Abstract
Drosophila melanogaster oocytes heterozygous for mutations in the α-tubulin 67C gene (αtub67C) display defects in centromere positioning during prometaphase of meiosis I. The centromeres do not migrate to the poleward edges of the chromatin mass, and the chromatin fails to stretch during spindle lengthening. These results suggest that the poleward forces acting at the kinetochore are compromised in the αtub67C mutants. Genetic studies demonstrate that these mutations also strongly and specifically decrease the fidelity of achiasmate chromosome segregation. Proper centromere orientation, chromatin elongation, and faithful segregation can all be restored by a decrease in the amount of the Nod chromokinesin. These results suggest that the accurate segregation of achiasmate chromosomes requires the proper balancing of forces acting on the chromosomes during prometaphase.
Keywords: chromosome, kinesin, meiosis, centromeres, meiotic spindle
The mechanisms by which chiasmate (or exchange) chromosomes achieve a stable bipolar orientation during meiotic prophase have been described by Nicklas 1974, Nicklas 1997. The kinetochores of the two homologous chromosomes, tethered together by chiasmata, make attachments to opposite poles of the bipolar spindle. The stability of metaphase chromosome positioning is achieved by balancing the poleward forces between two oppositely oriented centromeres connected by chiasmata. The proper segregation of achiasmate chromosomes poses a rather different problem, since homologues are, by definition, not connected by chiasmata.
Achiasmate segregation is a well-characterized component of the meiotic process in Drosophila melanogaster oocytes (Hawley and Theurkauf 1993; Dernburg et al. 1996; Karpen et al. 1996; Matthies et al. 1996), and we recently completed a screen for Drosophila mutants defective in this process (Sekelsky et al. 1999). Mutants were isolated that exhibited high levels of X and/or 4th chromosome nondisjunction in FM7/X females. FM7 is a rearranged X chromosome, often referred to as a balancer, which recombines with a normal sequence X homologue with a frequency of <1% of normal (Hawley et al. 1993). The use of this balancer chromosome allowed us to study the segregation of both the achiasmate X chromosomes and the obligately achiasmate 4th chromosomes (Hawley et al. 1993).
One of the mutants recovered from this screen, αtub67CP40, resulted from the insertion of the P{lacW} transposable element into the coding region of the α-tubulin 67C gene (αtub67C). This insertion occurred at position 2173, 39 bp upstream of the 3′ end, so that the last 13 amino acids of the normal αtub67C gene product are replaced by a novel sequence of five amino acids, followed by a stop codon. αtub67C, one of the most divergent of the α-tubulins (Kalfayan and Wensink 1982; Theurkauf et al. 1986), is transcribed primarily in the nurse cells, and is maternally loaded into the oocyte, eventually comprising ∼20% of the α-tubulin pool in normal oocytes (Matthews et al. 1993).
Materials and Methods
Drosophila Stocks
Two X chromosomes were used in this study: a normal sequence X chromosome marked with yellow (y) and white (w); and the FM7 balancer chromosome. The FM7 chromosome consists of three superimposed inversions: a nearly full-length paracentric inversion that moves most of the basal heterochromatin to the tip of the X and inverts all but the most distal bands of euchromatin; the dl-49 inversion; and a third inversion, broken in the distally located heterochromatin (region 20) and in the euchromatin at position 15 D. FM7 is as an excellent suppressor of exchange when heterozygous with a normal X chromosome.
Genetic Assays for Meiotic Nondisjunction
We monitor X and 4th chromosome disjunction by crossing X/X; pol/pol females to attached XY/0; C(4)RM, ci eyR males. The frequencies of nondisjunction were calculated as described in Hawley et al. 1993.
Confocal Cytology
Oocytes were prepared and examined as previously described with minor modifications (Theurkauf and Hawley 1992; Matthies et al. 1996). Egg chambers from 3–7-d-old females were extracted by quick pulses of a blender using a modified Robb's medium. The mixture was passed sequentially through a loose and fine mesh to separate late stage oocytes. The oocytes were fixed for 5 min on a rotator at room temperature in a hypertonic solution, therefore preventing hypotonic activation of the mature oocytes. After removal of the follicle cells, chorion, and vitelline membranes, the oocytes were permeabilized with 1% Triton X-100 in PBS. Oocytes were labeled with YL1/2 (1:200) and YOL1/34 (1:200) rat antitubulin mAbs (Accurate) and both Oligreen (Molecular Probes, Inc.; 1:10,000) and 1 MAB52 (1:500) anticore histone mouse monoclonal. In some experiments, oocytes were also labeled with MEI-S332 (1:500) guinea pig polyclonal antibody (generous gift of Drs. Tracy Tang and Terry Orr-Weaver, Massachusetts Institute of Technology, Cambridge, MA). Primary antibodies were then labeled with secondary antibodies (1:250) purchased from Jackson ImmunoResearch Laboratories, conjugated in the following manner: Cy2 to anti-mouse; Cy3 to anti-rat; and Cy5 to anti-guinea pig.
Oocytes were examined using an MRC-1024 BioRad confocal microscope (Kalman collection), and spindle and chromatin lengths and widths were determined using BioRad 3D software. Spindle and chromatin lengths were determined from maximum intensity projected spindles. Spindle lengths were measured from pole to pole and chromatin length from the sites of the chromatin found closest to either pole. Image stacks were converted to maximum intensity projections and subsequently converted to Photoshop Images (Adobe Systems Inc.). Final images were produced on a dye sublimation printer (Tektronics Phaser 440).
