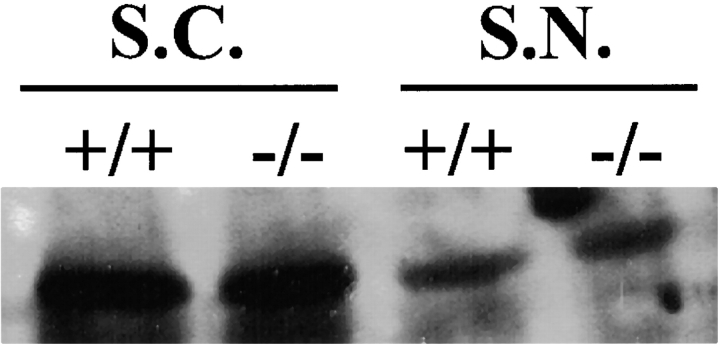Abstract
Mice incapable of synthesizing the abundant galactolipids of myelin exhibit disrupted paranodal axo-glial interactions in the central and peripheral nervous systems. Using these mutants, we have analyzed the role that axo-glial interactions play in the establishment of axonal protein distribution in the region of the node of Ranvier. Whereas the clustering of the nodal proteins, sodium channels, ankyrinG, and neurofascin was only slightly affected, the distribution of potassium channels and paranodin, proteins that are normally concentrated in the regions juxtaposed to the node, was dramatically altered. The potassium channels, which are normally concentrated in the paranode/juxtaparanode, were not restricted to this region but were detected throughout the internode in the galactolipid-defi- cient mice. Paranodin/contactin-associated protein (Caspr), a paranodal protein that is a potential neuronal mediator of axon-myelin binding, was not concentrated in the paranodal regions but was diffusely distributed along the internodal regions. Collectively, these findings suggest that the myelin galactolipids are essential for the proper formation of axo-glial interactions and demonstrate that a disruption in these interactions results in profound abnormalities in the molecular organization of the paranodal axolemma.
Keywords: paranodin, potassium channels, sodium channels, galactolipids, axo-glial interactions
Myelinated axons contain regularly spaced unmyelinated gaps known as nodes of Ranvier, which are critical for the proper function of the central nervous system (CNS) and the peripheral nervous system (PNS) (Morell et al. 1994). Both the structure and molecular organization of the nodal region are dependent on the formation of the appropriate axo-glial interactions. Structurally, these interactions establish the spacing of adjacent myelin segments and ultimately determine the position and length of the nodal gaps. In addition, septate-like junctions are formed at the interface between the myelin sheath and axon in the paranode (for review see Salzer 1997). Based on the location of these axo-glial junctions, Rosenbluth 1990 proposed that they function in the establishment and maintenance of axolemmal protein domains of the nodal and paranodal regions.
The most recognized domains of the nodal/paranodal region are those of the sodium and potassium channels. The positioning and segregation of these channels along the axonal membrane of myelinated fibers is crucial for the rapid conduction of nerve impulses in both the CNS and PNS (Black et al. 1990). Voltage-gated sodium channels, which are concentrated at the node of Ranvier are mainly responsible for the axonal depolarization that is required for action potential induction (for review see Ritchie 1995). In the paranodal and adjacent juxtaparanodal regions, Shaker-type Kv1.1 potassium channels cluster (Wang et al. 1993). The function of these channels is not completely understood, but they appear to prevent aberrant neuronal firing during development (Vabnick et al. 1999) and may modulate action potential duration and frequency (Hille 1992; Smart et al. 1998).
In addition to the sodium and potassium channels, other axolemmal proteins are also clustered in the region of the node of Ranvier of mature, myelinated axons (for review see Scherer 1996). Several of these proteins are initially distributed along the entire axon of unmyelinated fibers. Although these proteins become relocated to the nodal region, the mechanisms that mediate these events are poorly understood. Nodal clustering of neurofascin, an L1-associated protein that is tightly coupled to the cytoskeleton (Tuvia et al. 1997), precedes the elaboration of compact myelin and may play an important role in defining the site of node formation (Lambert et al. 1997). In contrast, the clustering of ankyrinG, a nodal protein that is associated with sodium channel distribution (Kordeli et al. 1990), appears to be dependent on the presence of the myelin sheath (Lambert et al. 1997). Similarly, paranodin, a 180–190-kD neuronal glycoprotein that is a potential cell adhesion molecule, accumulates in the paranodal axolemma after myelination (Einheber et al. 1997; Menegoz et al. 1997) and is a component of the paranodal septate-like junctions that may be involved in establishment and maintenance of the protein domains of the nodal and paranodal axolemma (Rosenbluth 1990). This protein was also identified as a partner of contactin, a glycosyl-phosphatidyl-inositol–anchored adhesion molecule, and termed contactin-associated protein (Caspr) (Peles et al. 1997). Nevertheless, the neuronal and/or glial molecules that are responsible for its localization in the paranodal junctions are not known.
