Abstract
Megakaryocytes release mature platelets in a complex process. Platelets are known to be released from intermediate structures, designated proplatelets, which are long, tubelike extensions of the megakaryocyte cytoplasm. We have resolved the ultrastructure of the megakaryocyte cytoskeleton at specific stages of proplatelet morphogenesis and correlated these structures with cytoplasmic remodeling events defined by video microscopy. Platelet production begins with the extension of large pseudopodia that use unique cortical bundles of microtubules to elongate and form thin proplatelet processes with bulbous ends; these contain a peripheral bundle of microtubules that loops upon itself and forms a teardrop-shaped structure. Contrary to prior observations and assumptions, time-lapse microscopy reveals proplatelet processes to be extremely dynamic structures that interconvert reversibly between spread and tubular forms. Microtubule coils similar to those observed in blood platelets are detected only at the ends of proplatelets and not within the platelet-sized beads found along the length of proplatelet extensions. Growth and extension of proplatelet processes is associated with repeated bending and bifurcation, which results in considerable amplification of free ends. These aspects are inhibited by cytochalasin B and, therefore, are dependent on actin. We propose that mature platelets are assembled de novo and released only at the ends of proplatelets, and that the complex bending and branching observed during proplatelet morphogenesis represents an elegant mechanism to increase the numbers of proplatelet ends.
Keywords: blood platelet, megakaryocyte, microtubules, actin cytoskeleton, thrombopoiesis
Blood platelets are small cells that lack a nucleus, but have a highly organized cytoskeleton, unique receptors, and specialized secretory granules (for review see Zucker-Franklin 1997). Nearly a trillion platelets circulate in an adult human and respond to blood vessel injury by changing shape, secreting granule contents, and aggregating. These responses are advantageous for hemostasis, but adverse when they cause tissue ischemia or infarction. Thus, the terminal differentiation of mammalian megakaryocytes into platelets represents a unique problem in cell biology with great relevance for human health. Each mature megakaryocyte produces and releases hundreds of platelets into the circulation (Kaufman et al. 1965; Harker and Finch 1969; Trowbridge et al. 1984), but the mechanisms underlying this remarkable culmination of cell differentiation are poorly understood, in part because of the extreme rarity of megakaryocytes in normal bone marrow (<0.1% of cells).
Over the years, many studies on specific aspects of platelet biogenesis have provided important insights and led to testable hypotheses. Megakaryocytes are polyploid cells whose size and DNA content correlate directly with the circulating platelet mass (Ebbe and Stohlman 1965; Paulus 1970). The cytoplasm of mature megakaryocytes is large, replete with platelet-specific granules, and harbors a unique and extensive system of internal membranes. According to one model of thrombopoiesis, these internal membranes demarcate fields or territories of prepackaged platelets, and fragmentation of the cytoplasm releases these platelets (Yamada 1957). An alternative flow model incorporates the observation that mature megakaryocytes extrude long cytoplasmic extensions, called proplatelets, that contain swellings encompassing platelet organelles (Becker and DeBruyn 1976). This model makes two distinct proposals: (1) that platelets assemble de novo within proplatelets, and (2) that the system of internal membranes is not simply a partition, but rather it serves as a reservoir for the plasma membranes of future blood platelets (Radley and Haller 1982). Recognizing that circulating platelets have a rich and unique cytoskeletal architecture is at the heart of the distinction between these alternative models because the problem of platelet genesis is not simply that of packaging cytoplasmic organelles. Rather, any model of thrombopoiesis requires elucidation of the mechanism for assembly and organization of a complex cytoskeleton and membrane skeleton. In this light, it is important to note that a developed platelet cytoskeleton is not readily apparent within the cytoplasm of terminally differentiated megakaryocytes.
The ability to expand and culture megakaryocytes in vitro in the presence of the growth factor thrombopoietin has greatly advanced the opportunity to study these processes in detail (reviewed in Kaushansky 1995). Cultured human and murine megakaryocytes terminate differentiation by producing large numbers of proplatelets (Choi et al. 1995; Cramer et al. 1997; Lecine et al. 1998). Initial studies that exploited this opportunity established that proplatelets express features of circulating blood platelets and outlined features of the proplatelet cytoskeleton (Choi et al. 1995; Cramer et al. 1997). This work has led investigators to consider proplatelet formation to be the physiologic basis of platelet release, and imparts renewed urgency to obtain a fuller understanding of the underlying mechanisms. Building on these initial studies, we report newly recognized aspects of proplatelet morphogenesis and provide detailed characterization of the structural basis of selected key features.
Materials and Methods
Megakaryocyte Cultures and Enrichment of Platelet-sized Particles
Livers were recovered from mouse fetuses and single cell suspensions were generated using methods described previously (Lecine et al. 1998). Between the fourth and sixth day of megakaryocyte culture, cells were placed on a 1.5–3.0% albumin step gradient and sedimented (Drachman et al. 1997) to obtain enriched populations of megakaryocytes. Platelet-sized particles were isolated from culture supernatants by centrifugation at 500 g, and when necessary, were washed using a metrizamide step gradient (Falet et al. 1998).
Video Microscopy
Isolated megakaryocytes were diluted into semi-solid medium containing 65% Leibowitz L-15 medium (GIBCO BRL) and 35% MethoCult (M-3230; Stem Cell Technologies). Megakaryocytes in suspension were pipetted into chambers formed by mounting a glass coverslip coated with 3% BSA onto a 10-mm petri dish with a 1-cm hole. Preparations were maintained at 37°C using a Bipolar temperature controller (Medical Systems Corp.), and examined on a Zeiss IM-35 inverted microscope equipped with a 40× phase-contrast long working distance condenser. Images were obtained using a Hamamatsu charged coupled device (CCD) camera and frames were captured at 1-, 5-, or 10-min intervals using a Power Macintosh 9500 equipped with a SCION LG3 Frame grabber. Movies were generated using NIH-Image 1.61 software.
Electron Microscopy
Samples were prepared for electron microscopy as described (Hartwig 1992). In brief, megakaryocytes in suspension were placed in wells of a 96-well microtiter plate, each containing a poly-l-lysine–coated coverslip, and the plate was centrifuged at 500 g for 5 min at 37°C. Some cells were fixed with 1% glutaraldehyde in PBS to study their surface topology. Cytoskeletons were isolated by permeabilizing cells with PHEM buffer (60 mM Pipes, 25 mM Hepes, 10 mM EGTA, and 2 mM MgCl2) containing 0.75% Triton X-100, 2 μM phallacidin, 20 μM taxol, and protease inhibitors for 2 min. Cytoskeletons were washed with PHEM buffer containing 0.1 μM phallacidin and 30 μM taxol, and fixed in PHEM buffer containing 1% glutaraldehyde for 10 min. To examine the membrane skeleton, cells were incubated with permeabilization buffer containing 0.1% glutaraldehyde for 2 min and fixed as described above. Coverslips of fixed cells and cytoskeletons were rapidly frozen, freeze-dried, and coated with tantalum-tungsten and carbon. Replicas were picked up on carbon-formvar–coated copper grids and examined with a JEOL JEM-1200 EX electron microscope at an accelerating voltage of 100 kV.
Immunofluorescence
Antibodies.
mAbs specific for β-tubulin were obtained from Amersham. Secondary antibodies, FITC, and TRITC conjugates of goat anti–rabbit and goat anti–mouse IgG were obtained from Sigma Immunochemical. Primary antibodies were used at 5 μg/ml in PBS + 1% BSA and secondary antibodies at 1:200 dilution in the same buffer.
Confocal Microscopy.
Megakaryocytes were fixed with 4% formaldehyde in Hanks' balanced salt solution (GIBCO BRL) for 20 min, cytocentrifuged at 500 g for 4 min onto coverslips previously coated with poly-l-lysine, permeabilized with 0.5% Triton X-100 in Hanks' containing 0.1 mM EGTA, and blocked with 0.5% BSA in PBS. The specimens were incubated in primary antibody for 3–6 h, washed, treated with the appropriate secondary antibody for 1 h, and washed extensively. Controls were processed identically except for omission of the primary antibody. Preparations were examined with a BioRad MRC 1024 laser scanning confocal microscope equipped with Lasersharp 3.1 software. Images were obtained using a Zeiss Axiovert S100 equipped with a 100× differential interference contrast (DIC) oil immersion objective (NA 1.4).