Results
The αtub67CP40Mutation Has a Dominant Effect on Chromatin Elongation
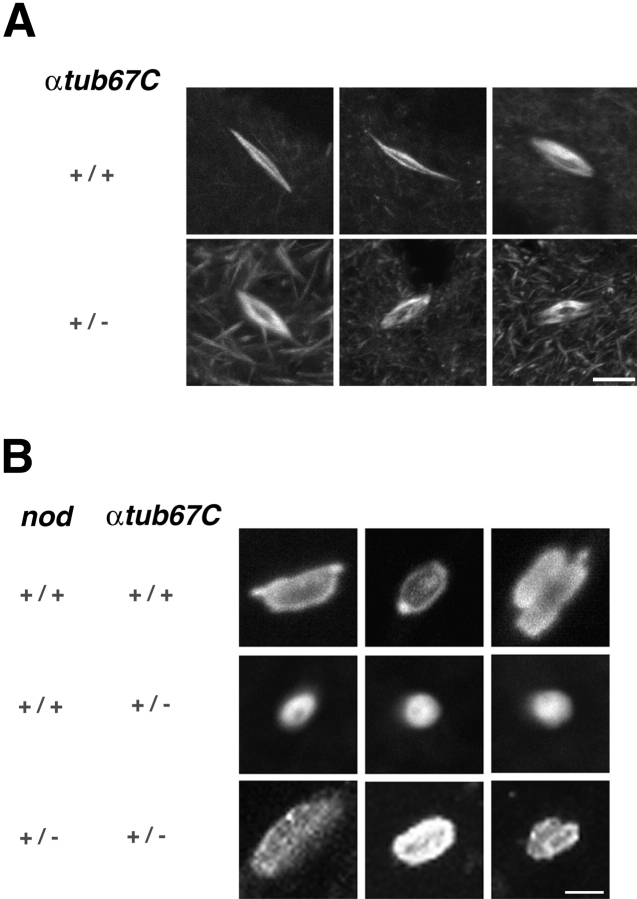
Although the spindles of FM7/X; αtub67CP40/+ oocytes are, on average, somewhat shorter than wild-type spindles (average spindle lengths are 12.2 versus 15.1 μm, respectively), confocal studies did not reveal obvious defects in spindle structure, as shown in Fig. 1 A. However, an examination of chromatin in FM7/X; αtub67CP40/+ prometaphase oocytes revealed a failure of the chromatin mass to elongate along the axis of the spindle (see Fig. 1 B; compare top two rows).
Figure 1.
Bipolar spindle assembly and failure of chromatin stretching in oocytes expressing αtub67C P40. A, Drosophila female meiotic spindles were examined by indirect immunofluorescence using antitubulin antibodies. Shown are maximum intensity projections of oocytes heterozygous for the X balancer chromosome, FM7, with either 2 copies of wild-type αtub67C genes (top row) or with one copy of wild-type αtub67C and one copy of the αtub67CP40mutant version of αtub67C (bottom row). Bar, 10 μm. B, FM7/X oocytes with the indicated genotypes were immunolabeled with both antitubulin and anticore histone antibodies and analyzed by confocal microscopy. Shown are maximum intensity projections of the chromatin masses of oocyte meiotic spindles. Chromatin masses from wild-type oocytes are shown in the top row and, in the next two rows, masses from oocytes heterozygous for the αtub67CP40mutation are shown. Chromatin masses from oocytes heterozygous for both nod and αtub67CP40 are shown in the bottom row. Bar, 4 μm. Oocytes were prepared and examined as previously described with minor modifications (Theurkauf and Hawley 1992; Matthies et al. 1996).
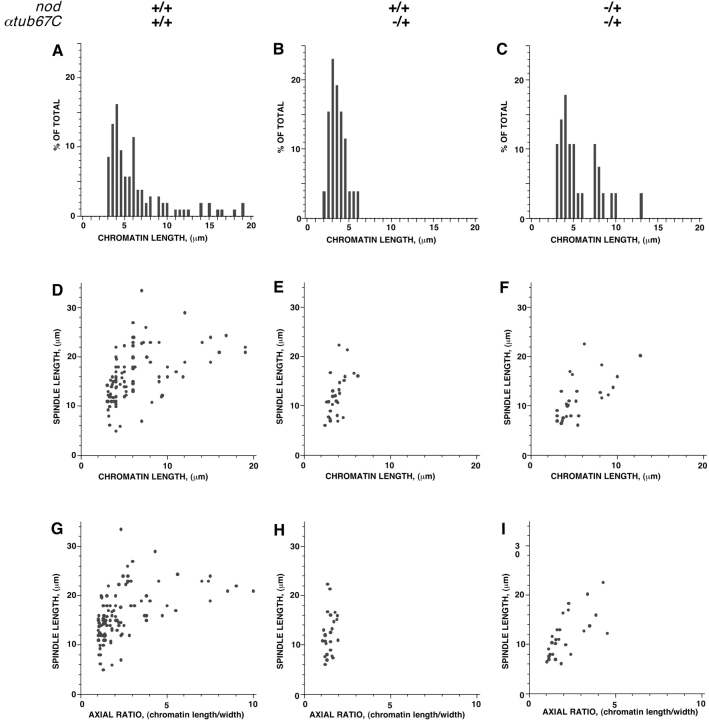
Chromatin masses in wild-type spindles appear almost spherical as spindle assembly initiates, but elongate as spindle assembly progresses. However, in αtub67CP40/+ oocytes, the chromatin remains almost spherical, even on fully elongated spindles. A plot of the chromatin mass length from oocytes with two wild-type copies of the αtub67C gene reveals a wide distribution of chromatin lengths (Fig. 2 A). In contrast, a plot of the chromatin mass length from oocytes heterozygous for αtub67CP40 exhibits a very narrow distribution (Fig. 2 B) that is centered at a much shorter length (Fig. 2 B). The chromatin mass ranged in length from 3.1–18 μm in control oocytes, whereas in FM7/X; αtub67CP40/+ oocytes, chromatin length was observed to vary from 2.4–5.8 μm. Thus, in oocytes heterozygous for the αtub67CP40mutation, the chromatin mass fails to elongate properly.