To further explore the role that axo-glial interactions play in the organization of axolemmal nodal proteins, we have analyzed the distribution of these proteins in mice that contain a disruption in the gene that encodes UDP-galactose–ceramide galactosyltransferase (CGT), an enzyme that is essential for the production of galactocerebroside (GalC) and sulfatide (Morell and Radin 1969). The galactolipid-deficient mice display several CNS nodal and paranodal abnormalities, including increased heminode formation, altered node length, and terminal loops that frequently face away from the axon (Dupree et al. 1998; Popko 2000). Moreover, the complete absence of CNS and PNS transverse bands, which are prominent components of the paranodal septate-like junctions, indicates that axo-glial interactions are severely disrupted in the galactolipid-deficient animals (Dupree et al. 1998; Dupree and Popko 1999). These studies described here should enhance our understanding of the role that axo-glial interactions play in the organization of axolemmal proteins.
Materials and Methods
Immunocytochemical Analysis
Spinal cords (C3) and sciatic nerves were harvested from 30-d-old CGT+/+ and CGT−/− mice that were perfused through the heart with either 4 or 1% paraformaldehyde in 0.1 M phosphate buffer (PB), pH 7.3. Spinal cords were postfixed for 1 h in the same fixative, cryopreserved in 30% sucrose in PB for 48 h at 4°C and sectioned at 10 μm. Sciatic nerves were teased apart to yield single fiber preparations. Spinal cord sections and sciatic teased fibers were blocked for 1 h in PB containing 10% normal goat serum, 5% fish gelatin, and 0.1% Triton X-100 and incubated in the primary antibody overnight at 4°C. The primary antibodies used in this study were directed against ankyrinG (mouse monoclonal, Zymed and gift from Dr. Van Bennett, Duke University, Durham, NC) (Kordeli et al. 1990), neurofascin (rabbit polyclonal, gift from Dr. Van Bennett) (Lambert et al. 1997), paranodin (rabbit polyclonal) (Menegoz et al. 1997), potassium channel Kv1.1 (rabbit polyclonal, gift from Dr. Bruce Tempel, University of Washington School of Medicine, Seattle, WA; mouse monoclonal, Upstate Biotechnology) (Wang et al. 1995), sodium channels (rabbit polyclonal, gift from Dr. Rock Levinson, Health Sciences Center, Denver, CO) (Dugandzija-Novakovic et al. 1995), and myelin basic protein (mouse monoclonal, Sternberger Monoclonal, Inc.; rabbit polyclonal, Dako, Inc.). After primary antibody incubation, the tissue was thoroughly rinsed in PB, blocked for 1 h as described previously, and incubated in the appropriate secondary antibody conjugated to biotin or Texas red for 2 h. After rinsing the tissue four times for 5 min, the tissue incubated in the secondary antibody conjugated to biotin was incubated in streptavidin fluorescein (diluted 1:200) for 30 min and thoroughly rinsed. The tissue was coverslipped using Vectashield (Vector Labs, Inc.). For double-labeling, the above procedure was repeated using a different species-specific antibody and the appropriate secondary antibody conjugated to the proper fluorochrome. When myelin basic protein was the second antigen to be labeled, the tissue was postfixed in 4% paraformaldehyde in PB for 10 min at −20°C, rinsed, and permeabilized in −20°C acetone for 10 min. All labeled slides were analyzed by both conventional fluorescent and scanning confocal microscopy. Images were generated on a Leica TCS-NT Laser Scanning microscope using a 40× oil objective and a pinhole size of 1.0 Airy disk units.
Western Blot Analysis
Spinal cord and sciatic nerve tissues were harvested from 30-d-old galactolipid-deficient and wild-type mice, diced into small pieces, homogenized in 1% SDS in PB, and placed in a boiling water bath for 10 min. Insoluble material was removed by centrifugation at 5,000 g for 10 min. Protein concentrations were determined using a modified Lowry assay (Bio-Rad, Inc.). 100 μg of protein was separated using a precast 4–15% gradient polyacrylamide gel (Jule, Inc.), transferred to nitrocellulose, and stained with Ponceau S. The nitrocellulose was blocked for 1 h in 5% milk solids, 5% normal goat serum, and 0.1% Triton X-100 in PB, incubated in antiparanodin (1:3,000) for 1 h at room temperature, rinsed in PB, blocked, incubated in goat anti–rabbit secondary antibody conjugated to HRP, rinsed in PB, and visualized using ECL™ according to the manufacturer's instructions (Amersham Pharmacia Biotech).
