyte survival. The conclusion that axons promote oligodendrocyte survival has been drawn by nearly every investigator who has examined optic nerves after transection. For instance, using electron microscopy, Fulcrand and Privat 1977 observed cells with the characteristic ultrastructure of oligodendrocytes undergoing degeneration after postnatal transection. EM is not sufficient to identify degenerating oligodendrocytes unambiguously, however; but using an antigenic identification, David et al. 1984 also concluded that axons likely promote oligodendrocyte survival because of the severe loss of oligodendrocytes despite some continued OPC proliferation after neonatal transection.
Oligodendrocytes in the rat optic nerve are normally generated predominantly between P5 and P45. Therefore, in order to determine whether axons help to promote oligodendrocyte survival, it is important to examine the effects of postnatal transection performed after P5. When P8 or P12 optic nerves are transected behind the eye so that the axons degenerate, the oligodendrocytes die (Barres et al. 1993b; Trapp et al. 1997). Within 3 d after transection, the number of cell undergoing apoptosis increases by more than fourfold, and many of the dying cells can be identified as oligodendrocytes based on expression of characteristic antigenic markers. P8 optic nerves have about 35,000 oligodendrocytes per nerve; when examined 10 d after a P8 transection on P18, the nerves contain only about 3,000 oligodendrocytes, compared with the 125,000 found in control P18 nerves (Barres et al. 1993b). Thus not only are few new oligodendrocytes generated after a P8 transection, but >90% of the oligodendrocytes present at P8 die. The death of oligodendrocytes after P8 transection is prevented if the levels of IGF-1, CNTF (Barres et al. 1993b), or neuregulins (NRG; P.-A. Fernandez, D. Tang, L. Cheng, A. Mudge, and M. Raff, manuscript submitted for publication) are experimentally elevated.
How do axons regulate oligodendrocyte survival? Oligodendrocytes do not die if the optic nerve is transected in WLD mutant mice in which the axons do not degenerate (Brown et al. 1991) and the ability of axons to promote oligodendrocyte survival does not depend on electrical activity in the axons (Barres et al. 1993b). Purified neurons, but not neuron-conditioned culture medium, promote the survival of purified oligodendrocytes in vitro (Barres et al. 1993b). These findings suggest that the axon-derived signal is contact-mediated and not dependent on electrical activity. Neuregulins have recently been proposed to be such a signal (see below).
How does one reconcile the findings that both astrocyte-derived and axon-derived signals seem to promote oligodendrocyte survival? It is possible that signals from both sources collaborate to promote the survival of oligodendrocytes; alternatively, newly formed oligodendrocytes may depend on the astrocyte-derived signals while more mature oligodendrocytes may lose their dependence on astrocytes and come to depend solely on axons for their survival.
Immature Schwann cells are also strongly dependent on axons for a survival signal (Ciutat et al. 1996; Grinspan et al. 1996; Syroid et al. 1996; Trachtenberg and Thompson 1996; Carroll et al. 1997; Nakao et al. 1997). Interestingly, one week after PNS myelination, Schwann cells no longer depend on axons for their survival (Grinspan et al. 1996) and may instead depend on autocrine signals (Cheng et al. 1998). The same is true for oligodendrocytes, many but not all of which survive transection in adult optic nerves (Vaughn and Pease 1970; Fulcrand and Privat 1977; McPhilemy et al. 1990; Ludwin 1990). These changes in survival requirements are reminiscent of the changes that occur in a number of types of neurons, including sensory DRG neurons and sympathetic neurons, that initially depend on target-derived signals for survival but lose this dependence in the adult (Acheson et al. 1995).