Flow Cytometry Studies
A fluorescein-phalloidin–based flow cytometric assay was employed to quantitate the actin filament content in blood and culture-derived platelets (Howard and Oresajo 1985). Platelets treated with and without 1 U/ml mouse thrombin (Sigma Chemical Co.) were fixed by the addition of an equal volume of 4% paraformaldehyde in platelet buffer (145 mM NaCl, 10 mM Hepes, 10 mM glucose, 0.5 mM Na2HPO4, 5 mM KCl, 2 mM MgCl2, and 0.3% BSA, pH 7.4) for 30 min at 37°C. Fixed platelets were permeabilized with 0.1% Triton X-100, labeled with 10 μM FITC-phalloidin for 1 h at room temperature, and washed twice with PBS. Samples were analyzed in a Becton Dickinson FACScan, gated for platelets, and a total of 10,000 events were analyzed using Lysis II software (Becton Dickinson). Experiments were repeated in triplicate and the results were averaged.
Cytoskeletal-disrupting Agents
Taxol, cytochalasin B and D, and nocodazole were obtained from Sigma Chemical Co.
Image Preparation
All images were imported into either Adobe Photoshop or Claris Draw and printed on a Kodak 8650 PS color printer.
Online Supplemental Material
The manuscript includes three movies supplied in Quick Time™ format.
Figure 1: Proplatelet formation contains 58 frames taken at 10-min intervals using a 40× phase-contrast objective. This movie shows a megakaryocyte undergoing proplatelet formation and demonstrates the dynamic behavior of proplatelets.
Figure 2: (a) Dynamic interconversion contains 30 frames taken at 10-min intervals and shows a higher magnification view of the reversible interconversion between spread lamellar segments and condensed proplatelet forms. (b) Bending/branching contains 10 frames taken at 10-min intervals and shows proplatelet bending and the bifurcation at high magnification. Videos are available at http://www.jcb.org/cgi/content/full/147/6/1299/DC1.
Results
Proplatelet Intermediates Are Remarkably Dynamic Structures
To date, proplatelets have been studied and described largely through static images, and most conclusions about mechanisms of proplatelet formation are inferred from studies conducted with only limited benefit of time-lapse cinematography. We used video-enhanced light microscopy of cultured mouse megakaryocytes to reveal a spectrum of highly reproducible morphogenetic changes and cellular movements during formation of proplatelets (Fig. 1 and accompanying video). A transformation of the entire megakaryocyte cytoplasm results in condensation of cell material into platelet-sized particles, which have the appearance of beads linked by thin cytoplasmic bridges. This process unfolds over 4–10 h, and initiates with erosion of one pole of the megakaryocyte cytoplasm. This generates a unique pseudopodial structure that elongates to yield slender tubules of uniform diameter of 2–4 μm; these in turn develop periodic densities along their length that impart the characteristic beaded appearance of proplatelets. Maturation of proplatelets ends in a rapid retraction that separates a variable portion of the proplatelets from the residual cell body, according to a process that presumably mirrors platelet release in vivo.
Figure 1.
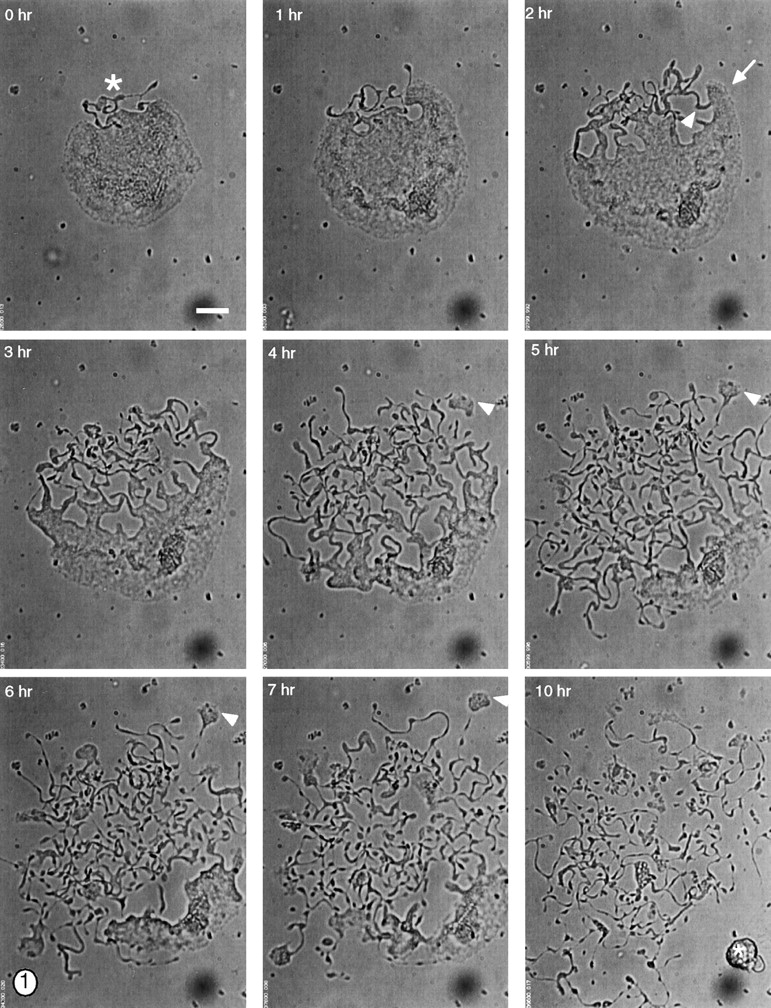
Video-enhanced light microscopy of a terminally differentiated mouse megakaryocyte forming proplatelets in vitro. During the initial stages of proplatelet formation, the megakaryocyte spreads and its cortical cytoplasm begins to unravel at one pole (this zone of erosion is labeled with an asterisk in the first panel). As the cell spreads, the cytoplasm at the erosion site is remodeled into large pseudopodia (white arrow at 2 h) that elongate and become thinner over time, forming narrow tubes of 1–4 μm diam. The proplatelet extensions frequently bend (white arrowhead at 2 h), and it is at sites of bending that the tube bifurcates to generate a new process. In this manner, the entire cytoplasmic space of the megakaryocyte is converted into anastomosing proplatelet extensions. Proplatelets also develop segmented constrictions along their length that impart a beaded appearance. The process of proplatelet elaboration culminates in a rapid retraction that separates the many strands of proplatelets from a residual nuclear mass (asterisk in last panel). Note the crawling of small cytoplasmic fragments (5–10 μm) at the ends of some of the proplatelet extensions (arrowheads at 4–7 h). Crawling begins when the end of the proplatelet adheres and flattens, forms a leading lamellipodia, and migrates away from the cell center, dragging a trail of proplatelets behind. Bar, 20 μm.
High resolution time-lapse analysis reveals several striking features that have not been recognized previously, and emphasizes the highly dynamic nature of thrombopoiesis. Remodeling of the megakaryocyte cytoplasm is accompanied by centrifugal spreading that increases the apparent cell surface area and, measured from representative cells (Fig. 1, note the expansion of the lower right hand cell margin), proceeds at a rate of ∼0.2 μm/min. In addition, portions of the proplatelet cytoplasm exhibit striking and dynamic interconversion between spread lamellar segments and condensed structures resembling platelets; this oscillation between proplatelet and spread morphologies may occur multiple times (Fig. 2 a and accompanying video). The distal ends of proplatelet processes periodically flatten to form fan-shaped sheets resembling lamellipodia and crawl away from the cell center, dragging a proplatelet trail (Fig. 1, arrowheads at 4–7 h). Interestingly, proplatelet fragments and the megakaryocyte cell body move at similar speeds (0.2–0.4 μm/min), and spreading movements are inhibited by 1–10 μM of cytochalasin B, suggesting that they require actin polymerization; upon removal of this drug, megakaryocytes recover the ability to spread on the surface (data not shown). The stable cytoskeletal architecture of mature blood platelets would seem to preclude the degree of dynamic cell motility revealed by these studies. Hence, platelet assembly must at best be incomplete during this phase of proplatelet morphogenesis, and platelets must be assembled de novo in the course of this transition; structural studies detailed below strongly support this conclusion.
Figure 2.