Results
Distribution of Ion Channels
To better understand the consequences that altered axo-glial interactions of the CNS (Dupree et al. 1998) and PNS (Dupree and Popko 1999) have on the establishment of ion channel domains, we analyzed the distribution of the nodally clustered voltage-gated sodium channels and the paranodal/juxtaparanodal Kv1.1 potassium channels in the CGT-deficient mice. In both the mutant and wild-type mice, CNS and PNS sodium channels were concentrated in small regions that were presumptive nodes of Ranvier (Fig. 1). Similar results have been reported previously regarding sodium channel distribution in the PNS of galactolipid-deficient mice (Bosio et al. 1998). Since we reported previously that CNS nodal length is increased in the galactolipid-deficient mice (Dupree et al. 1998), we measured the length and the width of the sodium channel domain and report node length as a function of axon caliber. In the CNS, the length to width ratio was significantly greater in the mutant (1.17 ± 0.05; n = 3 mice and 36 nodes; P < 0.02 by t test) compared with littermate wild-type (0.75 ± 0.13; n = 3 mice and 35 nodes) mice, indicating that the sodium channel domains, which likely corresponds to nodal length, were increased in the galactolipid-deficient animals.
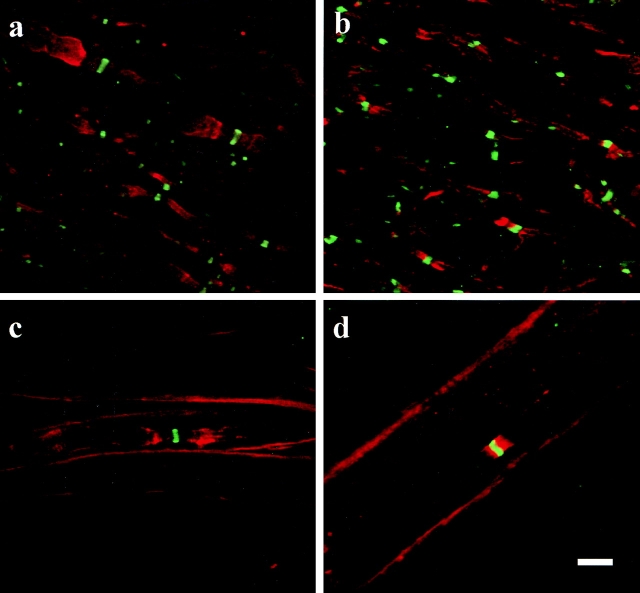
Figure 1.
(a) In the spinal cord of wild-type mice, potassium channels (red) are concentrated in the juxtaparanodes, and to a lesser extent, in the paranode, whereas the sodium channels (green) are restricted to the nodes of Ranvier. (b) In contrast, the potassium channels rarely accumulate in the juxtaparanodes of spinal cord tissue from the galactolipid-deficient mice. However, similar to the wild-type, the sodium channels cluster in the nodes but the sodium channel domains are slightly longer in the mutant as compared with the wild-type. The distribution of the potassium and sodium channels in the PNS is similar to the CNS for both the wild-type (c) and the mutant (d) mice. Note that in the CNS and the PNS of the galactolipid-deficient mice the channel domains occasionally overlap (yellow). a and b were generated as the compilation of eight consecutive images each 0.4 μm apart. c and d were generated as the compilation of 10 images each 0.26 μm apart. Bar, 5 μm.
In contrast, potassium channel distribution was dramatically altered in the CNS of the CGT-deficient mice. In the CNS and PNS of wild-type mice, intense labeling was commonly observed in the juxtaparanodal region (Fig. 1, a and c; Table ). The juxtaparanode was easily distinguished from the paranodal and nodal regions, since its diameter was conspicuously larger. Potassium channel antibody reactivity was occasionally observed in the paranodal region; however, the labeling intensity was greatly reduced. In the CNS of the mutant animals, fewer juxtaparanodal regions were immunolabeled with the Kv1.1 antibody (Fig. 1 b), whereas diffuse labeling over long stretches of axons was occasionally observed (see Fig. 3 b). When paranodal/juxtaparanodal potassium channel accumulations were present, the width of the labeling pattern did not change, indicating that the diameter of these axons does not change at the interface between the paranode and juxtaparanode in the galactolipid-deficient mice. In the PNS, paranodal/juxtaparanodal regions were typically labeled, although the labeling was frequently less intense and more diffuse as compared with the wild-type sciatic nerve fibers (Fig. 1c and Fig. d).
Table 1.