A Model for How Axons Control Oligodendrocyte Number
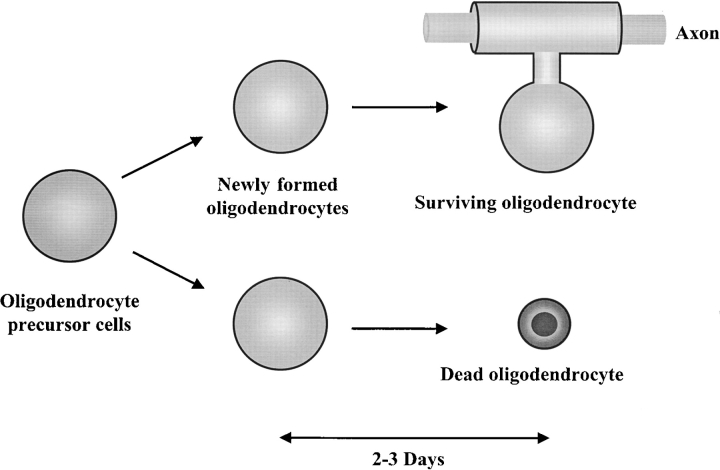
A tentative model for how a competition for axon-dependent survival signals may help to match oligodendrocyte and axon numbers during development has been proposed (Barres et al. 1993b; Barres and Raff 1994). Once an oligodendrocyte precursor cell stops dividing and begins to differentiate into an oligodendrocyte, its specific requirements for survival signals change: it rapidly loses its PDGF receptors, for example, so that PDGF can no longer promote its survival (Hart et al. 1989; McKinnon et al. 1990). It now has only 2–3 d to contact a nonmyelinated region of axon that provides new signals that are required for its continued survival. A cell that fails to find an axon will kill itself (Fig. 1). Forcing oligodendrocytes to compete for axon-dependent survival signals that are limited in amount or availability would help to ensure that the final number of oligodendrocytes is precisely matched to the number (and length) of axons requiring myelination. Importantly, according to this model, newly formed premyelinating oligodendrocytes depend on astrocyte-derived signals for their survival for about the first 2 d, whereas after 3 d the oligodendrocytes are more mature and depend primarily upon an axon-derived signal.
Figure 1.
A model for how oligodendrocyte number is matched to axonal surface area. Once an OPC stops dividing and differentiates into an oligodendrocyte (left side of figure), it has 2–3 d to contact an unmyelinated region of axon, which provides a new signal that the cell requires for continued survival. Astrocyte-derived signals, such as PDGF, can promote the survival of newly formed oligodendrocytes for at least 2 d (middle of figure). But as the newly formed oligodendrocytes undergo further maturation (right side of figure), they lose responsiveness to these astrocyte-derived signals and require an axonal signal to survive. Those that fail to contact an axon by 3 d after generation undergo apoptosis.
Much evidence supports such a model. The model explains why most developing oligodendrocytes that die do so 2 to 3 d after their generation and why most developing oligodendrocytes die after axotomy. It also explains why there appears to be a perfect matching between oligodendrocytes in the optic nerve and the number and lengths of axons (see Barres and Raff 1994); in normal CNS white matter, all mature oligodendrocytes that survive seem to myelinate axons. Since the proposal of this model, several of its predictions have been tested. One prediction is that if the number of axons is experimentally increased, then the number of oligodendrocytes that survive will increase proportionally. This has been found to be the case (Burne et al. 1996). Another prediction is that oligodendrocytes that succeed in contacting axons will preferentially survive, while those that don't will die. This has been tested and found to be true (Trapp et al. 1997). A last prediction is that if the number of oligodendrocytes generated is experimentally increased, increased death should reduce their numbers to normal. This has also been found to be the case, as overexpression of PDGF in transgenic mice initially leads to an enormous increase in oligodendrocyte numbers in the embryonic mouse spinal cord. All of the extra oligodendrocytes die, however, so that by a week or so after birth the number of oligodendrocytes is normal, as predicted by the model because the number of axons has not changed (Calver et al. 1998).
In a recent paper, Bruce Trapp and his colleagues reported that rat optic nerve oligodendrocytes develop in the absence of viable retinal ganglion cell axons (Ueda et al. 1999). To find out whether axons regulated oligodendrocyte development, they performed neonatal axotomy of the optic nerve. 7 d later, at P7, they found no change in the density of OPCs and a 50% decrease in the density of oligodendrocytes in optic nerve sections. However, because transection produces marked atrophy of the cut optic nerve, their data demonstrate a large decrease in the total number of OPCs and oligodendrocytes per axotomized nerve. Because they observed a fourfold decrease in the cross sectional area of the transected nerves, their OPC and oligodendrocyte density measurements indicate a fourfold reduction in the total number of OPCs and an eightfold reduction in the number of oligodendrocytes, by only one week after axotomy. The new findings of Ueda et al. 1999 therefore reconfirm the powerful role of axons in promoting the development of the oligodendrocyte lineage, although this is not the conclusion they draw. In fact, the presence of some oligodendrocyte lineage cells after neonatal transection (David et al. 1984; Ueda et al. 1999) is expected, as the early stages of the oligodendrocyte lineage (oligodendrocyte precursor cells and newly formed oligodendrocytes) are supported by astrocyte-derived signals such as PDGF.