Figure 2.
Quantitation of the failure of chromatin stretching due to the αtub67C P40 mutation. Shown are plots of chromatin length distributions in the indicated genotypes (A, B, and C), spindle versus chromatin length (D, E, and F), and finally, spindle length versus axial ratio of the chromatin (chromatin length/chromatin width) (G, H, and I).
To determine whether or not the observed defect in chromatin stretching was a consequence of the slightly shorter spindles found in FM7/X; αtub67CP40/+ oocytes (Fig. 2, compare D and E), we calculated the axial ratio (length over width) of the chromatin and plotted this value against spindle length. This metric of axial ratios allowed us to evaluate the stretching of the chromosomes in a manner independent of spindle length. For FM7/X; +/+ oocytes, the axial ratios range from one to ten, whereas in oocytes obtained from FM7/X; αtub67CP40/+ females, they range from one to two (Fig. 2, compare G and H). Thus, even when differences in spindle length are taken into account, oocytes from FM7/X; αtub67CP40/+ females still display an obvious defect in chromatin stretching. Even in those FM7/X; αtub67CP40/+ oocytes with the longest spindles, little or no stretching of the chromatin mass is observed and the chromatin remains almost spherical. Taken together, these data reveal a defect in the stretching of chromosomes during prometaphase in FM7/X; αtub67CP40/+ oocytes, which is clearly distinct from an effect on spindle lengthening.
The Defect in Chromatin Stretching in αtub67CP40/+ Oocytes Is Correlated with Abnormal Centromere Positioning
Lengthening of the spindle and chromatin during meiotic prometaphase is paralleled by the coalescence of a Drosophila centromere-resident protein, MEI-S332, at the most poleward ends of the chromatin mass (Moore et al. 1998; Tang et al. 1998). We examined the distribution of MEI-S332 protein in prometaphase oocytes bearing the αtub67CP40mutation by immunolocalization. In wild-type spindles, the MEI-S332 protein is distributed over the surface of the chromatin in discrete foci during early prometaphase, and coalesces symmetrically at the extreme poleward tips during spindle assembly and elongation (Moore et al. 1998; Tang et al. 1998).
As expected, MEI-S332 protein was found on the poleward edges of the chromatin masses in FM7/X; +/+ spindles (wild-type; see Fig. 3, bottom row). In contrast, the MEI-S332 localization pattern from oocytes derived from FM7/X; αtub67CP40/+ females indicated that the centromeres were positioned abnormally (Fig. 3, top two rows). In no case (0/15) was the MEI-S332 protein properly positioned at the distal tips of the elongating chromatin mass, as is observed in wild-type oocytes (Moore et al. 1998; Fig. 3, bottom row). Rather, MEI-S332 either failed to be completely localized at opposite poles of the main chromosome mass (Fig. 3, top row) or was found entirely on one side of the main chromatin mass (Fig. 3, second row). These cytological studies demonstrate that heterozygosity for the αtub67CP40mutation leads to a defect in both chromatin stretching and centromere positioning.
Figure 3.
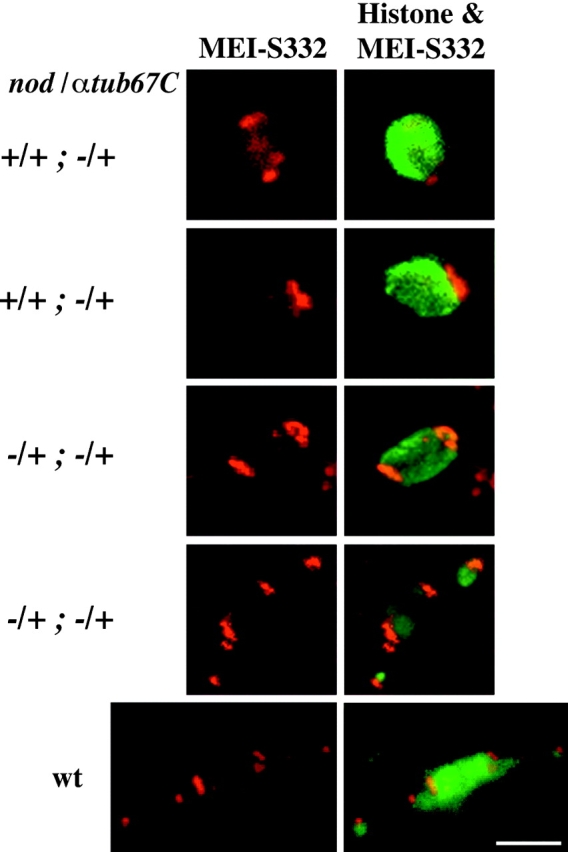
Failure of centromere positioning (MEI-S332 protein) in heterozygous αtub67C P40 mutant oocytes. FM7/X oocytes with the indicated genotypes were immunolabeled with anticore histone, antitubulin, and anti–MEI-S332 antibodies. The first column displays the projections of MEI-S332 alone (red, whereas the second columns displays the projections for both histone [green] and MEI-S332 [red]). In the first column are the projections of the MEI-S332 data, and the second column is the merged projection of the histone and MEI-S332 data. The top two rows depict oocytes heterozygous for αtub67CP40. In the oocyte displayed in the top row, a portion of the MEI-S332 immunoreactivity is found adjacent to the main chromosome mass, whereas the remainder of the protein is detected toward the center of the chromosomal mass. In the second panel, virtually all of the MEI-S332 immunoreactivity remains at one pole. The middle two rows are oocytes heterozygous for both αtub67CP40and nod (nodb17). In each of these oocytes, the MEI-S332 immunoreactivity is seen at opposite poles of the lengthening chromosomal mass, as is observed in wild-type oocytes (bottom row). Bar, 4 μm.