Quantitation of Potassium Channel Distribution
| Percent of total | ||||
|---|---|---|---|---|
| ND | Internodal | Paranodal | Juxtaparanodal | |
| CNS | ||||
| +/+ | 10.3 | 4.8 | 13.3 | 71.6 |
| −/− | 25.8 | 35.0 | 37.5 | 1.7 |
| PNS | ||||
| +/+ | 0.0 | 7.7 | 0.0 | 92.3 |
| −/− | 11.5 | 7.7 | 80.8 | 0.0 |
The quantitation of Kv1.1 potassium channel localization demonstrates that the channels are concentrated in the CNS and PNS juxtaparanodal regions in the wild-type (+/+) but not in the galatolipid-deficient (−/−) mice. In the CNS of the −/− animals, potassium channels are often not detected (ND), diffusely distributed throughout the internode, or restricted to the paranode but rarely limited to the juxtaparanodal region. In the mutant PNS, the channels were predominately concentrated in the paranodal region. For the CNS, 128 nodes from five wild-type mice and 120 nodes from four mutant mice were analyzed. For the PNS, 26 nodes from three mice were analyzed for both groups.
Figure 3.
Western blot analysis revealed no difference in the expression of paranodin between the wild-type (+/+) and the mutant (−/−) mice in either the spinal cord (S.C.) or the sciatic nerve (S.N.).
To verify the location of the sodium and potassium channel accumulations, we double-labeled CNS and PNS tissues with antibodies directed against the ion channels (Fig. 1). The findings from these double-labeling experiments supported the single immunolabeling experimental observations that the sodium channel distribution was not altered in the mutants, since these channels maintained a nodal localization in both the CNS and PNS. Using the sodium channel clustering as an indicator of node position, we have quantitatively demonstrated that the distribution of potassium channels is dramatically altered in both the CNS and PNS (Table ). In wild-type mice, potassium channels were primarily restricted to the juxtaparanodal regions with a minority of axons exhibiting paranodal distribution. In the galactolipid-deficient mice, the channels were rarely limited to the juxtaparanode. Instead, the potassium channels in the CNS were frequently clustered in the paranodal region, diffusely distributed along the internode, or were not detected, whereas in the PNS they were primarily observed only in the paranodes. In addition to facilitating the potassium channel quantitative analysis, the double-labeling approach also revealed that the prominent separation between the ion channel domains in the wild-type tissue is frequently absent in the mutant. In the mutant mice the potassium and sodium channel domains occasionally overlapped (Fig. 1).
Distribution of Cell Adhesion and Cell Adhesion–associated Molecules
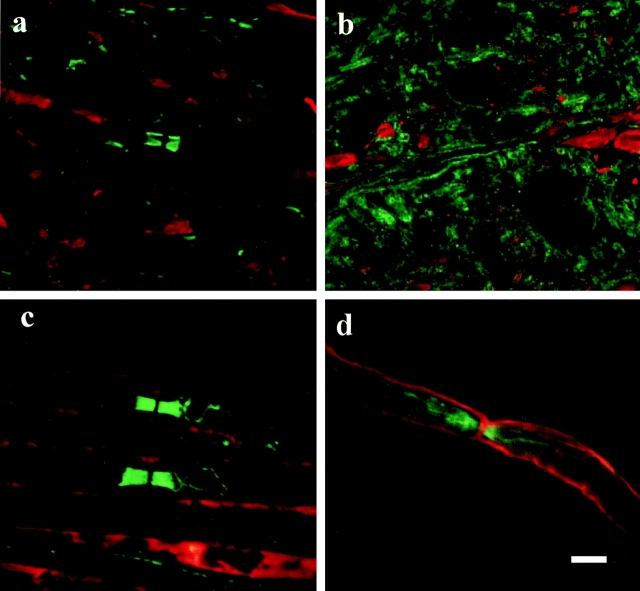
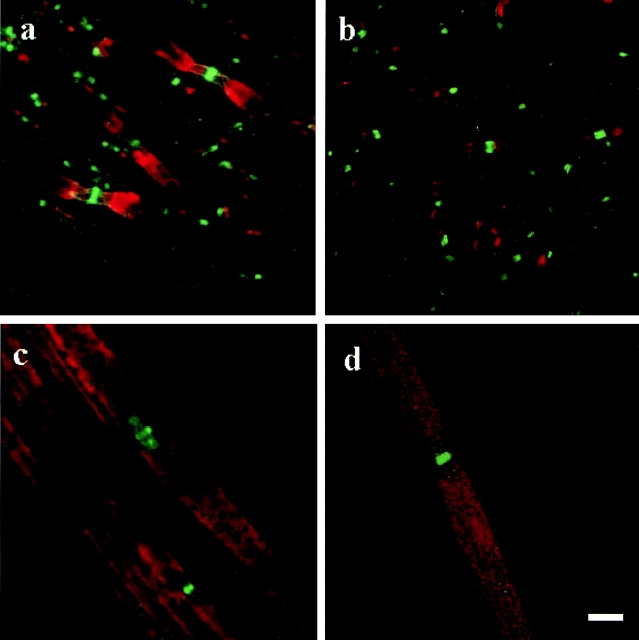
The structural abnormalities at the node of the galactolipid-deficient mice appear to be related to compromised axo-glial interactions, such that we have analyzed the distribution of two potential neuronal adhesion molecules: paranodin and neurofascin. In addition, we have determined the distribution of the cytoskeleton-associated molecule ankyrinG. Using a combination of immunocytochemical techniques and confocal microscopy, we demonstrated the complete absence of paranodin accumulation in the paranodal regions of the myelinated fibers of the spinal cord in the CGT−/− mouse (Fig. 2, a and b). In the galactolipid mutants, paranodin appeared to be diffusely distributed along the axon (Fig. 2c and Fig. d), resembling the expression pattern of unmyelinated fibers (Einheber et al. 1997). In the sciatic nerve, paranodin was localized to the paranodal region; however, the staining intensity was reduced and the border between the paranode and the juxtaparanode was not as clearly defined as in the wild-type sciatic nerve (Fig. 2c and Fig. d). Paranodin was not detected in the paranode of any of the CNS fibers examined and reduced accumulations of paranodin were always observed in the paranode of the PNS fibers observed. Western blot analysis revealed no difference in the level of paranodin expression between the galactolipid mutant and wild-type animals for either the spinal cord or the sciatic nerve (Fig. 3), indicating that the diminished immunoreactivity was a result of abnormal paranodal accumulations. In contrast, the distribution of the nodal proteins neurofascin (Fig. 4) and ankyrinG (data not shown) did not appear altered in either the spinal cord or the sciatic nerve of the mutant.