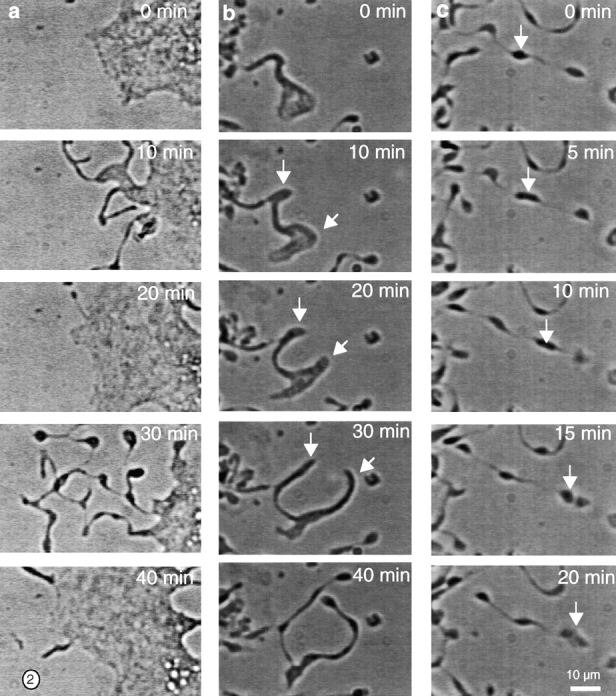
The dynamic behavior of proplatelets. (a) Interconversion between spread lamellar and condensed tubular forms. Phase-contrast images taken 10 min apart showing the dynamic interconversion between proplatelet morphologies. Proplatelets were observed to reversibly flatten, and then convert to thin tubular proplatelet processes. (b) Bifurcation of proplatelets. Phase-contrast images taken 10 min apart showing the bending and branching of a proplatelet extensions. Bends are converted into loops that become compressed and elongate, resulting in a bifurcation of the original tube. White arrows denote the branch points. (c) Platelet-sized particle movement along proplatelets. The white arrow denotes a platelet-sized nodule translocating along a proplatelet process, while the end of the proplatelet is stationary. In the last panel, this particle fuses with a stationary particle. The images are at 5-min intervals. Bar, 10 μm.
To better understand the dynamic aspects of proplatelet morphogenesis, we examined selected aspects at high resolution. Branching of proplatelets has been recognized previously (Haller and Radley 1983) but not investigated in detail. Proplatelet bifurcation occurs at a variable distance from the cell body, but only at sites of pronounced bending (Fig. 2 b and accompanying video). The sharp kinks elongate, forming a small loop, and a new proplatelet process formed by this loop extends from the original nexus. Newly generated extensions also develop the periodic constrictions characteristic of proplatelets. Platelet-sized particles and nodules of variable size translocate extensively along the length of megakaryocyte processes (Fig. 2 c), another feature that is incompatible with preassembly of a stable platelet cytoskeleton. Distinct segments move in either direction at an estimated rate of ∼1 μm/min, may move in opposite directions on a single extension, and can even reverse direction. The particles typically do not reveal changes in phase density as they move; however, we have observed several platelet-sized particles fuse together during translocation (Fig. 2 c, 20-min frame). Additionally, proplatelets undergo continuous cycles of extension and retraction that are most clearly evident in the video accompanying Fig. 1. Thus, these observations reveal proplatelet processes to be inherently unstable structures with a capacity for extensive morphogenetic changes.
The surprisingly dynamic character of proplatelet formation raises many important questions. (1) What is the structure of the intermediate, platelet-like particles detected within developing proplatelets? (2) Which cellular structures drive megakaryocyte spreading, elongation, and thinning of proplatelets? (3) What is the structural basis for the transition between proplatelets and mature platelets? (4) How does branching of proplatelet processes occur, and what is its significance? In the following experiments, we address some of these questions. Previous studies investigating platelet formation by cultured megakaryocytes have only partially assessed the structure and function of the released particles (Radley et al. 1991; Choi et al. 1995; Cramer et al. 1997). Therefore, we first investigated whether the shape changes and movements observed in our studies lead to the release of verifiable platelets.
Culture-derived Platelets Produced via Proplatelets Manifest Structural and Functional Features of Circulating Blood Platelets
Platelet-sized particles released by cultured mouse megakaryocytes exhibit each of the hallmark features of blood-derived platelets. They are disc-shaped, 2–3 μm in diameter, lack discernible membrane topology or protrusions (Fig. 3 a), and show periodic surface invaginations (Zucker-Franklin 1970) that demarcate entrances into the open canalicular system (Fig. 3 a, arrowheads). Removal of the plasma membrane exposes an elaborate membrane skeleton composed of 3–5-nm strands identical in appearance to the spectrin-based network of human platelets (Fig. 3 b), and a prominent marginal band (Haydon and Taylor 1965; White and Krivit 1967; Kenney and Linck 1985) derived from the apparent coiling of a single microtubule (Fig. 3 c). A complex three-dimensional network of actin filaments, similar to that observed in mature blood platelets (Fox et al. 1988; Nachmias and Yoshida 1988; Hartwig and DeSisto 1991; Fox et al. 1996), is also found beneath the membrane skeleton (Fig. 3 d). 200–225 platelet-sized microtubule coils may be seen extending from typical proplatelet-producing cells (see Fig. 5g and Fig. h), a value that is in good agreement with the proposed theoretical range of platelets produced per megakaryocyte (Kaufman et al. 1965; Harker and Finch 1969; Trowbridge et al. 1984; Stenberg and Levin 1989) and with observations in cultured human cells (Choi et al. 1995). Furthermore, the released particles respond to stimulation with thrombin by extending lamellipodia and filopodia (Fig. 3 e), forming microaggregates (Fig. 3 f), and exhibiting a twofold increase in their proportion of filamentous actin (data not shown), which is similar to observations in activated blood platelets (Carlsson et al. 1979; Fox and Phillips 1981; Fox et al. 1984; Karlsson et al. 1984). These particles showed increased expression of the activation-dependent antigens P-selectin and the functional fibrinogen receptor (data not shown), as previously reported for human proplatelets (Choi et al. 1995). Hence, platelets released in cell culture display all the morphological and functional criteria that distinguish blood platelets.
Figure 3.
Ultrastructural features and functional analysis of culture-derived mouse platelets. Structure of platelets released into the megakaryocyte culture media. This representative micrograph shows released particles to be disc-shaped after rapid freezing, freeze drying, and metal coating. Particles have smooth, featureless surfaces except for periodic pits that delineate entrances to the open canalicular system, a hallmark feature of platelets. Pits are indicated with arrowheads. (b) Electron micrograph showing that a culture-derived platelet has a membrane skeleton similar in structure to that of a blood-derived platelet. Careful removal of the membranes with the detergent Triton X-100 reveals a dense fibrous lamina that coats the underside of the plasma membrane and obscures the underlying actin cytoskeleton. The membrane skeleton is composed of thin strands that interconnect forming triangular pores. (c) Electron micrograph showing the presence of a peripheral microtubule coil in the detergent insoluble cytoskeleton from a resting, culture-derived platelet. The microtubule coil retains the dimensions of the platelet. Actin fibers are also visible. (d) The cytoskeleton of a representative culture-derived platelet after treatment with thrombin. Culture-derived platelets grow both lamellipodia and filopodia from their edges after treatment with thrombin, and assemble a dense actin filament based cytoskeleton. (e) Morphology of a culture-derived platelet after treatment with thrombin. In response to thrombin, platelet-sized particles convert from discoid shapes in active forms having broad flat lamellae and thin filopodia. (f) After treatment with 1 U/ml thrombin, culture-derived platelets form microaggregates containing 5–10 particles. (g and h) Microtubule coils at the ends of proplatelets. Antitubulin immunofluorescent staining of megakaryocytes at the peak time of proplatelet production. Each representative micrograph shows megakaryocytes in culture to produce hundreds of microtubule coils within the proplatelet extensions emanating from a single megakaryocyte. Each microtubule coil is similar in size and structure to those observed in mouse blood platelets (inset in g). Bars, 1 μm.
Figure 5.
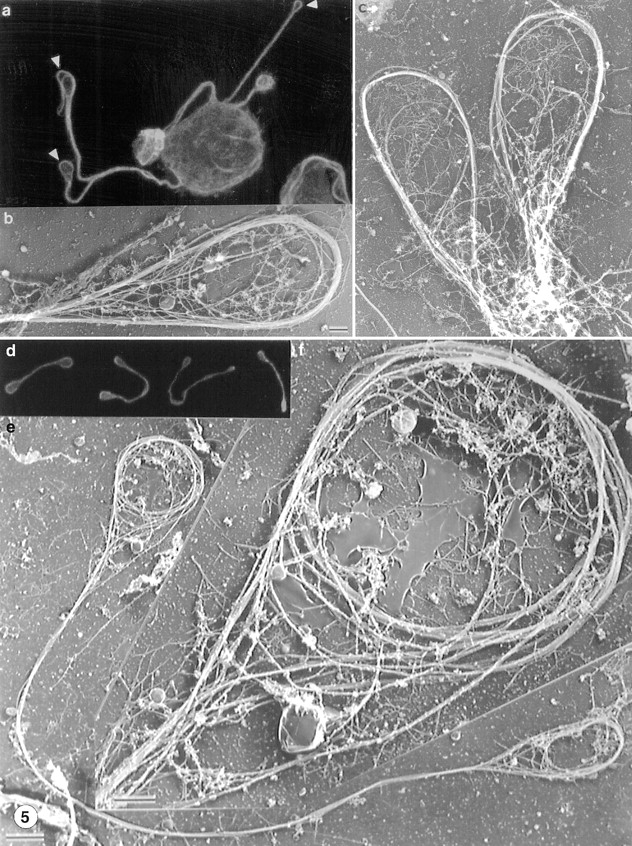
Organization of microtubules at the ends of proplatelet extensions (a–c) and in released proplatelet forms (d–g). (a) Antitubulin staining of a megakaryocyte and its early proplatelet extension. Microtubules concentrate along the edges of the megakaryocyte and enter into the proplatelet extensions. Bundles enter the projections from both sides and separate periodically within the extensions. The distal end of each proplatelet has a teardrop-shaped enlargement that contains a microtubule loop (arrowheads). (b and c) Representative electron micrographs showing the organization of microtubules within the tips of proplatelet termini. The end of each proplatelet contains a microtubule bundle that loops beneath the plasma membrane and reenters the shaft to form a teardrop-shaped structure. (d) Gallery of released platelet forms stained for tubulin by immunofluorescence confocal microscopy. Released platelet-sized particles are connected by thin cytoplasmic bridges, the most abundant being barbell shapes composed of two platelet-like particles connected by a single cytoplasmic strand. Microtubules line the shaft and rim the bulbous ends. (e) Low magnification electron micrograph showing the microtubule-based cytoskeleton of a representative released proplatelet form. A microtubule bundle lines the shaft of the proplatelet. Bar, 2 μm. (f) Higher magnification electron micrograph of one end of the proplatelet shown in e. Ends have microtubule bundles arranged into teardrop-shaped loops, similar to those at the ends of proplatelets extending from megakaryocytes. Within these loops are microtubule coils structurally similar to those observed in mature platelets isolated from the blood. Bar, 0.5 μm.
