Abstract
Human granulocytic anaplasmosis is an emerging tick-borne disease caused by Anaplasma phagocytophilum. A. phagocytophilum cells activate Toll-like receptor 2 signaling and possess mitogenic activity, and A. phagocytophilum infection in vivo activates NKT cells unrelated to major surface protein 2 (Msp2) hypervariable region expression. Thus, we hypothesized that lipoprotein or glycolipid components of A. phagocytophilum membranes could be important triggers of the innate immune response and immunopathology. A. phagocytophilum membranes depleted of Msp2 and protein antigens enhanced the proliferation of naïve mouse splenocytes beyond that of untreated membranes. Protein-depleted and polar lipid-enriched membranes from low-passage A. phagocytophilum cultures enhanced naïve splenocyte lymphoproliferation to a much greater degree than did these fractions from high-passage cultures of bacterial membranes (1.8- to 3.7-fold for protein-depleted fractions and 4.8- to ≥17.7-fold for polar lipid-enriched fractions). These results support the hypothesis that components that are enriched among polar lipids in the A. phagocytophilum membrane stimulate innate immune cell proliferation, possibly activating NKT cells that link innate and adaptive immunity, and immunopathology.
Human granulocytic anaplasmosis (HGA) is an emerging tick-borne disease caused by Anaplasma phagocytophilum, an obligate intracellular bacterium that infects neutrophils and lacks pathways for both lipopolysaccharide (LPS) and peptidoglycan biosynthesis (10, 16). Although the majority of human infections are mild, severe and even fatal infections complicated by interstitial pneumonitis, adult respiratory distress syndrome, and sepsis or toxic shock-like syndromes are well recognized (10, 20, 22, 30). The inflammatory basis of the disease is demonstrated in human histopathologic investigations and animals, including mouse models (4, 13, 15). In the mouse model, pathogenicity results from the induction of innate immunity and gamma interferon (IFN-γ), which drives local effectors and inflammatory tissue injury (3, 17, 18, 26). However, this inflammatory phenotype is also determined in part by bacterial factors, since infection of horses by isogenic A. phagocytophilum differing only in the length of in vitro passage results in differential clinical severity and hematologic derangements (21). A similar dichotomous phenotype was observed among B6 mice infected with A. phagocytophilum propagated for different lengths of time in vitro, resulting in the differential expansion of NK1.1+ cells and CD8+ splenic lymphocytes on days 2 to 7 (9). In fact, peak hepatic inflammation and plasma IFN-γ production with infection by low-passage bacteria occur on day 2, when adaptive immunity is very unlikely to be present, corresponding to approximately the same time that splenic NKT cells become activated.
T-cell responses to the immunodominant major surface protein 2 (Msp2) hypervariable regions that vary with in vitro propagation do not occur to any substantial degree, diminishing their importance as inflammatory stimuli (7). However, lymphoproliferative responses to whole-cell A. phagocytophilum cultures are detectable even among splenocytes from naïve mice. Owing to these data, the innate immune responses in mice infected with A. phagocytophilum, and our prior data implicating Toll-like receptor 2 (TLR2) but not TLR4 inflammatory signaling in human and murine macrophages exposed to A. phagocytophilum (8, 9, 26), we hypothesized that a lipoprotein or glycolipid component of A. phagocytophilum membranes is an important trigger of the innate immune response and immunopathology. Thus, we studied A. phagocytophilum to identify whether bacterial membranes and membrane components could initiate differential naïve immune cell proliferation that in part underlies the virulence observed with changing in vitro passage intervals.
MATERIALS AND METHODS
Preparation of whole A. phagocytophilum cultures and components.
Low-passage (passages 5 to 7) and high-passage (passage 22) A. phagocytophilum Webster strain-infected HL-60 cells were propagated in vitro as described previously (7, 21). Heavily infected cells (infection rate of >95%) were centrifuged (200 × g for 15 min), and the cell pellet was washed with phosphate-buffered saline (PBS). Cell-free bacteria were prepared by sonication lysis (output control of 2 for 30 s; Branson Sonifier 250) of heavily infected cells. The residual intact cells and fragments were removed by centrifugation at 150 × g, and the supernatant was centrifuged at 12,000 × g for 30 min to obtain a bacterial pellet that was washed twice in sterile PBS. The final pellet was resuspended in sterile PBS, and protein content was measured by the bicinchoninic assay method (Pierce Biotechnology, Rockford, IL).