Figure 2.
In the wild-type mice paranodin (green) is highly concentrated in the paranodal regions of spinal cord (a) and sciatic nerve (c) axons. In contrast, the galactolipid-deficient mice exhibit a more diffuse labeling pattern. In the mutant spinal cord (b) paranodin is evenly distributed in the axolemma throughout the internode. In the sciatic nerve (d) of these mice, paranodin is concentrated in the paranode but the interface between the paranode and the juxtaparanode is not clearly demarcated. a and b, eight images 0.26 μm apart; double-labeled for paranodin in green and phosphorylated neurofilament in red. c and d, eight images 0.4 μm apart; double-labeled for paranodin in green and myelin basic protein in red. Bar, 5 μm.
Figure 4.
In the spinal cord, neurofascin (green) was restricted to the nodes of Ranvier in both the wild-type (a) and the mutant (b) mice. a and b are also labeled for the potassium channels (red) in an effort to assist with the recognition of nodal/paranodal regions. In the sciatic nerve (c and d), neurofascin also is concentrated in the node; however, some protein is also located in the paranodal regions of the wild-type (c) and mutant (d) mice. a and b, six images 0.31 μm apart; double-labeled for neurofascin in green and potassium in red. c and d, four images 0.26 μm apart; double-labeled for neurofascin in green and myelin basic protein in red. Bar, 5 μm.
Discussion
We reported previously that mice incapable of synthesizing the myelin galactolipids GalC and sulfatide exhibit structural abnormalities of the nodal and paranodal regions that are likely due to compromised axo-glial interactions (Dupree et al. 1998; Dupree and Popko 1999). In this study, we demonstrate that in the CNS, the distribution of the Shaker-type Kv1.1 potassium channels is altered, whereas the clustering of the voltage-gated sodium channels is only mildly affected. Furthermore, we show a complete disruption in the axolemmal organization of the potential axo-glial adhesion molecule paranodin. In the PNS, we demonstrate similar trends with regard to ion channel organization and paranodin distribution; however, the abnormalities are less dramatic. The regional differences correlate well with the structural data, since the morphological abnormalities in the PNS are less severe. Taken together, we propose that the disruption in the distribution of paranodin is further evidence that axo-glial interactions are disrupted in the galactolipid-deficient mice, and that these aberrant interactions impair appropriate ion channel segregation.
Compromised Axo-glial Interactions Result in Abnormal Ion Channel Distribution
The pattern of potassium channel distribution that we report for the galactolipid-deficient mice is consistent with a previous report of shiverer mice (Wang et al. 1995), which also display compromised axo-glial interactions in the CNS (Rosenbluth 1980a). In both shiverer and galactolipid-deficient mice, potassium channels frequently do not cluster in the paranodal/juxtaparanodal region but are diffusely distributed throughout the internodal regions. Furthermore, the sciatic nerve of shiverer mice display elongated paranodal/juxtaparanodal potassium channel labeling (Wang et al. 1995). This alteration in ion channel distribution correlates well with the mild disruption in paranodal Schwann cell–axon interactions (Rosenbluth 1980b). Likewise, potassium channel distribution is altered in the PNS of the CGT−/− mice coinciding with compromised axo-glial interactions and reduced paranodal accumulation of paranodin.