Changes in the Microtubule Cytoskeleton during Proplatelet Morphogenesis
Microtubule assembly has been previously implicated in platelet formation (Handagama et al. 1987; Radley and Hatshorn 1987; Tablin et al. 1990), and our studies confirm its essential role in the progression of initial pseudopodia into proplatelets. The microtubule disrupting agent nocodazole (1–10 μM) completely inhibits formation of all megakaryocyte projections (Tablin et al. 1990). When nocodazole is added to megakaryocytes after they have formed proplatelet processes, particle transport is completely blocked (data not shown), indicating the requirement for a microtubule-based motile apparatus in this process. To understand the structural basis of the transitions between the megakaryocyte cell body, pseudopodia, proplatelet processes, and nascent platelets, we examined the microtubule cytoskeletons of proplatelet-producing megakaryocytes. Early in the maturation process, before spreading and erosion of the cell body begin, the megakaryocyte cytoplasm is replete with long individual arrays of microtubules that cluster around the nucleus and radiate toward the cell margins (Fig. 4, a and b). As large and blunt pseudopodia are formed in the area of erosion, the microtubules consolidate into cortical bundles situated just beneath the membrane surface of the protrusion (Fig. 4 c). As the pseudopodia extend and become thinner, they display a prominent band of microtubules along their edges; in some cases, these bundles are seen to curl or spiral inside the pseudopodia (Fig. 4 d). The next recognizable step is the conversion of the blunt pseudopodia, via elongation, into cytoplasmic extensions that continue to harbor thick bundles of microtubules (Fig. 5 a). These processes always have bulbous ends, and electron microscopy reveals a microtubule bundle that loops just beneath the plasma membrane and reenters the shaft to form a teardrop-shaped structure (Fig. 5b and Fig. c).
Figure 4.
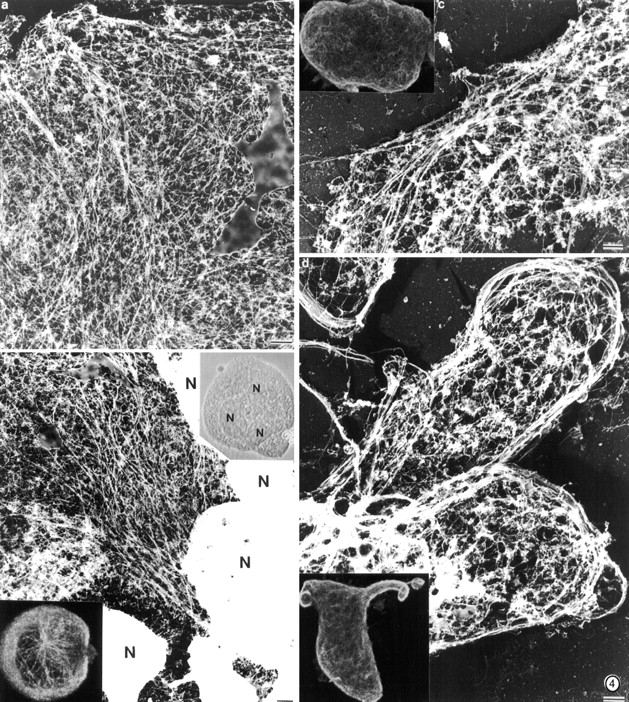
Structure of the megakaryocyte cytoskeleton before and during early proplatelet formation. (a and b) Structure of a megakaryocyte cytoskeleton lacking extensions. (a) Electron micrograph showing the structure of the cortical cytoskeleton in a megakarytocyte. A meshwork of actin filaments and microtubules is present in the cell cortex. Individual microtubules radiate outward from the cell center. (b) Electron micrograph showing the structure of the megakaryocyte cytoskeleton near its nuclear mass (N). Microtubules gather into arrays in the cell center. (right inset) Morphology of megakaryocyte in the light microscope photographed with phase contrast optics. (left inset) Confocal photograph of an early stage megakaryocyte stained with antitubulin antibody. Arrays of microtubules originate near the cell center and radiate out into the cell cortex. (c) Electron micrograph of a more mature megakaryocyte cytoskeleton before pseudopodia formation. Microtubules become densely packed into cortical bundles situated parallel to and just beneath the plasma membrane. (d) Organization of microtubules within pseudopodia. These structures contain dense rims composed of large bundles of microtubules. (Inset) Antitubulin immunofluorescent staining reveals the concentration of microtubules at the margins of the pseudopodia. Bars, 1 μm.
De Novo Assembly of Platelet Particles at Proplatelet Termini
Determining when and where platelet units are finally assembled during megakaryocyte differentiation has been controversial (Radley and Hatshorn 1987). At the heart of this question is understanding where and how the marginal microtubule band, a stable feature of terminally differentiated platelets (White 1968; Radley and Hatshorn 1987), is formed. If platelets preassemble within the megakaryocyte cytoplasm into platelet fields or territories, then microtubule coils would be expected to lie within these areas before cytoplasmic fragmentation. The dynamic process of proplatelet formation (Fig. 1 and Fig. 2) and the underlying progression of the microtubule cytoskeleton from pseudopodia to proplatelets (Fig. 4) argue strongly against this possibility. In addition, in scanning hundreds of antitubulin immunofluorescence profiles of megakaryocytes, we failed to identify microtubule coils within the cell bodies; instead, these are consistently and readily detected only in proplatelet extensions (Fig. 3g and Fig. h).
Most released platelet-sized particles remain connected by cytoplasmic bridges, the most abundant being barbell forms composed of two platelet-like particles joined by one cytoplasmic strand. To ask whether the bulbous ends and thickenings along the shaft of proplatelets show mature cytoskeletal features, we examined representative released particles by antitubulin immunofluorescence confocal microscopy (Fig. 5 d) and electron microscopy (Fig. 5e and Fig. f). A microtubule bundle forms the core of the proplatelet shaft, and the ends have microtubule bundles forming teardrop-shaped loops that are similar in both size (1–3 μm) and appearance to those described in mature blood platelets (White 1968). Distorted microtubule bundles are also visible in platelet-sized swellings along the shaft of the proplatelet; however, in contrast to the terminal rings, those observed along the proplatelet shafts are uniformly less distinct (Fig. 6). Whereas these platelet-sized nodules superficially appear to include microtubule rings, close inspection reveals that they are actually points where microtubule bundles simply diverge for a short distance but fail to form the mature teardrop shape (Fig. 6, c–g). These nodules consistently reveal membrane and other detergent-insoluble materials, presumably destined for final platelet assembly elsewhere (compare Fig. 6, a and b). These results are entirely consistent with the dynamic activity that is observed at the sites of the platelet-sized nodules, including branching (Fig. 2 b), interconversion between condensed and lamellipodial forms (Fig. 1 and Fig. 2 a), and rapid translocation of particles (Fig. 2 c), and indicate that they are structurally unstable. More importantly, they establish that the mature cytoskeletal feature of a microtubule coil is only detected at the ends of proplatelets (Fig. 5) and not along their lengths.
Figure 6.