Ueda et al. 1999 also addressed whether axons help to promote oligodendrocyte survival. In these experiments they transected P4 nerves rather than P0 nerves in order to allow time for at least some oligodendrocytes to be generated, and then examined the nerves at P7. They found no change in the density of surviving oligodendrocytes or the percentage of oligodendrocytes undergoing apoptosis, suggesting to them that axons do not control oligodendrocyte survival. Interpretation of these findings, however, is limited by the fact that very few oligodendrocytes are normally found in the optic nerve at P4 (Miller et al. 1985; Barres et al. 1992). Thus nearly all of the oligodendrocytes examined between P4 and P7 would be newly formed oligodendrocytes, which do not yet depend on axons to survive (Barres et al. 1993b). (That a small percentage of these oligodendrocytes are mature enough to depend on axons is suggested by the observation of Ueda et al. 1999 that there is a substantial increase in oligodendrocyte death one day after axotomy.) Clearly, for the effects of axons on oligodendrocyte survival to be examined meaningfully, axotomy must be performed at a later postnatal age after a significant number of oligodendrocytes have been generated, as was done previously (Fulcrand and Privat 1977; Privat et al. 1981; Barres et al. 1993b). Nonetheless, Trapp and colleagues concluded that their data “argue against axonal regulation of optic nerve oligodendrogenesis.” In fact, whereas the new data of Trapp and colleagues provides evidence that axons do not strongly promote the survival of just born oligodendrocytes, their new studies do not address whether axons promote the survival of oligodendrocytes that are at least 2 to 3 d beyond their birthday or older, as suggested by the studies of Barres et al. 1992, Barres et al. 1994(see below).
Ueda et al. 1999 suggest an alternative model for how oligodendrocyte numbers are controlled. They propose a density-dependent feedback mechanism where oligodendrocytes inhibit OPC expansion. Whereas such a mechanism might normally help to control the density of OPCs and newly formed oligodendrocytes in developing white matter (Zhang and Miller 1996) and might explain the relatively unaltered densities of OPCs and newly formed oligodendrocytes observed after axotomy by Ueda et al. 1999, it is not sufficient to explain how the final number of mature oligodendrocytes is determined. Even when the density of OPCs and newly formed oligodendrocytes is greatly increased by overexpression of PDGF, the final number of mature oligodendrocytes is unaltered (Calver et al. 1998). Moreover OPC proliferation continues in adult rodent optic nerves, but the number of mature oligodendrocytes does not change.
Neuregulin Is a Strong Candidate for an Axon-derived Promoter of Myelinating Cell Development
During the past several years, neuregulins (NRGs) have emerged as a likely candidate for an axonal signal that promotes both Schwann cell and oligodendrocyte development. NRGs are a large family of proteins related to epidermal growth factor. They occur in multiple isoforms, some membrane bound and some soluble, that are encoded by at least four alternatively spliced genes. They were first identified as potent mitogens for Schwann cells and astrocytes in culture and called glial growth factor (Raff et al. 1978; Brockes et al. 1980; Goodearl et al. 1993; Marchionni et al. 1993). In the developing CNS, NRGs are predominantly or entirely expressed by neurons, which target them to their axons throughout the CNS and PNS. NRGs promote the survival and proliferation of cells in the Schwann cell lineage by activating erbB2/erbB3 heterodimeric receptors (Morrissey et al. 1995; Grinspan et al. 1996; Minghetti et al. 1996). The entire Schwann cell lineage fails to develop in erbB3 deficient transgenic mice (Riethmacher et al. 1997) and in NRG-deficient transgenic mice (Meyer and Birchmeier 1995). Although, in principle, this finding might be explained by the ability of neuronally derived NRG to instruct multipotential neural crest cells to become Schwann cells (Shah et al. 1994), it is unlikely to be the entire explanation since in erbB2-deficient mice Schwann cell precursor cells develop within the DRG but fail to migrate into the peripheral nerves (Morris et al. 1999). Thus the lack of Schwann cell development in these transgenic mice may be accounted for by the loss of functional axonal NRG signaling. Axons have been shown to promote the survival and proliferation of Schwann cell lineage cells in vitro and in vivo; NRGs mediate these axonal effects in culture experiments (Morrissey et al. 1995; Dong et al. 1995, Dong et al. 1999), as well as after axotomy of developing peripheral nerves (Grinspan et al. 1996; Minghetti et al. 1996; Trachtenberg and Thompson 1996; Nakao et al. 1997; Kopp et al. 1997).