Bacterial membranes were prepared as described previously (2) by sonication on ice and separation from residual organisms by centrifugation. The bacterial lysate was layered onto a sucrose step gradient and centrifuged at 82,000 × g for 20 h at 4°C. After centrifugation, bands corresponding to approximately 1.15 and 1.22 g/cm3 were collected, suspended in cold 10 mM HEPES buffer (pH 7.4), and centrifuged at 177,000 × g for 1 h. Protein concentrations were determined, and the entire volume was subjected to centrifugation on a 25% sucrose gradient at 56,000 × g for 8 h to obtain both pellet (cytosol-enriched) and top (membrane-enriched) layers. Each layer was harvested, the protein content was determined and examined for protein distribution by Coomassie blue staining, and the Msp2 content was assessed by protein immunoblotting using Msp2 monoclonal antibody (MAb) 20B4. Some membrane fractions were treated with 1 μg/ml proteinase K for 1 h at 37°C followed by heat inactivation at 60°C for 2 h. All fractions used for splenocyte proliferation assays were tested for LPS contamination using the Limulus lysate assay (E-Toxoate; Sigma Chemical Co., St. Louis, MO).
To prepare polar lipid-enriched membrane fractions, 0.1 ml of packed A. phagocytophilum cells (low and high passage) freed from host cells was extracted with methanol and chloroform (24). Briefly, bacteria were adjusted to a final volume of 1.42 ml in ice-cold water and homogenized on ice. Four milliliters of methanol was added to this homogenate, vortexed vigorously, and allowed to come to room temperature. Two milliliters of chloroform was added to this suspension, followed by vigorous vortexing. The extraction mixture was then centrifuged at 425 × g for 15 min, after which the supernatant was removed and measured, and 0.17 parts of water was added, followed again by vigorous vortexing. After another centrifugation at 425 × g for 15 min, the upper polar lipid layer was harvested and evaporated to dryness and reconstituted in RMPI 1640 medium. Polar lipid-enriched membrane fractions purified in this way were examined for Msp2 content by protein immunoblotting and used as a stimulant for lymphoproliferation assays. Before use in splenocyte proliferation assays, the polar lipid-enriched preparations were tested for LPS contamination using the Limulus lysate assay.
Immunoblotting.
To assess the differential distribution of proteins into membranes or cytosol, and to assess the efficacy by which protease treatment and polar lipid extraction eliminated antigenic protein contamination, protein immunoblotting was used. Briefly, whole A. phagocytophilum cells or a preparation of bacterial membranes, cytosol, protease-treated membranes, or suspensions of polar lipids were resolved on 10% sodium dodecyl sulfate-polyacrylamide gels under reducing conditions, transferred onto nitrocellulose membranes, and then washed in a blocking solution containing 5% nonfat dried milk in Tris-buffered saline-Tween 20 (Fisher Biotech, Fair Lawn, NJ); some gels were stained directly with Coomassie blue for estimating protein content. After blocking, the membranes were incubated with either rabbit anti-A. phagocytophilum polyclonal serum, normal rabbit serum, A. phagocytophilum Msp2 MAb 20B4, or isotype-matched (immunoglobulin G2aκ) control MAb. The nitrocellulose blots were then washed and incubated with alkaline phosphatase-conjugated secondary antibody to mouse or rabbit immunoglobulin G (KPL, Gaithersburg, MD). Bands were detected by incubation with BCIP-NBT (Sigma Chemical Co., St. Louis, MO) as a substrate for alkaline phosphatase and color development. The density of the bands was quantified after densitometry by measuring the area under the curve established for the density of each band.
In vitro splenocyte proliferation assays.
Splenocytes (2 × 105 cells/ml) obtained from naïve B6 mice at necropsy were resuspended in RPMI 1640 containing 10% fetal bovine serum and 1× penicillin-streptomycin and seeded in duplicate into 96-well culture plates (9). After 24 h of incubation, the cell suspension was stimulated in duplicate wells by adding sterile medium, concanavalin A (ConA) (5 and 1 μg/ml; Sigma, St. Louis, MO), purified whole A. phagocytophilum at low and high passage, and membrane and cytosol proteins at concentrations of 50 to 60 and 25 to 30 μg/ml protein content. After 72 h of incubation, cells were used in cell proliferation assays (bromodeoxyuridine [BrdU] cell proliferation assay kit; Calbiochem, San Diego, CA) according to the manufacturer's recommendations. BrdU incorporation into cells was measured at dual wavelengths of 450 and 540 nm. Owing to the lower sensitivity and signal-to-noise ratio of the nonradiometric BrdU incorporation assay compared with [3H]thymidine incorporation (29), proliferation was expressed as overall or net optical density (instead of stimulation index) for each splenocyte preparation. Significant lymphoproliferative activity was determined to be present when the mean results of duplicate wells were greater than the means + 3 standard deviations of medium-only controls or when means of proliferation or proportional proliferation were significantly different using Student's t tests with P values of <0.05 and one-sided analyses when predicted to be directional.
