Abstract
We examined the association between haplotype tagging single-nucleotide polymorphisms in TLR4 and the pertussis toxin-specific immunoglobulin G response after whole-cell pertussis (wP) vaccination in 515 1-year-old children from the KOALA study. A lower titer was associated with the minor allele of rs2770150, supporting a role for Toll-like receptor 4 in the antibody response to wP vaccination.
Pertussis is a vaccine-preventable respiratory disease caused by Bordetella pertussis. Despite high vaccination coverage, pertussis is still prevalent in most countries, including The Netherlands, with epidemic peaks that occur every 2 to 3 years (12, 13, 27, 28). Susceptibility to B. pertussis and the course of infection vary between individuals. Studies in mice have provided evidence that murine host genes regulate susceptibility to B. pertussis infection (4, 19). Furthermore, animal studies indicated involvement of the Tlr4 gene in the infection process (5, 17, 23). In humans, two coding variants of TLR4 have been associated with enhanced susceptibility to infectious diseases, especially gram-negative infections, and with endotoxin hyporesponsiveness (1, 2, 29, 32). Since Tlr4 also plays a critical role in the response to whole-cell pertussis (wP) vaccination in mice (16; H. A. Banus, R. M. Strenger, E. R. Gremmer, J. Dormans, F. R. Mooi, T. G. Kimman, and R. J. Vandebriel, submitted for publication), we hypothesized that variation in the gene coding for Toll-like receptor 4 (TLR4) may account for some of the observed variability in the antibody response to this vaccine in humans. Furthermore, variation in response to vaccination may reflect differences in the course of infection (20). Here we studied the role of genetic variation in TLR4 in the response to wP vaccination in the Dutch KOALA Birth Cohort Study (7, 21). We therefore examined the association of single-nucleotide polymorphisms (SNPs) in TLR4 and pertussis toxin-specific immunoglobulin G (PT-IgG) following wP vaccination. The IgG antibody titer against PT, one of the dominant virulence factors of B. pertussis, correlates with protection against disease (11, 30, 31). We used vaccine-induced PT-IgG as a quantitative phenotype and compared the genotypes of high and low responders to PT. We hypothesized that minor TLR4 alleles that may affect promoter activity or receptor affinity of TLR4 are associated with an altered IgG titer to PT.
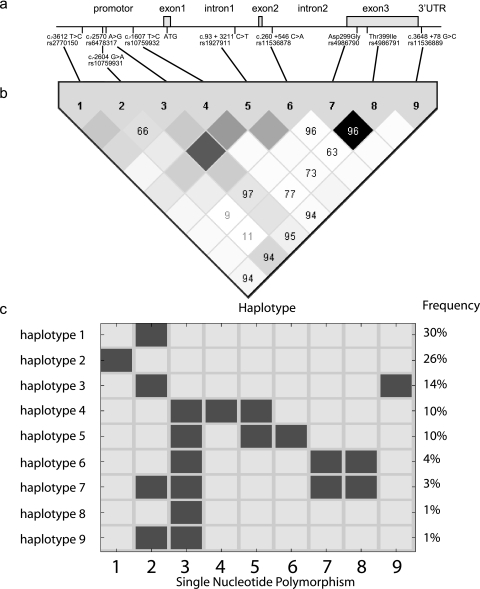
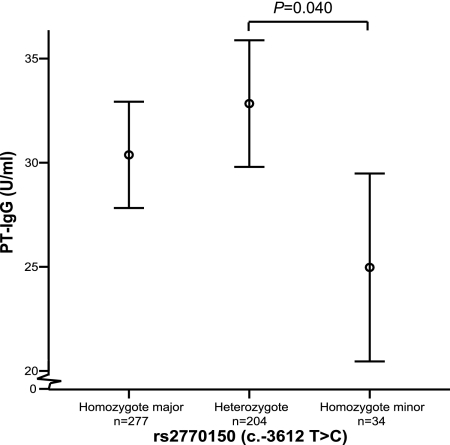
The level of PT-IgG was determined by an enzyme-linked immunosorbent assay (14) on capillary blood samples collected from 855 1-year-old children. One hundred fifty-one children were excluded from further analysis because their parents stated in the questionnaires that the child had not received the standard pertussis vaccination (15, 21). DNA was not available from 184 children, and a further five children were excluded because their PT-IgG level was above 200 U/ml, indicating natural infection (14). The remaining 515 children were genotyped by K-Biosciences (Cambridge, United Kingdom) for nine SNPs located on TLR4 (Fig. 1a). After Ln transformation, the PT-IgG levels were normally distributed according to Levene's test (P > 0.05). To examine possible confounding factors, we tested for associations between PT-IgG titer and the number of days between vaccination and blood sampling and infant gender using Pearson correlation. None of the factors tested influenced the PT-IgG level (P > 0.05). All SNPs were in Hardy Weinberg equilibrium (chi-square test, P > 0.05). Associations between nine SNPs in TLR4 and PT-IgG titers were assessed by analysis of variance (ANOVA), and the distribution of the genotypes among individuals with extreme 10th percentiles in PT-IgG titer was tested using Pearson's chi-square test (Table 1). The rs2770150 (c.