Similarly, the development of the oligodendrocyte lineage also depends on NRG signaling. In culture, NRG promotes the survival of oligodendrocytes and the proliferation of OPCs (Canoll et al. 1996; Raabe et al. 1997; Shi et al. 1998; Fernandez, P.-A., D. Tang, L. Cheng, A. Mudge, and M. Raff, submittedmanuscript for publication). When spinal cords from wild-type mice are cultured as explants, the oligodendrocyte lineage fails to develop if NRGs are neutralized (Vartanian et al. 1999). Moreover, the oligodendrocyte lineage does not develop in spinal cord explants obtained from NRG-deficient transgenic mice, but can be rescued by addition of recombinant NRG to the culture medium (Vartanian et al. 1999). The source of the spinal cord derived NRG that promotes OPC development is probably either motor neurons or ventral ventricular zone cells, both of which contain NRG immunoreactivity and are close to the site of OPC generation (Vartanian et al. 1999). These results clearly demonstrate the importance of NRG for either the differentiation or proliferation of OPCs. Recent experiments also provide strong support for the role of neuronally derived NRG in promoting oligodendrocyte survival as well. Retinal ganglion cells make NRG, which is targeted to their axons (Meyer and Birchmeier 1994; Shi et al. 1998). The survival-promoting effect of DRG axons in vitro is strongly inhibited if NRG is neutralized. In the developing optic nerve, neutralization of NRG increases normal oligodendrocyte death, whereas delivery of exogenous NRG decreases it; moreover, the oligodendrocyte death induced by nerve transection is almost completely abolished by delivery of exogenous NRG (Fernandez, P.-A., D. Tang, L. Cheng, A. Mudge, and M.C. Raff, manuscript submitted for publication). These results suggest that NRG is one of the major signals used by RGC axons to promote oligodendrocyte survival in the developing optic nerve.
In addition, other axonal signals are likely to participate. A recent study has suggested that integrin signaling helps to promote axon-mediated oligodendrocyte survival in DRG-oligodendrocyte co-cultures (Frost et al. 1999). This is interesting as in other tissues integrin signaling has been found to promote survival by enhancing responsiveness to trophic peptides (Ruoslahti and Reed 1994; Giancotti and Ruoslahti 1999). Thus it will be of great interest in future studies to explore whether NRG and integrin signaling are synergistic in promoting oligodendrocyte survival.
In summary, axons powerfully control the development of myelinating glial cells. Recent studies have revealed that axons promote oligodendrocyte development by helping to drive the proliferation of OPCs and by promoting the survival of mature, myelinating oligodendrocytes. Axonally derived NRG is a likely candidate signal that mediates these effects. Together, these recent studies provide strong support for a model in which the number of myelinating cells is matched during development to the axonal surface area requiring myelination.
Acknowledgments
We thank Pierre-Alain Fernandez and Anne Mudge for helpful comments on the manuscript.
Footnotes
Abbreviations used in this paper: BrdU, bromodeoxyuridine; OPCs, oligodendrocyte precursor cells; TTX, tetrodotoxin.
References
- Abney E.R., Bartlett P.P., Raff M.C. Astrocytes, ependymal cells, and oligodendrocytes develop on schedule in dissociated cell cultures of embryonic rat brain. Dev. Biol. 1981;83:301–310. doi: 10.1016/0012-1606(81)90476-0. [DOI] [PubMed] [Google Scholar]
- Acheson A., Conover J.C., Fandl J.P., DeChiara T.M., Russell M., Thadani A., Squinto S.P., Yancopoulos G.D., Lindsay R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature. 1995;374:450–453. doi: 10.1038/374450a0. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Raff M.C. Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature. 1993;361:258–260. doi: 10.1038/361258a0. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Raff M.C. Control of oligodendrocyte number in the developing rat optic nerve. Neuron. 1994;12:935–942. doi: 10.1016/0896-6273(94)90305-0. [DOI] [PubMed] [Google Scholar]