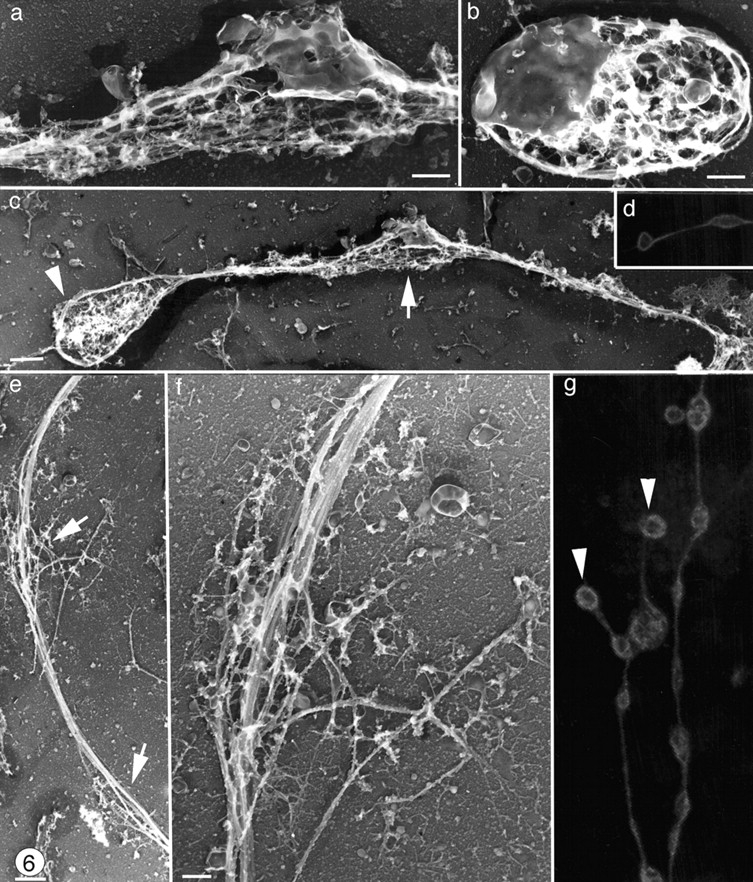
Organization of microtubules along the shaft of proplatelets. Swellings along the proplatelet (a) contain detergent-insoluble membrane and vesicles that are similar to those found in released platelets (b) in these samples. (c) Low magnification electron micrograph showing the microtubules bundles lining the shaft of a typical proplatelet. Although proplatelet ends (arrowhead) have distinct microtubule coils, microtubules observed in swellings along the shaft (arrow) are not coiled. (d and g) Proplatelets stained for tubulin by immunofluorescence and photographed on a confocal microscope. Proplatelets show periodic segments connected by thin cytoplasmic bridges, but only the ends (arrowheads) have microtubule bundles arranged into teardrop-shaped loops. (e and f) Higher magnification electron micrograph of a swelling along a proplatelet shaft showing that these are points where the microtubule bundle separates for a short distance (arrows) but does not form a loop. Bars: (b and f) 0.5 μm; (c and e) 1 μm.
The Microtubule and Actin Cytoskeletons Drive Distinct Aspects of Proplatelet Formation
The separation of proplatelet morphogenesis into stages characterized by specific alterations of the microtubule cytoskeleton and the recognition of distinct dynamic features provide approaches to investigation of the underlying molecular mechanisms. Megakaryocytes cultured in the presence of cytochalasin B, an inhibitor of actin assembly, retain the capacity to extend long, slender proplatelet-like projections (Fig. 7 a) but show specific abnormal features. The same results were observed with cytochalasin D. The cell body fails to spread, proplatelets extend from multiple points around the cell margin instead of the typical single erosion site, branching is completely inhibited, and although proplatelet processes retain bulbous ends, they harbor many fewer intermediate swellings. Thus, actin assembly is not required for megakaryocytes to extend proplatelets but is essential for proplatelet branching. Interestingly, one process associated with proplatelet bending is the attachment of a small region to the substratum over a period of 5–10 min and robust ruffling activity of this portion (data not shown), movements classically thought to be mediated by actin. In the electron microscope, the microtubule bundles composing the shaft routinely reveal small filamentous outpouchings (Fig. 8 a). At sites of bending, meshworks of actin filaments extrude processes that connect the microtubule bundles much like tendons attaching muscle to bone (Fig. 8 b). At more pronounced bends, the apparent sites of proplatelet branching, filamentous aggregates form a cusp between the microtubule bundles (Fig. 8 c). Considered together, these observations demonstrate that actin filaments are enriched at the sites of proplatelet bifuraction and probably required to execute this process.
Figure 7.
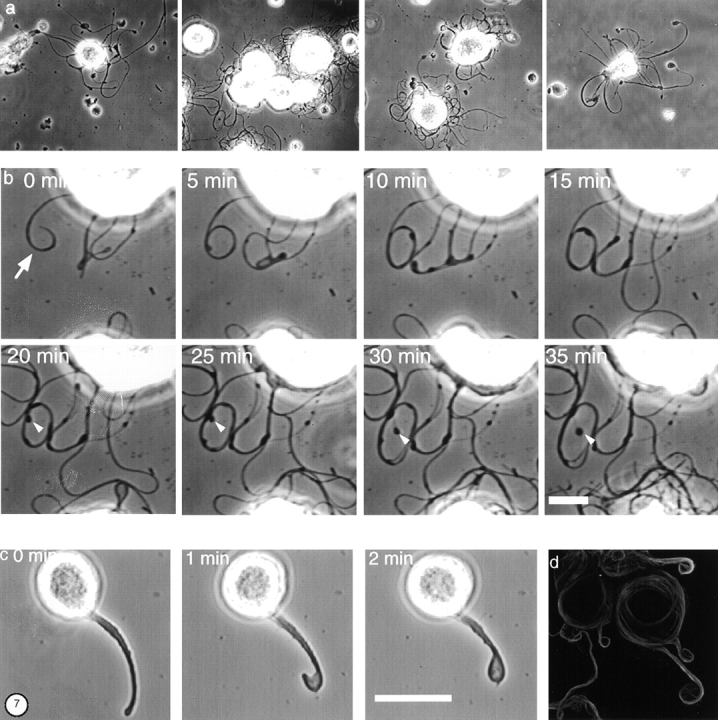
Effect of cytoskeletal disrupting agents on proplatelet growth. (a) Gallery of phase-contrast micrographs of megakaryocytes treated with 2 μM cytochalasin B. Extensions from the megakaryocyte surface show reduced beading and absence of branching. (b) Images showing a time series at a higher magnification of processes extending from the megakaryocyte surface in the presence of 2 μM cytochalasin B. The white arrowheads indicate the tip of one extension, which remains almost stationary while the diameter of the loop formed by the extension increases with time. (c) Effect of taxol on proplatelet formation. Phase-contrast images showing a time series of a tubelike extension growing from a taxol-treated megakaryocyte. In the presence of 10 μM taxol, the megakaryocyte forms a single pseudopodia. As this tube elongates, its distal tip bends and curves back on itself (0–1 min) until its tip contacts its shaft and appears to fuse (2 min). This forms a teardrop-shaped structure on the end of the projection. (d) Antitubulin immunofluorescence staining of a megakaryocyte cultured in the presence of 10 μM taxol. Taxol treatment reduces the number of extensions made by cells. Microtubule staining concentrates at the cell edge and in the proplatelet-like extensions grown from the cell surface. The microtubule bundles in the shafts are thickened compared with those extended by untreated cells, and are observed to curl within the bulbous ends. Bars: (b) 10 μm; (c) 25 μm.
Figure 8.
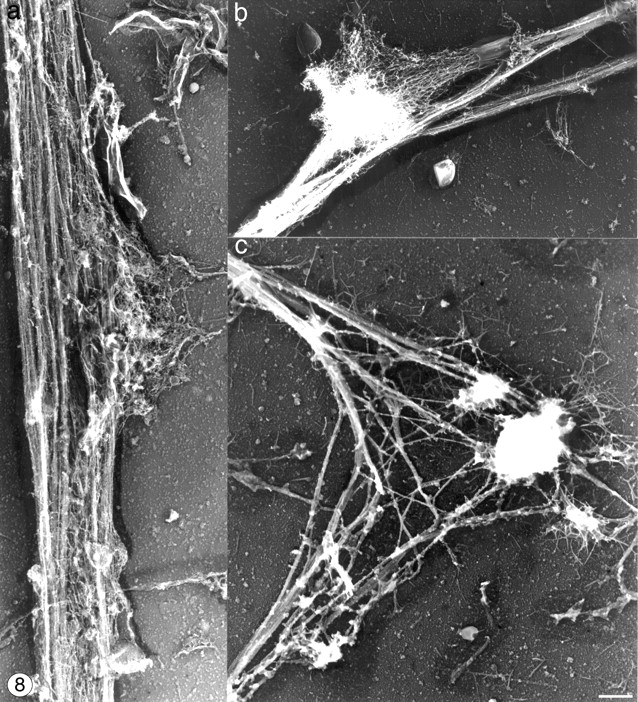
Cytoskeletal organization at proplatelet bends. (a) Representative micrograph showing the cytoskeleton of a proplatelet shaft. Parallel bundles of microtubules line the tube and prospective branch points are defined by outpouchings of 10-nm filaments. (b) Micrograph showing a platelet-sized swelling along the length of a proplatelet that has spread on the surface. This spread region contains a dense meshwork of F-actin, with ends that are collapsed and bound to the sides of the microtubule bundles. Microtubule bundles are bent and appear to have separated. A meshwork of spread actin filaments is observed extending from the platelet-sized segment. (c) Micrograph showing a pronounced bend in a proplatelet. The elbow of the bend contains aggregates of filamentous material, which contact the substratum in the elbow region. Bar, 1 μm.