RESULTS
Separation of components of low- and high-passage A. phagocytophilum cultures.
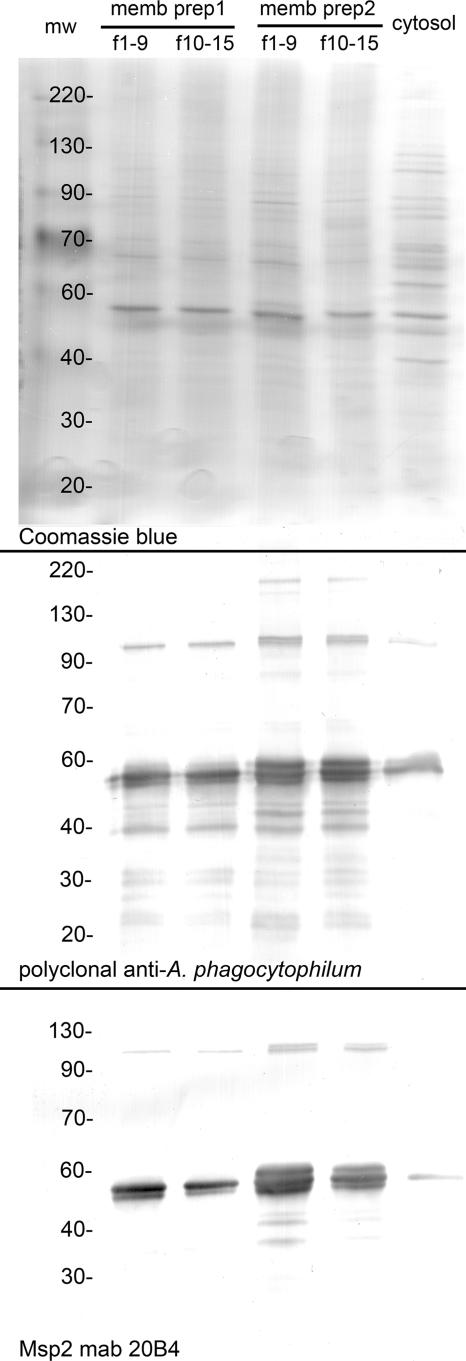
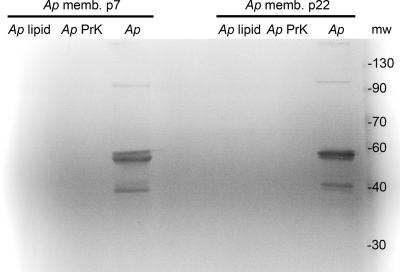
The overall protein contents were similar between the A. phagocytophilum membrane and cytosolic preparations. However, membranes were enriched for and cytosol was depleted of antigenic proteins, including the known surface-exposed protein Msp2 (Fig. 1, top). A. phagocytophilum antigens partitioned into the membranes by a factor of 4.1- to 5.3-fold more than in cytosol (Fig. 1, middle), as did Msp2, by a factor of 15.5- to 30.1-fold (Fig. 1, bottom). Both proteinase K digestion and methanol-chloroform extraction depleted proteins in membrane fractions to levels that were undetectable by Coomassie blue staining (<100 ng) (data not shown) and by Msp2 protein immunoblotting (<8 ng) (Fig. 2). Since Msp2 is the most abundant protein, comprising approximately 20 to 25% of total A. phagocytophilum membrane protein content (our unpublished data), these data show the nearly total depletion of proteins from the protease-treated and polar lipid-enriched membrane fractions.
FIG. 1.
Relative distribution of proteins, antigens, and Msp2 in membrane and cytosolic fractions of A. phagocytophilum. Lanes 1 and 2 and 3 and 4 represent membrane fractions 1 to 9 (f1-9) and 10 to 15 (f10-15), respectively, from separate preparations, and lane 5 represents the pooled cytoplasmic fractions from these preparations. The top panel shows a total protein Coomassie blue stain illustrating the overall similar protein contents but dissimilar protein profiles between membranes and bacterial cytosol. The middle panel is a protein immunoblot reacted with rabbit polyclonal antibody prepared for whole, purified A. phagocytophilum strain Webster cells, again depicting the 4.1- to 5.3-fold-greater distribution of antigens into the membrane fraction. The bottom panel shows protein immunoblotting with MAb 20B4 to A. phagocytophilum Msp2 and depicts even greater (15.5- to 30.1-fold) partitioning of Msp2 into bacterial membranes than into bacterial cytosol. Molecular weights (mw) (in thousands) are shown on the left side of each panel.