- Barres B.A., Hart I.K., Coles H.S., Burne J.F., Voyvodic J.T., Richardson W.D., Raff M.C. Cell death and control of cell survival in the oligodendrocyte lineage. Cell. 1992;70:31–46. doi: 10.1016/0092-8674(92)90531-g. [DOI] [PubMed] [Google Scholar]
- Barres B., Jacobson M., Schmid R., Sendtner M., Raff M. Does oligodendrocyte survival depend on axons? Curr. Biol. 3 1993. 489 497a [DOI] [PubMed] [Google Scholar]
- Barres B.A., Jacobson M.D., Schmid R., Sendtner M., Raff M.C. Does oligodendrocyte survival depend on axons? Curr. Biol. 3 1993. 489 497b [DOI] [PubMed] [Google Scholar]
- Barres B.A., Schmid R., Sendtner M., Raff M.C. Multiple extracellular signals are required for long-term oligodendrocyte survival Development. 118 1993. 283 295c [DOI] [PubMed] [Google Scholar]
- Barres B.A., Raff M.C., Gaese F., Bartke I., Dechant G., Barde Y.A. A crucial role for neurotrophin-3 in oligodendrocyte development. Nature. 1994;367:371–375. doi: 10.1038/367371a0. [DOI] [PubMed] [Google Scholar]
- Brockes J.P., Lemke G.E., Balzer D.R., Jr. Purification and preliminary characterization of a glial growth factor from the bovine pituitary. J. Biol. Chem. 1980;255:8374–8377. [PubMed] [Google Scholar]
- Brown M.C., Perry V.H., Lunn E.R., Gordon S., Heumann R. Macrophage dependence of peripheral sensory nerve regenerationpossible involvement of nerve growth factor. Neuron. 1991;6:359–370. doi: 10.1016/0896-6273(91)90245-u. [DOI] [PubMed] [Google Scholar]
- Burne J.F., Raff M.C. Retinal ganglion cell axons drive the proliferation of astrocytes in the developing rodent optic nerve. Neuron. 1997;18:223–230. doi: 10.1016/s0896-6273(00)80263-9. [DOI] [PubMed] [Google Scholar]
- Burne J.F., Staple J.K., Raff M.C. Glial cells are increased proportionally in transgenic optic nerves with increased numbers of axons. J. Neurosci. 1996;16:2064–2073. doi: 10.1523/JNEUROSCI.16-06-02064.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calver A.R., Hall A.C., Yu W.P., Walsh F.S., Heath J.K., Betsholtz C., Richardson W.D. Oligodendrocyte population dynamics and the role of PDGF in vivo. Neuron. 1998;20:869–882. doi: 10.1016/s0896-6273(00)80469-9. [DOI] [PubMed] [Google Scholar]
- Canoll P.D., Musacchio J.M., Hardy R., Reynolds R., Marchionni M.A., Salzer J.L. GGF/neuregulin is a neuronal signal that promotes the proliferation and survival and inhibits the differentiation of oligodendrocyte progenitors. Neuron. 1996;17:229–243. doi: 10.1016/s0896-6273(00)80155-5. [DOI] [PubMed] [Google Scholar]
- Carroll S.L., Miller M.L., Frohnert P.W., Kim S.S., Corbett J.A. Expression of neuregulins and their putative receptors, ErbB2 and ErbB3, is induced during Wallerian degeneration. J. Neurosci. 1997;17:1642–1659. doi: 10.1523/JNEUROSCI.17-05-01642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng L., Mudge A.W. Cultured Schwann cells constitutively express the myelin protein P0. Neuron. 1996;16:309–319. doi: 10.1016/s0896-6273(00)80049-5. [DOI] [PubMed] [Google Scholar]
- Cheng L., Esch F.S., Marchionni M.A., Mudge A.W. Control of Schwann cell survival and proliferationautocrine factors and neuregulins. Mol. Cell. Neurosci. 1998;12:141–156. doi: 10.1006/mcne.1998.0706. [DOI] [PubMed] [Google Scholar]
- Ciutat D., Caldero J., Oppenheim R.W., Esquerda J.E. Schwann cell apoptosis during normal development and after axonal degeneration induced by neurotoxins in the chick embryo. J. Neurosci. 1996;16:3979–3990. doi: 10.1523/JNEUROSCI.16-12-03979.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David S., Miller R.H., Patel R., Raff M.C. Effects of neonatal transection on glial cell development in the rat optic nerveevidence that the oligodendrocyte-type 2 astrocyte cell lineage depends on axons for its survival. J. Neurocytol. 1984;13:961–974. doi: 10.1007/BF01148596. [DOI] [PubMed] [Google Scholar]