The elongation of proplatelet extensions could be explained by forces derived from polymerization of tubulin subunits, from sliding of microtubules, or possibly a combination of both processes. Treatment with cytochalasin B results in substantially reduced complexity of proplatelets and permits isolated examination of the elongation process. In the representative sequence of micrographs shown in Fig. 7 b, we followed the growth of a single proplatelet. As this structure elongates, it rolls up upon itself into a loop. While the distal end subsequently remains stationary (Fig. 7 b, arrowheads), the process continues to elongate and causes the diameter of the loop to increase. This strongly suggests that extension of proplatelet processes can occur without growth at the tip.
The microtubule stabilizing agent taxol completely blocks bending and branching along the length of the proplatelet tube and increases both the diameter of individual tubes and the thickness of microtubule bundles within them. Megakaryocytes treated with taxol remain capable of projecting short, thickened extensions with large bulbous tips. Fig. 7 c shows representative video-enhanced light microscopy designed to investigate the mechanics of proplatelet extension after taxol treatment, and illustrates that the processes formed are thickened compared with normal. As processes elongate in the presence of taxol, their distal tip bends and curves back on itself until it makes contact with the shaft and appears to fuse with it to form a teardrop-shaped structure. In the presence of taxol, microtubules still align and compress in the cell cortex from which they enter pseudopodia (Fig. 7 d). In many cases, they do so from two sides and separate within the extension. Immunofluorescence reveals that microtubules either coil at the tips of projections or fold back on themselves to generate teardrop forms.
Discussion
The notion that mammalian platelets assemble through intermediate proplatelet structures was originally hypothesized by Becker and DeBruyn 1976, developed into a plausible model by Radley and co-workers (Radley and Scurfield 1980; Radley and Haller 1982), is strongly supported by the sum of many previous investigations, and is inconsistent with a competing model of thrombopoiesis that states that platelets are first formed as platelet territories within megakaryocytes. If the platelet territory model is true, as has been suggested by freeze-fracture and thin-section electron microscopy on the internal membranes of megakaryocytes (Yamada 1957; Zucker-Franklin and Petursson 1984), one would expect to find microtubule rings within such platelet fields (Radley and Hatshorn 1987). This simple model finds little support here, or historically, as microtubule coils have not been observed in the megakaryocyte body. In contrast, many observers have documented the elaboration of proplatelets in megakaryocyte cultures (Handagama et al. 1987; Leven and Yee 1987; Radley et al. 1987; Topp et al. 1990; An et al. 1994; Choi et al. 1995; Cramer et al. 1997), and occasional micrographs have captured human megakaryocytes extruding proplatelet-like processes into bone marrow sinusoids (Behnke 1968, Behnke 1969; Lichtman et al. 1978; Scurfield and Radley 1981). The infrequent sighting of this morphology in situ most likely results from geometric limitations of thin-section transmission electron microscopy. Proplatelets have been recognized in a wide range of mammalian species, including mice, rats, guinea pigs, dogs, cows, and humans; platelet territories are not observed in megakaryocytes from some of these species. Moreover, the early characterization of proplatelets revealed many features that are consistent with known aspects of thrombopoiesis, including the critical requirement for microtubule integrity (Handagama et al. 1987; Tablin et al. 1990; Choi et al. 1995; Cramer et al. 1997). Finally, mice lacking two distinct hematopoietic transcription factors have severe thrombocytopenia and fail to produce proplatelets in culture (Lecine et al. 1998). Taken together, these findings establish the central importance of proplatelets in mammalian thrombopoiesis.
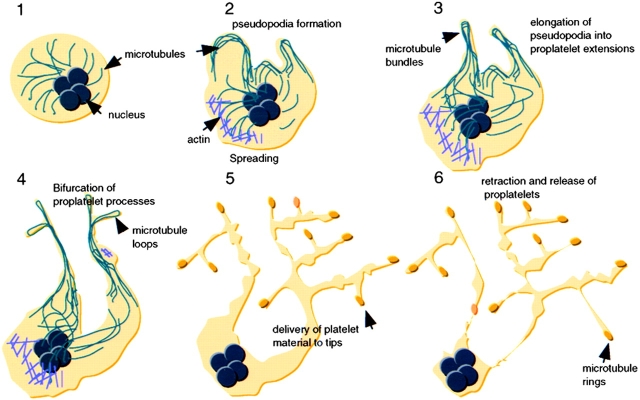
Here, we report detailed studies aimed at investigating discrete steps in platelet formation by terminally differentiated mouse megakaryocytes, and our analysis represents the first effort to dissect dynamic aspects of this process. Proplatelets extend by a microtubule-based system, bifurcate repeatedly to increase the number of ends, and deliver packets of platelet material to these ends. Proplatelets and their contents are highly dynamic structures that continuously engage in alternate extension and retraction, reversible spreading, and bidirectional transport of particles. This dynamic behavior is inconsistent with the notion of proplatelets as static, linear arrays of nascent blood platelets, and strongly suggests that platelets mature along and at the ends of proplatelets in the final stages of an elaborate process. Our analysis suggests the model for platelet formation shown in Fig. 9.
Figure 9.
Model of platelet production suggested by these experiments and previous studies. After commitment to the megakaryocyte lineage, cells undergo polyploidization and cytoplasmic maturation (Stage 1). During the initial stages of proplatelet formation, megakaryocytes remodel their cytoplasm into thick pseudopodia (Stage 2), which contain bundles of microtubules situated just beneath the protruding membrane. Blunt pseudopodia elongate into proplatelet processes, which harbor thick bundles of microtubules in their core and contain a teardrop-shaped loop at their distal tip (Stage 3). Proplatelets frequently bend and form a branched structure from which new processes extend (Stage 4). These proplatelet processes form constrictions along their length giving the beaded appearance to proplatelets (Stage 5). The swellings along proplatelets are unstable structures presumed to contain packets of platelet material in the process of being delivered to the ends. Proplatelets are released from the megakaryocyte body after a retraction (Stage 6), and may undergo further fragmentation to yield individual platelets.
Significance of the Dynamic Behavior of Proplatelet Morphogenesis
Segments of proplatelets clearly demonstrate the capacity to spread reversibly on the substratum and lose their characteristic tubular, beaded appearances. These changes occur both at the ends of tubes and along the shafts and are transient, with rapid reversion to the original, beads-on-a-string appearance (e.g., Fig. 1 and Fig. 2 a). This remarkable plasticity needs to be reconciled with the presence in blood platelets of an elaborate membrane skeleton, where molecules of filamin A (ABP-280) extend through pores in the spectrin network, connecting actin filaments to the cytoplasmic tail of the α chain of GPIb of the von Willebrand factor receptor, and create an arrangement that restricts the mobility of the membrane adherent spectrin lattice (Andrews and Fox 1991; Hartwig and DeSisto 1991; Kovacsovics and Hartwig 1996). Although not discussed here, the plasma membranes of proplatelet extensions have a spectrin lattice identical in appearance to that of the mature platelet (Italiano, J.E., R.A. Shivdasani, and J.H. Hartwig, unpublished observations). Our observations on the dynamic nature of proplatelet morphogenesis suggest that the linkages holding the membrane skeleton in compression between the plasma membrane and actin cytoskeleton must be formed only during the final stages of platelet maturation. Because lamellar spreading of proplatelets involves the dynamic assembly and disassembly of actin filaments, the filamin A–von Willebrand factor receptor linkage could only be established once the underlying actin cytoskeletal network is stabilized. This reinforces the notion that intermediate swellings do not correspond to assembled platelets, and suggests that platelet assembly completes just before release.