Chromosome Missegregation Is Restricted to Achiasmate Bivalents
The genetic studies of the αtub67CP40 mutation were carried out in two types of females: females that were heterozygous for a normal sequence X chromosome and an FM7 balancer chromosome, denoted FM7/X, and females carrying two normal sequence X chromosomes, denoted X/X. In X/X females the X chromosomes recombine at least once in >90% of these oocytes, while in FM7/X females, the presence of the multiply inverted balancer chromosome reduces the frequency of X chromosomal exchange to <1% of normal (Hawley and Theurkauf 1993). A comparison of the frequency of errors of meiotic segregation in FM7/X and X/X females allowed us to differentially assess the effects of mutations such as αtub67CP40 on the chiasmate and achiasmate segregation systems.
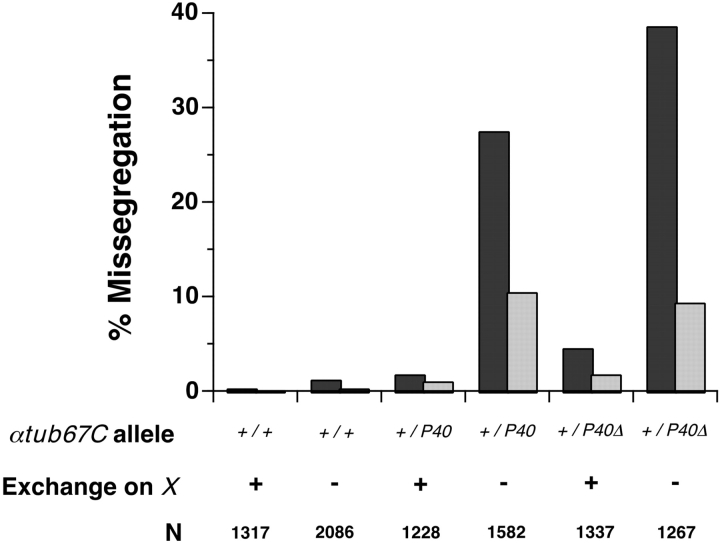
As shown in Fig. 4, FM7/X; αtub67CP40/+ females display 20-fold higher levels of X chromosome nondisjunction than do FM7/X; +/+ control females, suggesting that most, if not all, of these cases of X chromosome nondisjunction are due to a failure of achiasmate segregation. Indeed, even those few nondisjunction events that were observed in X/X; αtub67CP40/+ females occurred in the 5–8% of the oocytes in which the two X chromosomes failed to undergo exchange (data not shown). An additional mutant allele, derived from αtub67CP40and denoted αtub67CP40 Δ, displayed an enhancement of meiotic chromosome missegregation relative to the original P element allele (Fig. 4). The αtub67CP40 Δ allele differs from the original P element insertion only in that a substantial internal portion of the P element has been removed. Heterozygosity for αtub67C P40 Δ, as assayed in FM7/X females, leads to high levels of achiasmate nondisjunction; however, the αtub67CP40 Δ mutant has little or no effect on the segregation of chiasmate X chromosomes (in X/X females). For both of these alleles, the observed chromosome missegregation must primarily be due to nondisjunction rather than loss, as the frequency of diplo-X exceptions always equals or slightly exceeds that of nullo-X exceptions (data not shown).
Figure 4.
The αtub67CP40mutation leads to elevated levels of achiasmate chromosome missegregation. The frequencies of X (dark bars) and 4th (light bars) chromosome missegregation (nondisjunction) are displayed for various genotypes. The genotype at the αtub67C locus is represented as: +/+, two wild-type copies of the αtub67C gene; +/P40, heterozygous for the αtub67CP40 mutation; and +/P40Δ, heterozygous for the αtub67CP40 mutation. These experiments were done in both X/X and X/FM7 females, allowing us to examine segregation in oocytes with (+) or without (−) exchange on the X chromosome, respectively. N is the adjusted total of progeny scored (Hawley et al. 1993). Chromosome segregation was monitored by methods outlined in Hawley et al. 1993 and Sekelsky et al. 1999. Although females homozygous for αtub67CP40are sterile, αtub67CP40Δ/+ females are fully fertile and viable.