- Dong Z., Brennan A., Liu N., Yarden Y., Lefkowitz G., Mirsky R., Jessen K.R. Neu differentiation factor is a neuron-glia signal and regulates survival, proliferation, and maturation of rat Schwann cell precursors. Neuron. 1995;15:585–596. doi: 10.1016/0896-6273(95)90147-7. [DOI] [PubMed] [Google Scholar]
- Dong Z., Sinanan A., Parkinson D., Parmantier E., Mirsky R., Jessen K.R. Schwann cell development in embryonic mouse nerves. J. Neurosci. Res. 1999;56:334–348. doi: 10.1002/(SICI)1097-4547(19990515)56:4<334::AID-JNR2>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Frost E.E., Buttery P.C., Milner R., ffrench-Constant C. Integrins mediate a neuronal survival signal for oligodendrocytes. Curr. Biol. 1999;9:1251–1254. doi: 10.1016/s0960-9822(99)80506-5. [DOI] [PubMed] [Google Scholar]
- Fruttiger M., Karlsson L., Hall A.C., Abramsson A., Calver A.R., Bostrom H., Willetts K., Bertold C.H., Heath J.K., Betsholtz C., Richardson W.D. Defective oligodendrocyte development and severe hypomyelination in PDGF-A knockout mice. Development. 1999;126:457–467. doi: 10.1242/dev.126.3.457. [DOI] [PubMed] [Google Scholar]
- Fulcrand J., Privat A. Neuroglial reactions secondary to Wallerian degeneration in the optic nerve of the postnatal ratultrastructural and quantitative study. J. Comp. Neurol. 1977;176:189–222. doi: 10.1002/cne.901760204. [DOI] [PubMed] [Google Scholar]
- Giancotti F.G., Ruoslahti E. Integrin signaling. Science. 1999;285:1028–1032. doi: 10.1126/science.285.5430.1028. [DOI] [PubMed] [Google Scholar]
- Goodearl A.D., Davis J.B., Mistry K., Minghetti L., Otsu M., Waterfield M.D., Stroobant P. Purification of multiple forms of glial growth factor. J. Biol. Chem. 1993;268:18095–18102. [PubMed] [Google Scholar]
- Grinspan J.B., Marchionni M.A., Reeves M., Coulaloglou M., Scherer S.S. Axonal interactions regulate Schwann cell apoptosis in developing peripheral nerveneuregulin receptors and the role of neuregulins. J. Neurosci. 1996;16:6107–6118. doi: 10.1523/JNEUROSCI.16-19-06107.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart I.K., Richardson W.D., Heldin C.H., Westermark B., Raff M.C. PDGF receptors on cells of the oligodendrocyte-type-2 astrocyte (O-2A) cell lineage. Development. 1989;105:595–603. doi: 10.1242/dev.105.3.595. [DOI] [PubMed] [Google Scholar]
- Kopp D.M., Trachtenberg J.T., Thompson W.J. Glial growth factor rescues Schwann cells of mechanoreceptors from denervation-induced apoptosis. J. Neurosci. 1997;17:6697–6706. doi: 10.1523/JNEUROSCI.17-17-06697.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwin S.K. Oligodendrocyte survival in Wallerian degeneration. Acta Neuropathol. 1990;80:184–191. doi: 10.1007/BF00308922. [DOI] [PubMed] [Google Scholar]
- Marchionni M.A., Goodearl A.D., Chen M.S., Bermingham-McDonogh O., Kirk C., Hendricks M., Danehy F., Misumi D., Sudhalter J., Kobayashi K. Glial growth factors are alternatively spliced erbB2 ligands expressed in the nervous system. Nature. 1993;362:312–318. doi: 10.1038/362312a0. [DOI] [PubMed] [Google Scholar]
- McKinnon R.D., Matsui T., Dubois-Dalcq M., Aaronson S.A. FGF modulates the PDGF-driven pathway of oligodendrocyte development. Neuron. 1990;5:603–614. doi: 10.1016/0896-6273(90)90215-2. [DOI] [PubMed] [Google Scholar]
- McPhilemy K., Mitchell L.S., Griffiths I.R., Morrison S., Deary A.W., Sommer I., Kennedy P.G. Effect of optic nerve transection upon myelin protein gene expression by oligodendrocytesevidence for axonal influences on gene expression. J. Neurocytol. 1990;19:494–503. doi: 10.1007/BF01257239. [DOI] [PubMed] [Google Scholar]
- Meyer D., Birchmeier C. Distinct isoforms of neuregulin are expressed in mesenchymal and neuronal cells during mouse development. Proc. Natl. Acad. Sci. USA. 1994;91:1064–1068. doi: 10.1073/pnas.91.3.1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer D., Birchmeier C. Multiple essential functions of neuregulin in development. Nature. 1995;378:386–390. doi: 10.1038/378386a0. [DOI] [PubMed] [Google Scholar]