FIG. 2.
Methanol-chloroform extraction of polar lipids (Ap lipid) and protease treatment (Ap PrK) of purified A. phagocytophilum membranes from low-passage (Ap memb p7) and high-passage (Ap memb p22) bacteria removes detectable Msp2 by protein immunoblotting using MAb 20B4. Note that the typical monomeric and oligomeric Msp2 bands present without treatment are absent after treatment. mw, molecular weight (in thousands).
Membranes and polar lipids of low- and high-passage A. phagocytophilum cultures induce differential naïve splenocyte lymphoproliferation.
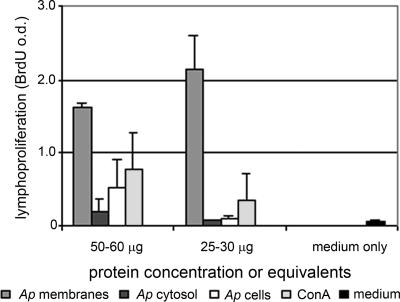
Splenocytes from naïve mice proliferated when exposed to 50 to 60 μg (based on protein content) of bacterial membranes, bacterial cytosol, or purified whole A. phagocytophilum cells and to 25 to 30 μg (based on protein content) of bacterial membranes as well as to ConA (Fig. 3). Considerably more proliferation in response to purified membrane proteins was detected than in response to A. phagocytophilum cells or cytosol (P < 0.017 by Student's t test), indicating the enrichment of endogenous mitogenic activity in A. phagocytophilum membranes.
FIG. 3.
Proliferation of naïve mouse splenic lymphocytes as reflected by the optical density (o.d.) of BrdU incorporation after exposure to A. phagocytophilum purified membranes (Ap membranes), cytosol (Ap cytosol), whole bacterial cells (Ap cells), ConA, and medium only. Significantly more proliferation in response to purified membranes than in response to either whole cells or cytosol was observed, suggesting the enrichment or availability of a membrane-associated mitogenic factor.
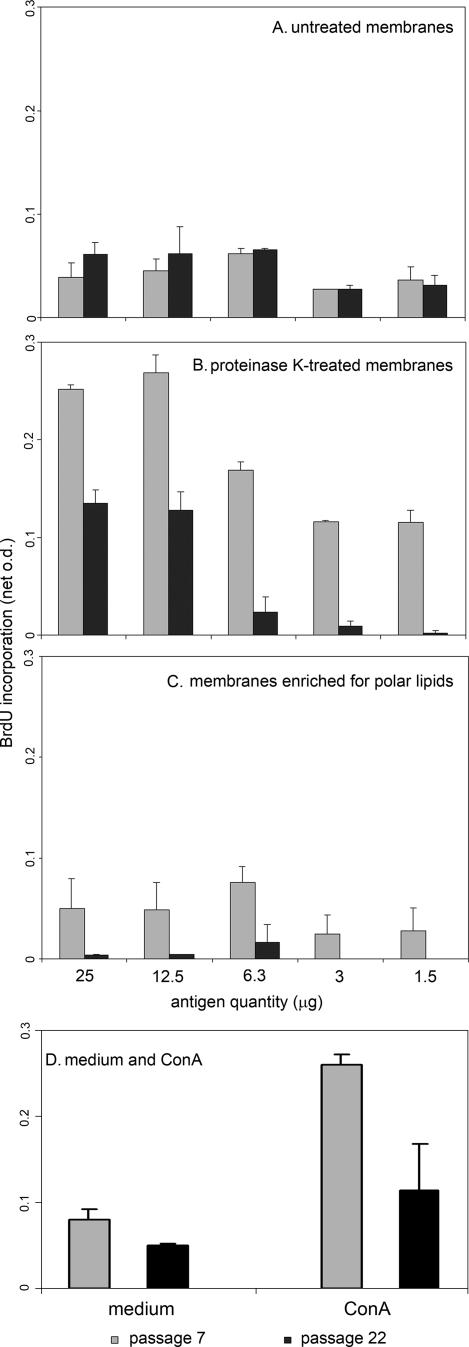
To determine whether protein or nonprotein components of A. phagocytophilum membranes possessed mitogenic activity, membranes, protease-treated membranes, and polar lipids obtained from equivalent starting amounts of A. phagocytophilum cells of passage 7 (low) and passage 22 (high) were prepared. These components were serially diluted and added to splenocytes from naïve mice that were assessed for relative proliferation by comparison with proliferation of splenocytes in response to medium only or ConA (Fig. 4). As anticipated, overall splenocyte proliferation in response to ConA was significantly more than that for medium alone (P < 0.010) (Fig. 4D). Proliferation in response to untreated membranes (Fig. 4A) and proteinase K-treated membranes (Fig. 4B) was significantly greater than that to medium alone for both low- and high-passage bacteria (P < 0.029 by Student's test, one-sided unequal variance). The maximum difference in proliferation between medium-only and untreated membranes was observed with 3 to 12.5 μg (P < 0.035 by one-sided, unequal-variance Student's t tests). While all concentrations of low-passage protease-treated membranes stimulated proliferation significantly beyond that of medium only (P < 0.009), more proliferation in response to high-passage-bacterium protease-treated membranes compared to medium was observed only with higher concentrations of stimulating protein equivalents (12.5 and 25 μg) (P < 0.011). Compared to medium only, overall lymphoproliferation was stimulated only when cells were exposed to membranes enriched for polar lipids from low-passage bacteria (P < 0.010), mostly with the equivalent of 6.3 μg of membrane protein (Fig. 4C). Overall proliferation in response to high-passage-bacterium polar lipid-enriched membranes did not differ from that of medium only (P = 0.139), although low-level proliferative activity was observed with high concentrations (equivalent to 12.5 to 25 μg) (P < 0.028).