Consistent with abnormal potassium channel distribution, particularly in conjunction with aberrant paranode structure, action potential duration is increased in the CNS of the galactolipid-deficient mice (Coetzee et al. 1996). In addition, action potential amplitude is decreased in the CNS (Coetzee et al. 1996), and to a lesser degree in the PNS (Dupree et al. 1998). Furthermore, the addition of 4-aminopyridine, an inhibitor of potassium channels, results in little or no change in PNS amplitude, whereas CNS amplitude increased 25%. This difference likely reflects the greater alteration of potassium channel distribution in the CNS compared with the PNS in these mutants.
Axo-glial interactions do not only influence potassium channel distribution. Clustering of sodium channels in the PNS also appears dependent upon the appropriate association of the Schwann cell with the axon (Dugandzija-Novakovic et al. 1995). In the CNS, Kaplan et al. 1997 reported that oligodendrocyte contact is not required for initial sodium channel clustering in vitro, but a recent report demonstrates that axo-glial contact, as indicated by paranodin and myelin-associated glycoprotein labeling, is required for the sodium channel accumulation in vivo (Rasband et al. 1999). In the galactolipid-deficient mice, sodium channels are concentrated in nodal regions. This finding demonstrates that normal paranodal axo-glial contacts are not essential for nodal clustering of sodium channels. Although gross clustering of sodium channels to the nodal gap is not dependent upon the formation of the paranodal septate-like junctions, these axo-glial junctions may be important in establishing and maintaining the interface between the sodium and potassium domains, since these domains occasionally overlap in both the CNS and the PNS of the galactolipid-deficient mutant.
Galactolipids Are Essential for Proper Formation of Axo-glial Interactions
Using ultrastructural analysis, we have shown previously that axo-glial interactions are disrupted in the galactolipid-deficient mice (Dupree et al. 1998; Dupree and Popko 1999). Here we provide evidence that these interactions are also disrupted at the molecular level, since paranodin, a known component of the septate-like junctions that form between the myelin sheath and the axolemma (Einheber et al. 1997), does not appropriately accumulate in the paranodal region. Presently, the mechanism responsible for proper axolemmal distribution of paranodin is not known. Since paranodin has multiple potential cell adhesion domains including an extracellular lectin-binding domain (Menegoz et al. 1997; Peles et al. 1997), an attractive model is that GalC and/or sulfatide directly bind paranodin and facilitate its accumulation in the paranodal axolemma. Nevertheless, there is no evidence to suggest that the galactolipids accumulate in the paranodal region. Another possibility centers on the role that the galactolipids play in detergent-insoluble-complex (DIGs) formation and trafficking. In oligodendrocytes, DIGs, which are raft-like microdomains composed of the myelin galactolipids and proteins, are thought to be responsible for the molecular organization of the myelin sheath (Kramer et al. 1997, Kramer et al. 1999). Therefore, in mice that lack GalC and sulfatide, an as yet unidentified paranodin ligand may be abnormally distributed in the oligodendrocyte of CGT−/− mice, resulting in the disruption of paranodin intracellular targeting.
The rearrangement of axolemmal proteins in the CGT−/− mice appears to be specific to the paranodal region, since the membrane arrangement of ankyrinG and neurofascin is not grossly affected. The clustering of neurofascin precedes myelination (Lambert et al. 1997), therefore it is not surprising that its distribution is not affected by a myelin gene mutation. In contrast, the clustering of ankyrinG and voltage-dependent sodium channels is temporally associated with the elaboration of myelin-associated glycoprotein-positive myelin-forming processes (Lambert et al. 1997). Therefore, if the distribution of sodium channels and ankyrinG is dependent on myelin, the mechanism by which these proteins are spatially organized apparently does not require the myelin galactolipids and is distinct from the process that regulates potassium channel and paranodin distribution.
In summary, mice that are incapable of producing the myelin galactolipids have compromised axo-glial interactions as evidenced by CNS and PNS structural abnormalities (Dupree et al. 1998; Dupree and Popko, in press). Furthermore, the distribution of paranodin, a prominent component of the paranodal septate-like junctions (Menegoz et al. 1997; Einheber et al. 1997), is dramatically altered with no paranodal accumulation in the CNS. The disruption in axo-glial interactions leads to the abnormal distribution of the Shaker-type Kv1.1 potassium channels in both the CNS and the PNS. Therefore, our data indicate that axo-glial interactions are essential not only for the proper myelin formation but also axolemmal organization.