- Miller R.H., David S., Patel R., Abney E.R., Raff M.C. A quantitative immunohistochemical study of macroglial cell development in the rat optic nervein vivo evidence for two distinct astrocyte lineages. Dev. Biol. 1985;111:35–41. doi: 10.1016/0012-1606(85)90432-4. [DOI] [PubMed] [Google Scholar]
- Minghetti L., Goodearl A.D., Mistry K., Stroobant P. Glial growth factors I-III are specific mitogens for glial cells. J. Neurosci. Res. 1996;43:684–693. doi: 10.1002/(SICI)1097-4547(19960315)43:6<684::AID-JNR5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Morris J.K., Lin W., Hauser C., Marchuk Y., Getman D., Lee K.F. Rescue of the cardiac defect in ErbB2 mutant mice reveals essential roles of ErbB2 in peripheral nervous system development. Neuron. 1999;23:273–283. doi: 10.1016/s0896-6273(00)80779-5. [DOI] [PubMed] [Google Scholar]
- Morrissey T.K., Levi A.D., Nuijens A., Sliwkowski M.X., Bunge R.P. Axon-induced mitogenesis of human Schwann cells involves heregulin and p185erbB2. Proc. Natl. Acad. Sci. USA. 1995;92:1431–1435. doi: 10.1073/pnas.92.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao J., Shinoda J., Nakai Y., Murase S., Uyemura K. Apoptosis regulates the number of Schwann cells at the premyelinating stage. J. Neurochem. 1997;68:1853–1862. doi: 10.1046/j.1471-4159.1997.68051853.x. [DOI] [PubMed] [Google Scholar]
- Noble M., Murray K., Stroobant P., Waterfield M.D., Riddle P. Platelet-derived growth factor promotes division and motility and inhibits premature differentiation of oligodendrocyte-type-2 astrocyte progenitor cells. Nature. 1988;333:560–562. doi: 10.1038/333560a0. [DOI] [PubMed] [Google Scholar]
- Privat A., Valat J., Fulcrand J. Proliferation of neuroglial cell lines in the degenerating optic nerve of young rats. A radioautographic study. J. Neuropathol. Exp. Neurol. 1981;40:46–60. [PubMed] [Google Scholar]
- Purves D. Body and BrainA Trophic Theory of Neural Connections. Harvard University Press; Cambridge, MA: 1988. [DOI] [PubMed] [Google Scholar]
- Raabe T.D., Suy S., Welcher A., DeVries G.H. Effect of neu differentiation factor isoforms on neonatal oligodendrocyte function. J. Neurosci. Res. 1997;50:755–768. doi: 10.1002/(SICI)1097-4547(19971201)50:5<755::AID-JNR12>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Raff M.C., Abney E., Brockes J.P., Hornby-Smith A. Schwann cell growth factors. Cell. 1978;15:813–822. doi: 10.1016/0092-8674(78)90266-0. [DOI] [PubMed] [Google Scholar]
- Raff M.C., Abney E.R., Fok-Seang J. Reconstitution of a developmental clock in vitroa critical role for astrocytes in the timing of oligodendrocyte differentiation. Cell. 1985;42:61–69. doi: 10.1016/s0092-8674(85)80101-x. [DOI] [PubMed] [Google Scholar]
- Raff M.C., Abney E.R., Miller R.H. Two glial cell lineages diverge prenatally in rat optic nerve. Dev. Biol. 1984;106:53–60. doi: 10.1016/0012-1606(84)90060-5. [DOI] [PubMed] [Google Scholar]
- Raff M.C., Barres B.A., Burne J.F., Coles H.S., Ishizaki Y., Jacobson M.D. Programmed cell death and the control of cell survivallessons from the nervous system. Science. 1993;262:695–700. doi: 10.1126/science.8235590. [DOI] [PubMed] [Google Scholar]
- Raff M.C., Lillien L.E., Richardson W.D., Burne J.F., Noble M.D. Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature. 1988;333:562–565. doi: 10.1038/333562a0. [DOI] [PubMed] [Google Scholar]
- Raff M.C., Miller R.H., Noble M. Glial cell lineages in the rat optic nerve Cold Spring Harb. Symp. Quant. Biol. 48 1983. 569 572a [DOI] [PubMed] [Google Scholar]
- Raff M.C., Miller R.H., Noble M. A glial progenitor cell that develops in vitro into an astrocyte or an oligodendrocyte depending on culture medium Nature. 303 1983. 390 396b [DOI] [PubMed] [Google Scholar]