FM7/X; αtub67CP40/+ and FM7/X; αtub67CP40 Δ /+ females also display elevated levels of 4th chromosome nondisjunction (again with an approximate equality of diplo-4 and nullo-4 exceptions). In both cases, the effect on 4th chromosome missegregation was less than half of the effect on X chromosome segregation. Two lines of evidence suggest that most of the observed 4th chromosome nondisjunction events are a secondary consequence of failures of X chromosome missegregation and not a direct effect of heterozygosity for the αtub67C mutations. First, 4th chromosome nondisjunction is rarely observed in females bearing structurally normal (i.e., recombining) X chromosomes, despite the fact that the 4th chromosomes themselves are always achiasmate. Second, most of the 4th chromosome nondisjunction occurs in oocytes that were simultaneously nondisjunctional for the X chromosomes, and reflects cases where the two 4th chromosomes segregate away from the two nondisjoining X chromosomes (data not shown).
We also analyzed the meiotic effects of four other female-sterile recessive alleles of the αtub67C gene obtained by Matthews et al. 1993. When heterozygous, these alleles induce only low levels of X or 4th chromosome nondisjunction in females bearing two normal sequence X chromosomes (Komma and Endow 1997). However, in the presence of the FM7 balancer chromosome, heterozygosity for the αtub67C2allele increases X nondisjunction to levels comparable to those observed in FM7/X; αtub67CP40/+ females, whereas the αtub67C1, αtub67C3, and αtub67C4 alleles exhibited intermediate levels (7.1–8.2%) of X chromosome (data not shown). These data demonstrate that disruption of achiasmate segregation is a property of many alleles of the αtub67C locus. The dominant effects of the αtub67C alleles appear to be antimorphic, since heterozygosity for a deficiency of the αtub67C locus (Df(3L)AC1) had no effects on achiasmate segregation (data not shown).
The Effect of the αtub67C Mutations on Achiasmate Chromosome Segregation Is Suppressed by Decreasing the Dose of the Nod Chromokinesin Protein
The Nod kinesin-like protein is specifically required for achiasmate segregation in Drosophila (Carpenter 1973; Zhang and Hawley 1990; Zhang et al. 1990). Homozygosity for loss-of-function nod mutations specifically causes achiasmate chromosomes to nondisjoin or to be lost at high frequencies (Carpenter 1973; Zhang and Hawley 1990). Cytological studies of prometaphase oocytes from homozygous nod females reveal that achiasmate chromosomes are found precociously at the poles of the developing meiotic spindle and frequently dissociate from the spindle (Theurkauf and Hawley 1992). During prometaphase, Nod protein is localized along the entire lengths of oocyte chromosomes (Afshar et al. 1995a,Afshar et al. 1995b). Here, it acts to retard the poleward migration of achiasmate chromosomes and serves, in essence, as a stabilizing plateward force.
As shown in Fig. 5 A, heterozygosity for a recessive loss-of-function mutation of nod (nodb17) strongly suppressed the meiotic effects of the αtub67CP40 and αtub67CP40 Δ alleles. All four of the αtub67C mutations isolated by Matthews et al. 1993 are also suppressed by heterozygosity for a loss-of-function nod allele (data not shown). This effect is not allele-specific with respect to nod, because all three nod alleles tested, including a deficiency (Df(1)nod), exhibit a similar capability to suppress the phenotype of the αtub67CP40allele. These data are consistent with the view that the relative abundance of functional αtub67C and Nod proteins is critical to the faithful segregation of achiasmate chromosomes.
Figure 5.
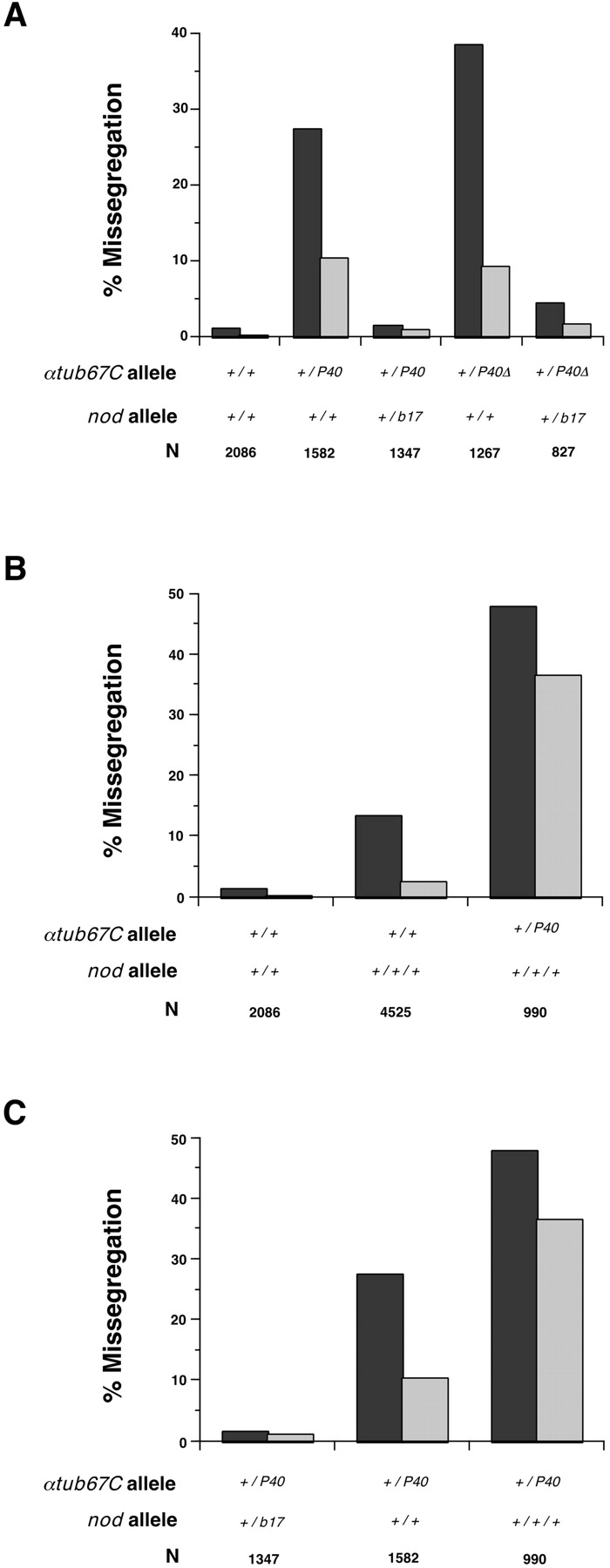
The fidelity of achiasmate chromosome segregation is sensitive to the dose of both Nod and αtub67C proteins. Dark and light bars represent the frequencies of X and 4th chromosome missegregation, respectively, in the indicated genotypes. A, The effects of heterozygosity for αtub67C mutations on chromosome missegregation can be suppressed by heterozygosity for a loss-of-function allele of nod (nodb17). B, Increasing the dose of nod + to three copies (by use of a duplication) increases the frequency of X and 4th chromosomal missegregation, both in the females carrying two normal copies of the αtub67C gene, and, to an even greater extent, in females heterozygous for a mutation in the αtub67C gene. This enhancement of missegregation by the nod + duplication can be eliminated by using females that simultaneously bear a nod mutation on one of the two X chromosomes (i.e., flies that only carry two copies of nod +, despite carrying the nod + duplication; data not shown). This demonstrates that the enhancement observed in the presence of the duplication is due to the extra copy of nod +, since both X and 4th chromosome nondisjunction occur with the same frequencies in flies carrying two alleles of nod +, regardless of whether one comes from a duplication or from both the endogenous genes on the same chromosome. C, Comparison of the effects of one, two, and three doses of nod + in FM7/X; +/P40 females. N is the adjusted total of progeny scored (Hawley et al. 1993).