FIG. 4.
Splenocyte proliferation with exposure to untreated low- and high-passage A. phagocytophilum membranes (A), protease-treated bacterial membranes (B), and membranes enriched for polar lipids (C). Although proliferations in response to untreated membranes are similar when splenocytes are stimulated with membranes from low- and high-passage bacterial cultures, depletion of proteins enhances proliferation more, and enrichment for polar lipids retains more proliferative activity for low-passage than for high-passage bacterial cultures. D illustrates the lymphoproliferative activity of splenocytes exposed to medium only or ConA for experiments that used low- or high-passage A. phagocytophilum-derived antigens, although bacterial antigens were not used in the control cultures.
When low- and high-passage A. phagocytophilum cultures were compared, proliferation was not different from that for untreated bacterial membranes (P = 0.389). Protease-treated membranes from low-passage A. phagocytophilum cultures stimulated 1.8- to 3.7-fold more proliferation (P < 0.001) than did protease-treated membranes from high-passage bacterial cultures (Fig. 4B). Overall, bacterial membranes enriched for polar lipids from low-passage A. phagocytophilum cultures induced 4.8- to ≥17.7-fold more splenocyte proliferation than those from high-passage bacterial cultures (P < 0.001) (Fig. 4C). Net proliferation was lower for untreated and polar lipid-enriched membranes than for proteinase K-treated membranes. The differences between proliferation in response to protease-treated and polar lipid-enriched membranes perhaps resulted from the inevitable lipid losses during extraction. These data provide evidence that nonimmune splenocyte proliferation can be driven by a non-Msp2, nonprotein component of A. phagocytophilum membranes that is enriched in the polar lipid fraction. Moreover, the degree of proliferation-inducing activity differs among the membranes and polar lipid-enriched membranes of A. phagocytophilum passaged for different lengths of time in vitro.
DISCUSSION
Despite its ability to avoid clearance by innate immune responses, A. phagocytophilum virulence is mediated in part by inducing host innate immunopathology (8, 9, 18, 27, 28). The resulting proinflammatory response leads to the recruitment of additional potential hosts and retention of infected cells in the vasculature, possibly to increase accessibility to tick bite wound vascular pools (1, 5, 6, 25). The inflammatory side effects are the foundation for histopathologic injury and disease, but the mechanisms of inflammation induction are not clear. In fact, the best candidate ligand to drive this process, the immunodominant A. phagocytophilum surface-exposed protein Msp2, is probably not an important stimulus for tissue inflammation and the generation of inflammatory or immune effectors (7). Moreover, owing to the dichotomous disease phenotypes with ongoing in vitro cultivation, microbial factors that drive immunopathologic responses must also vary with isogenic A. phagocytophilum passage (9, 21). Since severe consequences include septic or toxic shock-like presentations, interstitial pneumonitis, or other significant inflammatory pathologies, and given the lack of both LPS and peptidoglycan in A. phagocytophilum cell walls, identification of the antigens or stimulants that initiate innate immunity and immunopathologic responses becomes an important goal.