- Richardson W.D., Pringle N., Mosley M.J., Westermark B., Dubois-Dalcq M. A role for platelet-derived growth factor in normal gliogenesis in the central nervous system. Cell. 1988;53:309–319. doi: 10.1016/0092-8674(88)90392-3. [DOI] [PubMed] [Google Scholar]
- Riethmacher D., Sonnenberg-Riethmacher E., Brinkmann V., Yamaai T., Lewin G.R., Birchmeier C. Severe neuropathies in mice with targeted mutations in the ErbB3 receptor. Nature. 1997;389:725–730. doi: 10.1038/39593. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E., Reed J.C. Anchorage dependence, integrins, and apoptosis. Cell. 1994;77:477–478. doi: 10.1016/0092-8674(94)90209-7. [DOI] [PubMed] [Google Scholar]
- Shah N.M., Marchionni M.A., Isaacs I., Stroobant P., Anderson D.J. Glial growth factor restricts mammalian neural crest stem cells to a glial fate. Cell. 1994;77:349–360. doi: 10.1016/0092-8674(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Shi J., Marinovich A., Barres B.A. Purification and characterization of adult oligodendrocyte precursor cells from the rat optic nerve. J. Neurosci. 1998;18:4627–4636. doi: 10.1523/JNEUROSCI.18-12-04627.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syroid D.E., Maycox P.R., Burrola P.G., Liu N., Wen D., Lee K.F., Lemke G., Kilpatrick T.J. Cell death in the Schwann cell lineage and its regulation by neuregulin. Proc. Natl. Acad. Sci. USA. 1996;93:9229–9234. doi: 10.1073/pnas.93.17.9229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple S., Raff M.C. Clonal analysis of oligodendrocyte development in cultureevidence for a developmental clock that counts cell divisions. Cell. 1986;44:773–779. doi: 10.1016/0092-8674(86)90843-3. [DOI] [PubMed] [Google Scholar]
- Trachtenberg J.T., Thompson W.J. Schwann cell apoptosis at developing neuromuscular junctions is regulated by glial growth factor. Nature. 1996;379:174–177. doi: 10.1038/379174a0. [DOI] [PubMed] [Google Scholar]
- Trapp B.D., Nishiyama A., Cheng D., Macklin W. Differentiation and death of premyelinating oligodendrocytes in developing rodent brain. J. Cell Biol. 1997;137:459–468. doi: 10.1083/jcb.137.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda H., Levine J., Miller R., Trapp R. Rat optic nerve oligodendrocytes develop in the absence of viable retinal ganglion cell axons. J. Cell Biol. 1999;146:1365–1374. doi: 10.1083/jcb.146.6.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valat J., Privat A., Fulcrand J. Experimental modifications of postnatal differentiation and fate of glial cells related to axo-glial relationships. Int. J. Dev. Neurosci. 1988;6:245–260. doi: 10.1016/0736-5748(88)90005-6. [DOI] [PubMed] [Google Scholar]
- Vartanian T., Fischbach G., Miller R. Failure of spinal cord oligodendrocyte development in mice lacking neuregulin. Proc. Natl. Acad. Sci. USA. 1999;96:731–735. doi: 10.1073/pnas.96.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughn J.E., Pease D.C. Electron microscopic studies of Wallerian degeneration in rat optic nerves. II. Astrocytes, oligodendrocytes and adventitial cells. J. Comp. Neurol. 1970;140:207–226. doi: 10.1002/cne.901400205. [DOI] [PubMed] [Google Scholar]
- Wallace V.A., Raff M.C. A role for Sonic hedgehog in axon-to-astrocyte signalling in the rodent optic nerve. Development. 1999;126:2901–2909. doi: 10.1242/dev.126.13.2901. [DOI] [PubMed] [Google Scholar]
- Wang S., Sdrulla A.D., diSibio G., Bush G., Nofziger D., Hicks C., Weinmaster G., Barres B.A. Notch receptor activation inhibits oligodendrocyte differentiation. Neuron. 1998;21:63–75. doi: 10.1016/s0896-6273(00)80515-2. [DOI] [PubMed] [Google Scholar]
- Wender M., Kozik M., Goncerzewicz A., Mularek O. Neuroglia of the developing optic nerve in the course of Wallerian degeneration. J. Hirnforsch. 1980;21:417–428. [PubMed] [Google Scholar]
- Zhang H., Miller R.H. Density-dependent feedback inhibition of oligodendrocyte precursor expansion. J. Neurosci. 1996;16:6886–6895. doi: 10.1523/JNEUROSCI.16-21-06886.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]