Acknowledgments
The authors thank Dr. Jim Salzer for his insightful suggestions and his advice with the preparation of the sciatic teased fibers. We also thank Drs. Rock Levinson, Vann Bennett, and Bruce Tempel for kindly supplying us with antibodies. In addition, we wish to thank Dr. Michael Chua for his assistance with preparing the confocal microscopy images, and Ms. Jill Marcus for her critical reviews of the manuscript.
This work was supported by the National Institutes of Health (NS27336 to B. Popko). J.L. Dupree is supported by an Advanced Post-doctoral Fellowship Award from the National Multiple Sclerosis Society.
Footnotes
Abbreviations used in this paper: Caspr, contactin-associated protein; CGT, ceramide galactosyltransferase; CNS, central nervous system; GalC, galactocerebroside; PB, phosphate buffer; PNS, peripheral nervous system.
References
- Black J.A., Kocsis J.D., Waxman S.G. Ion channel organization of the myelinated fiber. Trends Neurosci. 1990;13:48–54. doi: 10.1016/0166-2236(90)90068-l. [DOI] [PubMed] [Google Scholar]
- Bosio A., Bussow H., Adam J., Stoffel W. Galactosphingolipids and axono-glial interaction in myelin of the central nervous system. Cell Tissue Res. 1998;292:199–210. doi: 10.1007/s004410051051. [DOI] [PubMed] [Google Scholar]
- Coetzee T., Fujita N., Dupree J., Shi R., Blight A., Suzuki K., Suzuki K., Popko B. Myelination in the absence of galactocerbroside and sulfatidenormal structure with abnormal function and regional instability. Cell. 1996;86:209–219. doi: 10.1016/s0092-8674(00)80093-8. [DOI] [PubMed] [Google Scholar]
- Dugandzija-Novakovic S., Koszowski A.G., Levinson S.R., Shrager P. Clustering of Na+ channels and node of Ranvier formation in remyelinating axons. J. Neurosci. 1995;15:492–503. doi: 10.1523/JNEUROSCI.15-01-00492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree J.L., Coetzee T., Blight A., Suzuki K., Popko B. Myelin galactolipids are essential for proper node of ranvier formation in the CNS. J. Neurosci. 1998;18:1642–1649. doi: 10.1523/JNEUROSCI.18-05-01642.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupree J.L., Popko B. Genetic dissection of myelin galactolipid function. J. Neurocytol. 1999;28:271–279. doi: 10.1023/a:1007049310758. [DOI] [PubMed] [Google Scholar]
- Einheber S., Zanazzi G., Ching W., Scherer S., Milner T.A., Peles E., Salzer J.L. The axonal membrane protein Caspr, a homologue of neurexin IV, is a component of the septate-like paranodal junctions that assemble during myelination. J. Cell Biol. 1997;139:1495–1506. doi: 10.1083/jcb.139.6.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille B. Ionic Channels of Excitable Membranes. 2nd ed. Sinauer Associates, Inc; Sunderland, MA: 1992. [Google Scholar]
- Kaplan M.R., Meyer-Franke A., Lamber S., Bennett V., Duncan I.D., Levinson S.R., Barres B.A. Induction of sodium channel clustering by oligodendrocytes. Nature. 1997;386:724–728. doi: 10.1038/386724a0. [DOI] [PubMed] [Google Scholar]
- Kordeli E., Davis J., Trapp B., Bennett V. An isoform of abkyrin is localized at nodes of Ranvier in myelinated axons of central and peripheral nerves. J. Cell Biol. 1990;110:1342–1352. doi: 10.1083/jcb.110.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer E.-M., Kocj T., Niehaus A., Trotter J. Oligodendrocytes direct glycosyl phosphatidylinositol-anchored proteins to the myelin sheath in glycosphingolipid-rich complexes. J. Biol. Chem. 1997;272:8937–8945. doi: 10.1074/jbc.272.14.8937. [DOI] [PubMed] [Google Scholar]
- Kramer E.-M., Klein C., Koch T., Boytinck M., Trotter J. Compartmentalization of Fyn kinase with glycosylphosphatidylinositol-anchored molecules in oligodendrocytes facilitates kinase activation during myelination. J. Biol. Chem. 1999;274:29042–29049. doi: 10.1074/jbc.274.41.29042. [DOI] [PubMed] [Google Scholar]