Based on the recognition that IFN-γ; TLR2 signaling; NK, NKT, and CD8 T cells; and pathogen in vitro propagation appear to play a role in inflammatory signaling and histopathologic severity (8, 9, 18, 21), we first determined that most lymphoproliferative activity for splenocytes was present in purified bacterial membranes. We previously showed that lymphoproliferation in response to highly purified, recombinant Msp2 hypervariable region proteins did not occur but that whole A. phagocytophilum cells possessed mitogenic activity (7). Thus, we sought to develop evidence that most of this activity could not be accounted for by either Msp2 or other proteins. Protease digestion results in significant increases in lymphoproliferative activity beyond that with purified membranes alone. Thus, these data strongly imply a minimal or negligible role for proteins, including other antigens detected in protein immunoblots (Fig. 1), although a role for an A. phagocytophilum protein that suppresses lymphoproliferative activity cannot be excluded. In concert with the dichotomous inflammation phenotype, mitogenic activity is also altered with in vitro propagation since more extensive proliferation was observed when naïve splenocytes were exposed to protease-treated and polar lipid-enriched membranes from low-passage A. phagocytophilum cultures than from high-passage A. phagocytophilum cultures.
Coupled with our recent description of NKT cell activation in the murine model of HGA and the recognition that another related Anaplasmataceae family member possesses NKT cell ligands, these data suggest that A. phagocytophilum mitogenic activity could be related to polar lipids in the bacterial membrane (9, 19). In keeping with this hypothesis and with the observation that A. phagocytophilum-infected mice develop more severe and earlier hepatic histopathology (7, 9), purified membrane polar lipids from low-passage bacteria account for a substantially higher proportion of membrane mitogenic activity than do polar lipids from high-passage A. phagocytophilum membranes. Whether these membrane polar lipids are good ligands for NKT cell activation and whether a role exists for NKT cells in the development of histopathologic lesions in the murine model need to be assessed (9).
These data provide several important observations that may facilitate further work toward understanding the basis of disease in HGA and in potentially managing adverse complications and outcomes. First, innate mechanisms are sufficient for generating in vitro activities that correlate with the immunopathologic response to A. phagocytophilum. Second, at least part of the activity observed in immunologically naïve cells results from exposure to A. phagocytophilum membranes depleted of proteins and enriched for polar lipids that are thought to be important ligands for NKT cells or for innate immune responses via TLR2. While this observation is not new for bacterial infections, in fact, only a few NKT ligands have been demonstrated to exist among clinically significant bacterial infections (11, 12, 14), and their precise identification in A. phagocytophilum could expand that list and investigative opportunities. Finally, in vitro analysis confirms that the polar lipid components that drive lymphocyte proliferation vary in activity with in vitro passage. This variance perhaps owes to changes in abundance or structure induced by unknown selective or permissive forces applied in culture systems. The latter point is also important since the clinical manifestations of HGA are extremely variable among human and animal populations, and natural variation in the activity of specific metabolic pathways could account for disparities in pathogen virulence (23). Obtaining evidence that supports such a microbial genetic component to the disease and focuses attention on appropriate inflammation-stimulating ligands, structures, or pathways could advance management and control strategies for this emerging vector-transmitted pathogen and investigation of inflammatory disease with LPS- and peptidoglycan-deficient bacteria.
Acknowledgments
This work was supported by grant R01 AI41213 to J.S.D. from the National Institutes of Allergy and Infectious Diseases.