-3612 T>C SNP) was significantly associated with the lowest 10th percentile (low responders) compared to the highest 10th percentile (high responders) titer of PT-IgG (P = 0.027). Subjects homozygous for the minor C allele of this SNP had a significantly lower PT-IgG titer upon pertussis vaccination (P = 0.040) compared with persons heterozygous for this allele (Fig. 2). To examine whether the effect of rs2770150 could be due to a more distant variant, we performed haplotype analysis. We constructed haplotypes for the genotyped SNPs and tested them for association with PT-IgG titers using WHAP (http://pngu.mgh.harvard.edu/purcell//whap/). There is strong linkage disequilibrium between most genotyped SNPs within TLR4 (Fig. 1b) (6). Therefore, the selected SNPs represent nine different haplotypes encompassing 99% of the haplotypes in our cohort (Fig. 1c). No significant associations were found between haplotypes and the PT-IgG titer (data not shown). This could be due to the small size of our cohort or the need for two alleles (recessive model) to obtain an effect on titer which would be missed when analyzing haplotypes.
FIG. 1.
Position, linkage disequilibrium, and haplotypes of the nine SNPs within TLR4. (a) TLR4 is located on chromosome 9 (9q32) 119,506,431. Nine haplotype-tagging SNPs were selected from the Innate Immunity website (http://www.innateimmunity.net/data/homology). All SNPs are in Hardy Weinberg equilibrium (P > 0.05). 3′UTR, 3′ untranslated region. (b) Pairwise linkage disequilibrium plot according to Haploview3.32 (6). D′ is presented as a number if it deviates from 100. D′ is 100 when no recombination has occurred between two SNPs. The measure of r2 is represented by color, changing from dark gray when r2 = 1 (the minor alleles at two SNP positions are always present on the same haplotype) to white when r2 = 0 (the minor alleles are always on separate haplotypes). (c) The frequency of the major haplotypes of TLR4 present in the KOALA cohort as identified by the nine tagging SNPs genotyped in our study. Black boxes represent minor alleles.
TABLE 1.
Summary of TLR4 SNPs tested for association with PT-IgG values
| SNPa | Minor allele frequency |
P value
|
Allele | No. of children with genotype | Ln(PT-IgG) titerd | PT-IgG titer (U/ml) | |
|---|---|---|---|---|---|---|---|
| Continuousb | Percentilec | ||||||
| rs2770150 c.-3612 T>C | 0.26 | 0.083 | 0.027 | TT | 277 | 3.24 | 30.38 |
| CT | 204 | 3.31 | 32.84 | ||||
| CC | 34 | 3.09 | 24.97 | ||||
| rs10759931 c.-2604 G>A | 0.42 | 0.906 | 0.656 | GG | 187 | 3.26 | 31.03 |
| GA | 210 | 3.24 | 29.87 | ||||
| AA | 108 | 3.25 | 31.85 | ||||
| rs6478317 c.-2570 A>G | 0.35 | 0.957 | 0.676 | AA | 203 | 3.26 | 31.40 |
| GA | 255 | 3.25 | 30.61 | ||||
| GG | 52 | 3.28 | 31.48 | ||||
| rs10759932 c.-1607 T>C | 0.13 | 0.720 | 0.269 | TT | 365 | 3.25 | 30.71 |
| CT | 115 | 3.30 | 32.15 | ||||
| CC | 5 | 3.19 | 27.80 | ||||
| rs1927911 c.93 + 3211 C>T | 0.25 | 0.630 | 0.491 | CC | 276 | 3.25 | 31.25 |
| CT | 207 | 3.23 | 29.49 | ||||
| TT | 23 | 3.34 | 33.74 | ||||
| rs11536878 c.260 + 546 C>A | 0.12 | 0.620 | 0.649 | CC | 378 | 3.25 | 30.76 |
| CA | 103 | 3.24 | 29.80 | ||||
| AA | 7 | 3.04 | 21.57 | ||||
| rs4986790 Asp299Gly | 0.07 | 0.836 | 0.548 | AA | 441 | 3.25 | 30.86 |
| GA | 68 | 3.26 | 30.60 | ||||
| GG | 3 | 3.45 | 35.33 | ||||
| rs4986791 Thr399Ile | 0.07 | 0.659 | 0.518 | CC | 431 | 3.24 | 30.59 |
| CT | 66 | 3.30 | 33.29 | ||||
| TT | 3 | 3.45 | 35.33 | ||||
| rs11536889 c.3648 + 78 G>C | 0.14 | 0.308 | 0.502 | GG | 373 | 3.27 | 31.66 |
| CG | 128 | 3.19 | 28.44 | ||||
| CC | 8 | 3.36 | 32.63 | ||||
SNPs were named according to the Human Genome Variation Society guidelines (http://www.hgvs.org/mutnomen/recs.html).