To test this hypothesis further, we measured chromosome segregation in oocytes with various levels of nod + and αtub67C. We kept tubulin levels constant, and tested the effect of increasing the dose of nod +. Using a duplication of nod +, we showed that three wild-type copies of nod + in an otherwise wild-type background increased chromosome missegregation (see Fig. 5 B). The frequencies of X and 4th missegregation were lower than, but qualitatively similar to, the effect on achiasmate chromosome nondisjunction observed in oocytes from females heterozygous for αtub67CP40. We also compared the effect of three copies of nod + in the presence of different levels of wild-type αtub67C. X and 4th chromosome nondisjunction levels were substantially elevated in FM7/X; αtub67CP40/+ females carrying the nod + duplication, compared with flies with the nod duplication and two wild-type copies of αtub67C (Fig. 5 B). Finally, we determined the effect of varying the number of copies of nod + in the presence of constant levels of wild-type αtub67C, i.e., in αtub67CP40/+ oocytes. Again, in the presence of comparable αtub67CP40levels, the levels of chromosome missegregation showed an almost linear relationship with the levels of nod (Fig. 5 C).
The Defect in Chromatin Stretching and Centromere Positioning can be Suppressed by Reducing the Number of Copies of the nod+ Gene
Reducing the copy number of nod + suppresses the chromosome missegregation in oocytes from FM7/X; αtub67CP40/+ females. Therefore, we determined what effect a loss-of-function allele of nod (nodb17) has on the cytological defects observed in FM7/X; αtub67CP40/+ oocytes. We measured chromatin mass and spindle lengths in oocytes derived from FM7, nodb17/X; αtub67CP40/+ females (Fig. 1 B, bottom row and Fig. 2C, Fig. F, and Fig. I), and found that chromatin mass length in FM7, nodb17/X; αtub67CP40/+ oocytes paralleled those of wild-type and ranged in length from 3–12.5 μm, with no chromatin mass shorter than 3 μm (Fig. 2, compare A and C). Plotting of the chromatin length or axial ratio versus the spindle length demonstrated a parallel elongation of chromatin and spindles in nod-suppressed oocytes (Fig. 2, compare D–F and G–I). Heterozygosity for nod with two copies of wild-type αtub67C has no effect on chromatin mass elongation (data not shown). Moreover, in FM7, nodb17/X; αtub67CP40/+ oocytes, MEI-S332 protein is once again normally localized (Fig. 3, third and fourth rows).
Reducing Nod levels restores the ability of chromatin to be elongated in mature spindles (Fig. 2, G–I) and restores proper centromere positioning. However, the effect of the αtub67CP40mutation on decreasing overall spindle length (average = 12.2 μm) in oocytes from αtub67CP40/+ females was not suppressed by reducing the dosage of nod + (average spindle length = 11.1 μm). Thus, reducing the dose of nod + can suppress the chromosome missegregation phenotype, the defect in chromatin elongation, and centromere mispositioning, but does not suppress the reduction in spindle length created by αtub67CP40. Altering Nod dosage in oocytes bearing the αtub67C mutations indicates that Nod plays a role in centromere positioning; this effect would have been impossible to detect using loss-of-function nod alleles alone.
Discussion
A Model in Which the Balancing Of Forces Is Required for the Segregation of Achiasmate Chromosomes
Our data demonstrate that the fidelity of achiasmate chromosome segregation is sensitive to the relative levels of functional αtub67C and Nod proteins. The consequences of altering the level of one of these two proteins are ameliorated or exacerbated by changes in the level of the other. We propose that when the level of wild-type αtub67C protein is reduced, the poleward force(s) are compromised, and this leads to a failure of chromatin elongation and centromere positioning.