We thank Nicole Barat for excellent technical support, Robert Yolken for his generous sharing of laboratory equipment, and Ron Schnaar for advice.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Akkoyunlu, M., S. E. Malawista, J. Anguita, and E. Fikrig. 2001. Exploitation of interleukin-8-induced neutrophil chemotaxis by the agent of human granulocytic ehrlichiosis. Infect. Immun. 69:5577-5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown, W. C., V. Shkap, D. Zhu, T. C. McGuire, W. Tuo, T. F. McElwain, and G. H. Palmer. 1998. CD4+ T-lymphocyte and immunoglobulin G2 responses in calves immunized with Anaplasma marginale outer membranes and protected against homologous challenge. Infect. Immun. 66:5406-5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Browning, M. D., J. W. Garyu, J. S. Dumler, and D. G. Scorpio. 2006. Role of reactive nitrogen species in development of hepatic injury in a C57bl/6 mouse model of human granulocytic anaplasmosis. Comp. Med. 56:55-62. [PubMed] [Google Scholar]
- 4.Bunnell, J. E., E. R. Trigiani, S. R. Srinivas, and J. S. Dumler. 1999. Development and distribution of pathologic lesions are related to immune status and tissue deposition of human granulocytic ehrlichiosis agent-infected cells in a murine model system. J. Infect. Dis. 180:546-550. [DOI] [PubMed] [Google Scholar]
- 5.Choi, K. S., J. Garyu, J. Park, and J. S. Dumler. 2003. Diminished adhesion of Anaplasma phagocytophilum-infected neutrophils to endothelial cells is associated with reduced expression of leukocyte surface selectin. Infect. Immun. 71:4586-4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi, K. S., D. J. Grab, and J. S. Dumler. 2004. Anaplasma phagocytophilum infection induces protracted neutrophil degranulation. Infect. Immun. 72:3680-3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi, K. S., D. G. Scorpio, N. C. Barat, and J. S. Dumler. 2007. Msp2 variation in Anaplasma phagocytophilum in vivo does not stimulate T cell immune responses or interferon-gamma production. FEMS Immunol. Med. Microbiol. 49:374-386. [Epub ahead of print.] [DOI] [PubMed] [Google Scholar]
- 8.Choi, K. S., D. G. Scorpio, and J. S. Dumler. 2004. Anaplasma phagocytophilum ligation to Toll-like receptor (TLR) 2, but not to TLR4, activates macrophages for nuclear factor-kappa B nuclear translocation. J. Infect. Dis. 189:1921-1925. [DOI] [PubMed] [Google Scholar]
- 9.Choi, K.-S., T. Webb, M. Oelke, D. G. Scorpio, and J. S. Dumler. 2007. Differential innate immune cell activation and proinflammatory response in Anaplasma phagocytophilum infection. Infect. Immun. 75:3124-3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dumler, J. S., K. S. Choi, J. C. Garcia-Garcia, N. S. Barat, D. G. Scorpio, J. W. Garyu, D. J. Grab, and J. S. Bakken. 2005. Human granulocytic anaplasmosis and Anaplasma phagocytophilum. Emerg. Infect. Dis. 11:1828-1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fischer, K., E. Scotet, M. Niemeyer, H. Koebernick, J. Zerrahn, S. Maillet, R. Hurwitz, M. Kursar, M. Bonneville, S. H. Kaufmann, and U. E. Schaible. 2004. Mycobacterial phosphatidylinositol mannoside is a natural antigen for CD1d-restricted T cells. Proc. Natl. Acad. Sci. USA 101:10685-10690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guinet, F., C. Ronet, M. Mempel, M. Huerre, E. Carniel, and G. Gachelin. 2002. NKT cells-containing inflammatory lesions induced by Yersinia pseudotuberculosis glycolipids. Immunol. Lett. 80:113-118. [DOI] [PubMed] [Google Scholar]
- 13.Hodzic, E., J. W. Ijdo, S. Feng, P. Katavolos, W. Sun, C. H. Maretzki, D. Fish, E. Fikrig, S. R. Telford III, and S. W. Barthold. 1998. Granulocytic ehrlichiosis in the laboratory mouse. J. Infect. Dis. 177:737-745. [DOI] [PubMed] [Google Scholar]
- 14.Kinjo, Y., E. Tupin, D. Wu, M. Fujio, R. Garcia-Navarro, M. R. Benhnia, D. M. Zajonc, G. Ben-Menachem, G. D. Ainge, G. F. Painter, A. Khurana, K. Hoebe, S. M. Behar, B. Beutler, I. A. Wilson, M. Tsuji, T. J. Sellati, C. H. Wong, and M. Kronenberg. 2006. Natural killer T cells recognize diacylglycerol antigens from pathogenic bacteria. Nat. Immunol. 7:978-986. [DOI] [PubMed] [Google Scholar]