An additional dynamic feature of intermediate proplatelet segments is their rapid internal translocation. Viewed in the light microscope, nodular segments move bidirectionally along the proplatelet shaft, collide into each other, and fuse. These observations suggest that they are packets of material destined for assembly into nascent platelets, and the linear arrays of microtubules present throughout proplatelet processes probably serve as tracks for the transport of membrane, organelles, and granules into developing platelets. Therefore, we interpret the observed movement of particles (e.g., Fig. 2 c) to result from a combination of translocation along the microtubule tracks and proplatelet extension by microtubule growth.
Structural Insights into Proplatelet Formation
Proplatelet processes are generated by the elongation and thinning of blunt pseudopodia, which first appear at one pole of the cell and exhibit a unique organization of microtubule bundles parallel to the cell margins. Pseudopodial growth is associated with extension and apparent compression of these cortical microtubule bundles, and it is likely that the final orientation of microtubule bundles in the shafts of thinner proplatelet processes result from a continuation of this compression reaction. These structural details highlight earlier work (Radley and Haller 1982; Handagama et al. 1987; Tablin et al. 1990) demonstrating the central role of microtubules in proplatelet formation; indeed, disassembly of cytoplasmic microtubules with nocodazole blocks formation of pseudopodia, whereas taxol treatment results in the formation of very few abnormal protrusions. Proplatelets terminate in bulbous structures invariably lined by a bundle of microtubules that originate in the shaft, form a teardrop-shaped loop within the bulb, and reenter the shaft. The loop itself reveals at least two discrete bundles, which must have opposing polarity, within the distal portion of the proplatelet.
Whether loop formation is part of the mechanism that drives tube extension or a structural consequence of this process is unclear. In the simplest model, assembly of microtubules at their plus ends, either at the tip or tube base, could generate the forces leading to proplatelet extension. The invariable finding of microtubule loops at the termini, however, argues against a model of plus end–driven tip growth, such as that which occurs in axonal extension. Moreover, extension of the proplatelet per se does not require tip elongation because time-lapse microscopy reveals stationary tips during periods of intense shaft growth (e.g., Fig. 7 b). Alternatively, tubulin subunits could add selectively to microtubules at the base and thereby elongate the tube; this model makes the testable prediction that the plus ends (the preferred sites of microtubule assembly) of proplatelet microtubules lie near the cell body, an unlikely possibility. Therefore, we favor the third possibility that tubulin subunits add to the plus ends of microtubules dispersed throughout the proplatelet shaft, and that microtubule motor proteins aid in tube elongation by sliding individual microtubules past one another. Such sliding would both lengthen the tube and reduce its diameter, consistent with the present observations, while microtubule-based motor activity might also contribute to the observed bends in proplatelet extensions and deliver platelet components to the ends.
Our examination of the sequence of events leading to proplatelet bending and bifurcation reveals critical details and again suggests mechanistic possibilities. For a proplatelet extension to bifurcate into two processes, three unique events must occur. First, the proplatelet shaft must bend sharply until it folds over on itself to form a loop that in turn elongates until it is structurally indistinguishable from its parent form. Second, microtubules within the tube must fragment, branch, or bend into loops, as depicted in Fig. 5, which elongate to form new processes. Third, either two sections of the shaft must fuse or looping must occur within the confines of the proplatelet shaft. The transient cell spreading and actin-based movements observed at sites of bifurcation may be necessary to accomplish this feat. The mechanical forces leading to tube branching require the activity of the actin cytoskeleton because treatment with cytochalasins prevents all branch formation. Regions with focal actin filament assembly may, thus, transmit contractile forces to the microtubule bundle to help compress it during loop formation.
In the last step, proplatelets separate from the residual cell mass and are released into the culture medium by a retractile mechanism. The tension generated by this retraction can be observed as a snapping of the strands between individual proplatelet extensions (data not shown). Although individual platelet-sized particles frequently appear in the culture, most of the released material consists of chains containing two or more platelet-sized particles. Release of individual platelet-sized particles in vivo may be accelerated by a fragmentation mechanism that is not expressed in cell culture or by shear forces normally encountered in the circulation. The process of proplatelet formation may also be modified by factors present in vivo. The bone marrow environment is composed of a complex adherent cell population containing endothelial cells, macrophages, and preadipocytes, which could play a role in platelet formation by direct cell contact or secretion of cytokines.
Toward a Modified Proplatelet Model of Thrombopoiesis
Our observations support the view that proplatelets are essential intermediates in platelet assembly, but refute the widely assumed concept that proplatelets are simply chains of fully and equally mature blood platelets. While platelet-sized units found along the shaft of proplatelets may represent intermediate stages in platelet maturation, these segments are surprisingly motile and unstable. Therefore, we suggest a conservative reinterpretation of previous models that imply that each platelet-sized swelling observed along proplatelet shafts is a nascent blood platelet. Only the particles at the ends of proplatelets consistently display the mature feature of a single microtubule rolled into a coil. This finding posits the ends of proplatelets as the best sites of platelet assembly. One implication of these results is that proplatelet branching represents an elegant mechanism designed to increase the number of termini for productive thrombopoiesis. The process of proplatelet branching, therefore, appears to play an essential role in platelet biogenesis and provides a mechanism for increasing the final number of released platelets. By looping microtubules, the mechanism of proplatelet bifurcation may also initiate the process of rolling individual microtubules into the marginal ring destined to be incorporated into each mature blood platelet.
Reconstitution of platelet formation from murine megakaryocytes in vitro provides a powerful system for identifying the cytoskeletal components involved in the remarkable mechanism of platelet formation and release. More detailed structural analysis, coupled with the ability to study knockout mice lacking specific proteins will allow us to probe the contribution of individual components in thrombopoiesis. The principles learned from studying cytoskeletal dynamics in megakaryocytes are likely to provide insights into mechanisms of shape changes and movements of other cells.
Acknowledgments
We thank Ezra Poister (both from Brigham and Women's Hospital) for superb and invaluable assistance with culture of mouse megakaryocytes, Dr. Thomas P. Stossel for his supportive environment and helpful criticisms, and Dr. Kurt Barkalow (Dana-Farber Cancer Institute) for many valuable suggestions.
This work was supported by the National Institutes of Health grant HL56252 (to J. Hartwig) and the Edwin S. Webster Foundation. We thank Ned Hiam for generously supporting this research. J. Italiano was supported by a training grant (HLO7680) and a postdoctoral fellowship from the National Institutes of Health; P. Lecine was supported by a fellowship from the American Society of Hematology; and R.A. Shivdasani was supported in part by faculty development awards from Harcourt General Foundation and the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation.
Footnotes
The online version of this article contains supplemental material.