We can imagine four mechanisms to explain the disruption of poleward forces by αtub67C mutations. The first proposes that the presence of the aberrant αtub67CP40 subunits results in reduced production of poleward force from a minus end directed microtubule-based motor: a kinesin or a dynein (Komma and Endow 1997; Starr et al. 1998). Failure of these motors to generate poleward force could prevent the migration of kinetochores and/or the elongation of chromosome arms. The second hypothesis suggests reduced coupling between a plus end directed kinesin at the kinetochore and kinetochore microtubules (Duesbery et al. 1997; Schaar et al. 1997; Wood et al. 1997). This could lead to poor coupling of kinetochore microtubules to the kinetochore, resulting in a failure of the mechanism that actually provides force for poleward kinetochore motility. A third possibility is that a plus end directed motor (Kashina et al. 1996) responsible for sliding antiparallel microtubules is compromised and this prevents sliding of microtubules bound to the chromatin. Finally, the observed impairment in poleward movement may reflect reduced binding of a microtubule-associated protein(s) or kinesin that regulates poleward flux (Wilson et al. 1994; Wilson and Forer 1997; Desai et al. 1998) or microtubule dynamics (Karabay and Walker 1999). Nod could also play a role in microtubule dynamics, and thereby regulate poleward and antipoleward forces.
As noted above, cytological and genetic studies are consistent with a model in which Nod functions in wild-type spindles to provide both a plateward force and a function which is important for centromere positioning. When poleward forces and chromatin stretching are reduced by heterozygosity for αtub67CP40, decreasing the dose of nod + ameliorates this defect. We speculate that this suppression occurs because the reduced plateward forces now more closely equal the impaired poleward forces. Similarly, increasing the amount of Nod in both wild-type and in αtub67CP40/+ oocytes should, and more importantly does, increase the frequency of meiotic errors in oocytes. These observations are consistent with a model in which Nod protein serves as a stabilizing plateward force for the segregation of achiasmate chromosomes, and that this force is balanced by poleward forces which are dependent on the level of functional αtub67C protein.
The model presented in the preceding paragraph does not require the physical interaction of the αtub67C and Nod proteins, only their separate roles in creating opposing forces. It is, however, at least possible that the two proteins do indeed physically interact. In this case, the observed defects in chromosome and centromere movements in oocytes carrying mutations in αtub67C gene might be the result of poisonous or rigor-like interactions between Nod protein bound to the chromatin and the mutant αtub67C protein.
The observation that the effects of these α-tubulin mutations is restricted to achiasmate chromosomes is puzzling in terms of the more global defects observed cytologically. We can only surmise that the presence of chiasmata is prophylactic to the kinds of errors created by these tubulin mutations. Perhaps the types of kinetochore orientation mechanisms that successfully ensure the segregation of chiasmate bivalents are to some degree more fail-safe than those ensuring the segregation of achiasmate homologues.
Acknowledgments
We thank Drs. Kenneth Burtis, Don Cleveland, Larry Goldstein, Frank McNally, Jodi Nunnari, Kim McKim, Lesilee Rose, Jon Scholey, Bill Theurkauf, Jeff Sekelsky, and all of the members of the Hawley and Scholey laboratories, especially Christina Boulton, for stimulating discussions. We especially would like to thank Drs. Tracy Tang and Terry Orr-Weaver, who generously supplied the MEI-S332 antibody, and Dr. Kathy Matthews for various alleles of αtub67C.
This work was supported by a grant from the National Institutes of Health to R.S. Hawley.
Footnotes
Heinrich J.G. Matthies and Lisa G. Messina contributed equally to this manuscript.
Abbreviation used in this paper: αtub67C, α-tubulin 67C gene.
References
- Carpenter A.T. A meiotic mutant defective in distributive disjunction in Drosophila melanogaster . Genetics. 1973;73:393–428. doi: 10.1093/genetics/73.3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dernburg A.F., Sedat J.W., Hawley R.S. Direct evidence of a role for heterochromatin in meiotic chromosome segregation. Cell. 1996;86:135–146. doi: 10.1016/s0092-8674(00)80084-7. [DOI] [PubMed] [Google Scholar]
- Desai A., Maddox P.S., Mitchison T.J., Salmon E.D. Anaphase A chromosome movement and poleward spindle microtubule flux occur at similar rates in Xenopus extract spindles. J. Cell Biol. 1998;141:703–713. doi: 10.1083/jcb.141.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesbery N.S., Choi T., Brown K.D., Wood K.W., Resau J., Fukasawa K., Cleveland D.W., Vande Woude G.F. CENP-E is an essential kinetochore motor in maturing oocytes and is masked during mos-dependent, cell cycle arrest at metaphase II. Proc. Natl. Acad. Sci. USA. 1997;94:9165–9170. doi: 10.1073/pnas.94.17.9165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley R.S., Theurkauf W.E. Requiem for the distributive systemachiasmate segregation in Drosophila females. Trends Gen. 1993;9:310–317. doi: 10.1016/0168-9525(93)90249-h. [DOI] [PubMed] [Google Scholar]