- Lambert S., Davis J.Q., Bennett V. Morphogenesis of the nod of Ranvierco-clusters of ankyrin and ankyrin-binding integral proteins define early developmental intermediates. J. Neurosci. 1997;17:7025–7036. doi: 10.1523/JNEUROSCI.17-18-07025.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menegoz M., Gaspar P., Le Bert M., Galvez T., Burgaya F., Palfrey C., Ezan P., Arnos F., Girault J.-A. Paranodin, a glycoprotein of neuronal paranodal membranes. Neuron. 1997;19:319–331. doi: 10.1016/s0896-6273(00)80942-3. [DOI] [PubMed] [Google Scholar]
- Morell P., Radin N.S. Synthesis of cerebroside by brain from uridine diphosphate galactose and ceramide containing hydroxy fatty acid. Biochemistry. 1969;8:506–512. doi: 10.1021/bi00830a008. [DOI] [PubMed] [Google Scholar]
- Morell P., Quarles R.H., Norton W.T. Myelin formation, structure, and biochemistry. In: Siegel G.J., Agranoff B.W., Albers R.W., Molinoff P.B., editors. Basic NeurochemistryMolecular, Cellular, and Medical Aspects. Raven Press; New York: 1994. pp. 117–143. [Google Scholar]
- Peles E., Nativ M., Lustig M., Grumet M., Schilling J., Martinez R., Plowman G.D., Schlessinger J. Identification of a novel contactin-associated transmembrane receptor with multiple domains implicated in protein-protein interactions. EMBO (Eur. Mol. Biol. Organ.) J. 1997;16:978–988. doi: 10.1093/emboj/16.5.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popko B. Myelin galactolipidsmediators of axo-glial interactions? Glia. 2000;29:149–153. doi: 10.1002/(sici)1098-1136(20000115)29:2<149::aid-glia8>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Rasband M.N., Peles E., Trimmer J.S., Levinson S.R., Lux S.E., Shrager P. Dependence of nodal sodium channel clustering on paranodal axoglial contact in the developing CNS. J. Neurosci. 1999;19:7516–7528. doi: 10.1523/JNEUROSCI.19-17-07516.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie J. Physiology of axons. In: Waxman S.G., Kocsis J.D., Stys P.K., editors. The AxonStructure, Function and Pathophysiology. Oxford University Press; New York: 1995. pp. 68–96. [Google Scholar]
- Rosenbluth J. Central myelin in the mouse mutant shiverer J. Comp. Neurol. 194 1980. 639 648a [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Peripheral myelin in the mouse mutant Shiverer J. Comp. Neurol. 194 1980. 729 739b [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Axolemmal abnormalities in myelin mutants. Ann. N.Y. Acad. Sci. 1990;605:194–214. doi: 10.1111/j.1749-6632.1990.tb42393.x. [DOI] [PubMed] [Google Scholar]
- Salzer J.L. Clustering of sodium channels at the node of Ranvierclose encounters of the axon-glia kind. Neuron. 1997;18:843–846. doi: 10.1016/s0896-6273(00)80323-2. [DOI] [PubMed] [Google Scholar]
- Scherer S.S. Molecular specialization at nodes and paranodes in peripheral nerve. Microsc. Res. Tech. 1996;34:452–461. doi: 10.1002/(SICI)1097-0029(19960801)34:5<452::AID-JEMT5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Smart S.L., Lopantsev V., Zhang C.L., Robbins C.A., Wang H., Chiu S.Y., Schwartzkroin P.A., Messing A., Tempel B. Deletion of the Kv 1.1 potassium channel causes epilepsy in mice. Neuron. 1998;20:809–819. doi: 10.1016/s0896-6273(00)81018-1. [DOI] [PubMed] [Google Scholar]
- Tuvia S., Garver T.D., Bennett V. The phosphorylation state of the FIGQY tyrosine of beurofascin determines ankyrin-binding activity and patterns of cell segregation. Proc. Natl. Acad. Sci. USA. 1997;94:12957–12962. doi: 10.1073/pnas.94.24.12957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vabnick I., Trimmer J.S., Schwarz T.L., Levinson S.R., Risal D., Shrager P. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J. Neurosci. 1999;19:747–758. doi: 10.1523/JNEUROSCI.19-02-00747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Kunkel D.D., Martin T.M., Schwartzkroin P.A., Tempel B.L. Heteromultimeric K+ channels in terminal and juxtaparanodal regions of neurons. Nature. 1993;365:75–79. doi: 10.1038/365075a0. [DOI] [PubMed] [Google Scholar]
- Wang H., Allen M.L., Grigg J.J., Noebels J.L., Tempel B.L. Hypomyelination alters K+ channel expression in mouse mutants shiverer and Trembler . Neuron. 1995;15:1337–1347. doi: 10.1016/0896-6273(95)90012-8. [DOI] [PubMed] [Google Scholar]