References
- An E., Ogata K., Kuriya S., Nomura T. Interleukin-6 and erythropoietin act as direct potentiators and inducers of in vitro cytoplasmic process formation on purified mouse megakaryocytes. Exp. Hematol. 1994;22:149–156. [PubMed] [Google Scholar]
- Andrews R.K., Fox J.B. Interaction of purified actin-binding protein with glycoprotein Ib-IX complex. J. Biol. Chem. 1991;266:7144–7147. [PubMed] [Google Scholar]
- Becker R.P., DeBruyn P.P. The transmural passage of blood cells into myeloid sinusoids and the entry of platelets into the sinusoidal circulationa scanning electron microscopic investigation. Am. J. Anat. 1976;145:1046–1052. doi: 10.1002/aja.1001450204. [DOI] [PubMed] [Google Scholar]
- Behnke O. An electron microscope study of megakaryocytes of rat bone marrow. I. The development of the demarcation membrane system and the platelet surface coat. J. Ultrastruct. Res. 1968;24:412–428. doi: 10.1016/s0022-5320(68)80046-2. [DOI] [PubMed] [Google Scholar]
- Behnke O. An electron microscope study of the rat megakaryocyte. II. Some aspects of platelet release and microtubules. J. Ultrastruct. Res. 1969;26:111–129. doi: 10.1016/s0022-5320(69)90039-2. [DOI] [PubMed] [Google Scholar]
- Carlsson L., Markey F., Blikstad I., Persson T., Lindberg U. Reorganization of actin in platelets stimulated by thrombin as measured by the DNase I inhibition assay. Proc. Natl. Acad. Sci. USA. 1979;76:6376–6380. doi: 10.1073/pnas.76.12.6376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi E.S., Nichol J.L., Hokom M.M., Hornkohl A.C., Hunt P. Platelets generated in vitro from proplatelet-displaying human megakaryocytes are functional. Blood. 1995;85:402–413. [PubMed] [Google Scholar]
- Cramer E., Norol F., Guichard J., Breton-Gorius J., Vainchenker W., Massé J.M., Debili N. Ultrastructure of platelet formation by human megakaryocytes cultured with the Mpl ligand. Blood. 1997;89:2336–2346. [PubMed] [Google Scholar]
- Drachman J., Sabath D., Fox N., Kaushansky K. Thrombopoietin signal transduction in purified murine megakaryocytes. Blood. 1997;89:483–492. [PubMed] [Google Scholar]
- Ebbe S., Stohlman F. Megakaryocytopoiesis in the rat. Blood. 1965;26:20–34. [PubMed] [Google Scholar]
- Falet H., Pain S., Rendu F. Tyrosine unphosphorylated platelet SHP-1 is a substrate for calpain. Biochem. Biophys. Res. Commun. 1998;252:51–55. doi: 10.1006/bbrc.1998.9593. [DOI] [PubMed] [Google Scholar]
- Fox J., Phillips D.R. Inhibition of actin polymerization in blood platelets by cytochalasins. Nature. 1981;292:650–652. doi: 10.1038/292650a0. [DOI] [PubMed] [Google Scholar]
- Fox J., Koyles J., Reynolds C., Phillips J. Actin filament content and organization in unstimulated platelets. J. Cell Biol. 1984;98:1985–1991. doi: 10.1083/jcb.98.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., Aggerbeck L., Berndt M. Structure of the glycoprotein Ib-IX complex from platelet membranes. J. Biol. Chem. 1988;263:4882–4890. [PubMed] [Google Scholar]
- Fox J., Shattil S., Kinlough-Rathbone R., Richardson M., Packham M., Sanan D. The platelet cytoskeleton stabilizes the interaction between αIIbβ3 and its ligand and induces selective movement of ligand-occupied integrin. J. Biol. Chem. 1996;271:7004–7011. doi: 10.1074/jbc.271.12.7004. [DOI] [PubMed] [Google Scholar]
- Haller C., Radley J. Time-lapse cinemicrography and scanning electron microscopy of platelet formation by megakaryocytes. Blood Cells. 1983;9:407–418. [PubMed] [Google Scholar]
- Handagama P., Feldman B., Jain N., Farver T., Kono C. In vitro platelet release by rat megakaryocyteseffect of metabolic inhibitors and cytoskeletal disrupting agents. Am. J. Vet. Res. 1987;48:1142–1146. [PubMed] [Google Scholar]
- Harker L.A., Finch C.A. Thrombokinetics in man. J. Clin. Invest. 1969;48:963–974. doi: 10.1172/JCI106077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J. Mechanism of actin rearrangements mediating platelet activation. J. Cell Biol. 1992;118:1421–1442. doi: 10.1083/jcb.118.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig J., DeSisto M. The cytoskeleton of the resting human blood plateletstructure of the membrane skeleton and its attachment to actin filaments. J. Cell Biol. 1991;112:407–425. doi: 10.1083/jcb.112.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haydon G., Taylor D. Microtubules in hamster platelets. J. Cell Sci. 1965;26:673–676. doi: 10.1083/jcb.26.2.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T., Oresajo C. A method for quantifying F-actin in chemotactic peptide activated neutrophilsstudy of the effect of tBOC peptide. Cell Motil. 1985;5:545–557. doi: 10.1002/cm.970050609. [DOI] [PubMed] [Google Scholar]
- Karlsson R., Lassing I., Hoglund A.-S., Lindberg U. The organization of microfilaments in spreading plateletsa comparison with fibroblasts and glial cells. J. Cell Physiol. 1984;121:96–113. doi: 10.1002/jcp.1041210113. [DOI] [PubMed] [Google Scholar]
- Kaufman R.M., Airo R., Pollack S., Crosby W.H. Circulating megakaryocytes and platelet release in the lung. Blood. 1965;26:720–728. [PubMed] [Google Scholar]
- Kaushansky K. Thrombopoietinthe primary regulator of platelet production. Blood. 1995;85:419–431. [PubMed] [Google Scholar]
- Kenney D., Linck R. The cytoskeleton of unstimulated blood plateletsstructure and composition of the isolated marginal microtubular band. J. Cell Sci. 1985;78:1–22. doi: 10.1242/jcs.78.1.1. [DOI] [PubMed] [Google Scholar]
- Kovacsovics T.J., Hartwig J.H. Thrombin-induced GPIb-IX centralization on the platelet surface requires actin assembly and myosin II activation. Blood. 1996;87:618–629. [PubMed] [Google Scholar]
- Lecine P., Villeval J., Vyas P., Swencki B., Xu Y., Shivdasani R.A. Mice lacking transcription factor NF-E2 provide in vivo validation of the proplatelet model of thrombocytopoiesis and show a platelet production defect that is intrinsic to megakaryocytes. Blood. 1998;92:1608–1616. [PubMed] [Google Scholar]
- Leven R.M., Yee M.K. Megakaryocyte morphogenesis stimulated in vitro by whole or partially fractionated thrombocytopenic plasmaa model system for the study of platelet formation. Blood. 1987;69:1046–1052. [PubMed] [Google Scholar]
- Lichtman M., Chamberlain J., Simon W., Santillo P. Parasinusoidal location of megakaryocytes in marrowa determinant of platelet release. Am. J. Hematol. 1978;4:303–312. doi: 10.1002/ajh.2830040402. [DOI] [PubMed] [Google Scholar]
- Nachmias V.T., Yoshida K.-I. The cytoskeleton of the blood plateleta dynamic structure. Adv. Cell Biol. 1988;2:181–211. [Google Scholar]
- Paulus J. DNA metabolism and development of organelles in guinea-pig megakaryocytesa combined ultrastructural, autoradiographic and cytophotometric study. Blood. 1970;35:298–311. [PubMed] [Google Scholar]
- Radley J.M., Scurfield G. The mechanism of platelet release. Blood. 1980;56:996–999. [PubMed] [Google Scholar]
- Radley J.M., Haller C.J. The demarcation membrane system of the megakaryocytea misnomer? Blood. 1982;60:213–219. [PubMed] [Google Scholar]
- Radley J.M., Hatshorn M. Megakaryocyte fragments and the microtubule coil. Blood Cells. 1987;12:603–608. [PubMed] [Google Scholar]
- Radley J.M., Hatshorn M., Green S. The response of megakaryocytes with processes to thrombin. Thromb. Haemostasis. 1987;58:732–736. [PubMed] [Google Scholar]
- Radley J.M., Rogerson J., Ellis S., Hasthorpe S. Megakaryocyte maturation in long-term marrow culture. Exp. Hematol. 1991;19:1075–1078. [PubMed] [Google Scholar]
- Scurfield G., Radley J.M. Aspects of platelet formation and release. Am. J. Hematol. 1981;10:285–296. doi: 10.1002/ajh.2830100308. [DOI] [PubMed] [Google Scholar]
- Stenberg P.E., Levin J. Mechanisms of platelet production. Blood Cells. 1989;15:23–31. [PubMed] [Google Scholar]
- Tablin F., Castro M., Leven R.M. Blood platelet formation in vitrothe role of the cytoskeleton in megakaryocyte fragmentation. J. Cell Sci. 1990;97:59–70. doi: 10.1242/jcs.97.1.59. [DOI] [PubMed] [Google Scholar]
- Topp K., Tablin F., Levin J. Culture of isolated bovine megakaryocytes on reconstituted basement membrane matrix leads to proplatelet process formation. Blood. 1990;76:912–924. [PubMed] [Google Scholar]
- Trowbridge E.A., Martin J.F., Slater D.N., Kishk Y.T., Warren C.W., Harley P.J., Woodcock W. The origin of platelet count and volume. Clin. Phys. Physiol. Meas. 1984;5:145–156. doi: 10.1088/0143-0815/5/3/007. [DOI] [PubMed] [Google Scholar]
- White J. Effects of colchicine and vinca alkaloids on human platelets. Am. J. Pathol. 1968;53:281–291. [PMC free article] [PubMed] [Google Scholar]
- White J., Krivit W. An ultrastructural basis for the shape changes induced in platelets by chilling. Blood. 1967;30:625–635. [PubMed] [Google Scholar]
- Yamada F. The fine structure of the megakaryocyte in the mouse spleen. Acta Anat. 1957;29:267–290. doi: 10.1159/000141169. [DOI] [PubMed] [Google Scholar]
- Zucker-Franklin D. The ultrastructure of megakaryocytes and platelets. In: Gordon A., editor. Regulation of Hematopoiesis. Vol. 55. Appleton-Century-Crofts; New York: 1970. pp. 1553–1586. [Google Scholar]
- Zucker-Franklin D. Platelet structure and function. In: Kuter D., Hunt P., Sheridan W., Zucker-Franklin D., editors. Thrombopoiesis and Thrombopoietins. Humana Press; Totowa, NJ: 1997. pp. 41–62. [Google Scholar]
- Zucker-Franklin D., Petursson S. Thrombocytopoiesis—analysis by membrane tracer and freeze-fracture studies on fresh human and cultured mouse megakaryocytes. J. Cell Biol. 1984;99:390–402. doi: 10.1083/jcb.99.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]