- Hawley R.S., Irick H., Zitron A.E., Haddox D.A., Lohe A., New C., Whitley M., Arbel T., Jang J., McKim K. There are two mechanisms of achiasmate segregation in Drosophila, one of which requires heterochromatic homology. Dev. Gen. 1993;13:440–467. doi: 10.1002/dvg.1020130608. [DOI] [PubMed] [Google Scholar]
- Kalfayan L., Wensink P.C. Developmental regulation of Drosophila α-tubulin genes. Cell. 1982;29:91–98. doi: 10.1016/0092-8674(82)90093-9. [DOI] [PubMed] [Google Scholar]
- Karabay A., Walker R.A. The Ncd tail domain promotes microtubule assembly and stability. Biochem. Biophys. Res. Comm. 1999;258:39–43. doi: 10.1006/bbrc.1999.0572. [DOI] [PubMed] [Google Scholar]
- Karpen G.H., Le M.H., Le H. Centric heterochromatin and the efficiency of achiasmate disjunction in Drosophila female meiosis. Science. 1996;273:118–122. doi: 10.1126/science.273.5271.118. [DOI] [PubMed] [Google Scholar]
- Kashina A.S., Baskin R.J., Cole D.G., Wedaman K.P., Saxton W.M., Scholey J.M. A bipolar kinesin. Nature. 1996;379:270–272. doi: 10.1038/379270a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komma D.J., Endow S.A. Enhancement of the ncdD microtubule motor mutant by mutants of α-Tub67C. J. Cell Sci. 1997;110:229–237. doi: 10.1242/jcs.110.2.229. [DOI] [PubMed] [Google Scholar]
- Matthews K.A., Rees D., Kaufman T.C. A functionally specialized α-tubulin is required for oocyte meiosis and cleavage mitoses in Drosophila . Development. 1993;117:977–991. doi: 10.1242/dev.117.3.977. [DOI] [PubMed] [Google Scholar]
- Matthies H.J., McDonald H.B., Goldstein L.S., Theurkauf W.E. Anastral meiotic spindle morphogenesisrole of the nonclaret disjunctional kinesin-like protein. J. Cell Biol. 1996;134:455–464. doi: 10.1083/jcb.134.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D.P., Page A.W., Tang T.T., Kerrebrock A.W., Orr-Weaver T.L. The cohesion protein MEI-S332 localizes to condensed meiotic and mitotic centromeres until sister chromatids separate. J. Cell Biol. 1998;140:1003–1012. doi: 10.1083/jcb.140.5.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas R.B. Chromosome segregation mechanisms. Genetics. 1974;78:205–213. doi: 10.1093/genetics/78.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklas R.B. How cells get the right chromosomes. Science. 1997;275:632–637. doi: 10.1126/science.275.5300.632. [DOI] [PubMed] [Google Scholar]
- Schaar B.T., Chan G.K., Maddox P., Salmon E.D., Yen T.J. CENP-E function at kinetochores is essential for chromosome alignment. J. Cell Biol. 1997;139:1373–1382. doi: 10.1083/jcb.139.6.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekelsky J.J., McKim K.S., Messina L., Arbel T., Chin G., French R.L., Hari K.L., Hurley W., Jang J.-K., Laurençon A. Identification of novel meiotic genes recovered in a P element screen. Genetics. 1999;152:529–542. doi: 10.1093/genetics/152.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr D.A., Williams B.C., Hays T.S., Goldberg M.L. ZW10 helps recruit dynactin and dynein to the kinetochore. J. Cell Biol. 1998;142:763–774. doi: 10.1083/jcb.142.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang T.T.L., Bickel S.E., Young L.M., Orr-Weaver T.L. Maintenance of sister-chromatid cohesion at the centromere by the Drosophila MEI-S332 protein. Genes Dev. 1998;12:3843–3856. doi: 10.1101/gad.12.24.3843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W.E., Hawley R.S. Meiotic spindle assembly in Drosophila femalesbehavior of nonexchange chromosomes and the effects of mutations in the Nod kinesin-like protein. J. Cell Biol. 1992;116:1167–1180. doi: 10.1083/jcb.116.5.1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theurkauf W.E., Baum H., Bo J., Wensink P.C. Tissue-specific and constitutive α-tubulin genes of Drosophila melanogaster code for structurally distinct proteins. Proc. Natl. Acad. Sci. USA. 1986;83:8477–8481. doi: 10.1073/pnas.83.22.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson P.J., Forer A. Effects of nanomolar taxol on crane-fly spermatocyte spindles indicate that acetylation of kinetochore microtubules can be used as a marker of poleward tubulin flux. Cell Motil. Cytoskel. 1997;37:20–32. doi: 10.1002/(SICI)1097-0169(1997)37:1<20::AID-CM3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Wilson P.J., Forer A., Leggiadro C. Evidence that kinetochore microtubules in crane-fly spermatocytes disassemble during anaphase primarily at the poleward end. J. Cell Sci. 1994;107:3015–3027. doi: 10.1242/jcs.107.11.3015. [DOI] [PubMed] [Google Scholar]
- Wood K.W., Sakowicz R., Goldstein L.S., Cleveland D.W. CENP-E is a plus end-directed kinetochore motor required for metaphase chromosome alignment. Cell. 1997;91:357–366. doi: 10.1016/s0092-8674(00)80419-5. [DOI] [PubMed] [Google Scholar]
- Zhang P., Hawley R.S. The genetic analysis of distributive segregation in Drosophila melanogaster. II. Further genetic analysis of the nod locus. Genetics. 1990;125:115–127. doi: 10.1093/genetics/125.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang P., Knowles B.A., Goldstein L.S., Hawley R.S. A kinesin-like protein required for distributive chromosome segregation in Drosophila . Cell. 1990;62:1053–1062. doi: 10.1016/0092-8674(90)90383-p. [DOI] [PubMed] [Google Scholar]