- 15.Lepidi, H., J. E. Bunnell, M. E. Martin, J. E. Madigan, S. Stuen, and J. S. Dumler. 2000. Comparative pathology, and immunohistology associated with clinical illness after Ehrlichia phagocytophila-group infections. Am. J. Trop. Med. Hyg. 62:29-37. [DOI] [PubMed] [Google Scholar]
- 16.Lin, M., and Y. Rikihisa. 2003. Ehrlichia chaffeensis and Anaplasma phagocytophilum lack genes for lipid A biosynthesis and incorporate cholesterol for their survival. Infect. Immun. 71:5324-5331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Martin, M. E., J. E. Bunnell, and J. S. Dumler. 2000. Pathology, immunohistology, and cytokine responses in early phases of human granulocytic ehrlichiosis in a murine model. J. Infect. Dis. 181:374-378. [DOI] [PubMed] [Google Scholar]
- 18.Martin, M. E., K. Caspersen, and J. S. Dumler. 2001. Immunopathology and ehrlichial propagation are regulated by interferon-gamma and interleukin-10 in a murine model of human granulocytic ehrlichiosis. Am. J. Pathol. 158:1881-1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattner, J., K. L. Debord, N. Ismail, R. D. Goff, C. Cantu III, D. Zhou, P. Saint-Mezard, V. Wang, Y. Gao, N. Yin, K. Hoebe, O. Schneewind, D. Walker, B. Beutler, L. Teyton, P. B. Savage, and A. Bendelac. 2005. Exogenous and endogenous glycolipid antigens activate NKT cells during microbial infections. Nature 434:525-529. [DOI] [PubMed] [Google Scholar]
- 20.Paparone, P. W., P. Ljubich, G. A. Rosman, and N. T. Nazha. 1995. Ehrlichiosis with pancytopenia and ARDS. N. J. Med. 92:381-385. [PubMed] [Google Scholar]
- 21.Pusterla, N., J. E. Madigan, K. M. Asanovich, J. S. Chae, E. Derock, C. M. Leutenegger, J. B. Pusterla, H. Lutz, and J. S. Dumler. 2000. Experimental inoculation with human granulocytic Ehrlichia agent derived from high- and low-passage cell culture in horses. J. Clin. Microbiol. 38:1276-1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Remy, V., Y. Hansmann, S. De Martino, D. Christmann, and P. Brouqui. 2003. Human anaplasmosis presenting as atypical pneumonitis in France. Clin. Infect. Dis. 37:846-848. [DOI] [PubMed] [Google Scholar]
- 23.Reubel, G. H., R. B. Kimsey, J. E. Barlough, and J. E. Madigan. 1998. Experimental transmission of Ehrlichia equi to horses through naturally infected ticks (Ixodes pacificus) from Northern California. J. Clin. Microbiol. 36:2131-2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnaar, R. L. 1994. Isolation of glycosphingolipids. Methods Enzymol. 230:348-370. [DOI] [PubMed] [Google Scholar]
- 25.Scorpio, D. G., M. Akkoyunlu, E. Fikrig, and J. S. Dumler. 2004. CXCR2 blockade influences Anaplasma phagocytophilum propagation but not histopathology in the mouse model of human granulocytic anaplasmosis. Clin. Diagn. Lab. Immunol. 11:963-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Scorpio, D. G., F. D. von Loewenich, C. Bogdan, and J. S. Dumler. 2005. Innate immune tissue injury and murine HGA: tissue injury in the murine model of granulocytic anaplasmosis relates to host innate immune response and not pathogen load. Ann. N. Y. Acad. Sci. 1063:425-428. [DOI] [PubMed] [Google Scholar]
- 27.Scorpio, D. G., F. D. von Loewenich, H. Gobel, C. Bogdan, and J. S. Dumler. 2006. Innate immune response to Anaplasma phagocytophilum contributes to hepatic injury. Clin. Vaccine Immunol. 13:806-809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Loewenich, F. D., D. G. Scorpio, U. Reischl, J. S. Dumler, and C. Bogdan. 2004. Frontline: control of Anaplasma phagocytophilum, an obligate intracellular pathogen, in the absence of inducible nitric oxide synthase, phagocyte NADPH oxidase, tumor necrosis factor, Toll-like receptor (TLR)2 and TLR4, or the TLR adaptor molecule MyD88. Eur. J. Immunol. 34:1789-1797. [DOI] [PubMed] [Google Scholar]
- 29.Wemme, H., S. Pfeifer, R. Heck, and J. Muller-Quernheim. 1992. Measurement of lymphocyte proliferation: critical analysis of radioactive and photometric methods. Immunobiology 185:78-89. [DOI] [PubMed] [Google Scholar]
- 30.Wong, S., and L. J. Grady. 1996. Ehrlichia infection as a cause of severe respiratory distress. N. Engl. J. Med. 334:273. [DOI] [PubMed] [Google Scholar]