The statistical differences in phenotypes (Ln-transformed PT-IgG titers) were assessed by ANOVA.
Distribution of the Pt-IgG titers among the extreme percentiles was tested by Pearson chi-square test.
Ln(PT-IgG), Ln-transformed PT-IgG.
FIG. 2.
Pertussis toxin-specific IgG titer per genotype. Circles represent the means of the PT-IgG titer, and the standard deviations are represented by the vertical error bars. Horizontal lines represent a statistical difference (P < 0.05) between groups according to the least significant difference post hoc test (ANOVA, SPSS).
Although all participants had PT-IgG titers that can be considered protective at the time of blood sampling (11) (approximately 1 month after receiving the fourth vaccination), TLR4 polymorphisms may be important in the duration of protective immunity. Future work should indicate whether this and other polymorphisms in TLR4 have clinical relevance either by affecting the antibody response following vaccination, during the waning of the antibody response, or by affecting the outcome of infection itself irrespective of vaccination.
The children in this study were vaccinated four times (when they were 2, 3, 4, and 11 months old) with wP vaccine that contains the TLR4 ligands lipopolysaccharide (LPS) and PT (18, 25, 26). In mice, Tlr4 affected the vaccination response after vaccination with both the LPS-containing wP vaccine and the (LPS-free) acellular vaccine. Both vaccines induced less bacterial clearance in Tlr4 defective C3H/HeJ mice (Tlr4Lps-d) than in wild-type mice (Tlr4Lps-n) (16; Banus et al., submitted), suggesting that not only the interaction between TLR4 and LPS but also the interaction between TLR4 and PT is important in the generation of vaccine-induced immunity.
The SNP that was associated with the PT-IgG titer, rs2770150, is characterized by a T-to-C substitution in the promoter region of TLR4 (position −3612). This SNP may therefore be involved in transcriptional regulation, suggesting that subjects with a minor allele of this SNP have lower expression of the gene. We have shown that in mice Tlr4 mRNA expression is upregulated 1.5 times after B. pertussis infection, suggesting that transcriptional activation of Tlr4 is involved in the response to B. pertussis infection (5). The results of the present study may be explained by altered transcriptional activation of TLR4 upon wP vaccination in humans.
PT-IgG levels have been shown to correlate with protection after vaccination, both in humans (11, 30, 31) and mice (8). Cell-mediated immunity, however, does also critically contribute to protection in both humans (3, 9, 22) and mice (24). The association between PT-IgG levels and protective immunity is most apparent early after vaccination (10). We speculate that genetic diversity in TLR4 indeed affects antibody titers after wP vaccination but that the variation in response of 1-year-old children is limited due to the many booster vaccinations. Therefore, the Dutch Vaccination Program comprising four vaccinations during the first year of life may adequately address the genetic variation in the most vulnerable age group, at least regarding pertussis. It remains to be established, however, whether the same holds true for the persistence of the PT-specific antibody response and the response to other vaccines.
In conclusion, we demonstrate that genetic variation in TLR4 is associated with the wP vaccination response in 1-year-old children. To our knowledge, this is the first study to report the involvement of TLR4 in the induction of the antibody response after vaccination against B. pertussis in humans.
Acknowledgments
We thank the children and parents of the KOALA study for their participation. In addition, we thank the KOALA field team for their efforts in sample collection and B. Elvers for advice and coordination concerning PT-IgG determinations. We also acknowledge H. van Houwelingen for help with the statistical analysis.
The study was supported by ZonMW (grant 912-03-031) and The Netherlands Asthma Foundation (grant 3.2.03.48).
The study was approved by the medical ethics committee of Maastricht University.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.Agnese, D. M., J. E. Calvano, S. J. Hahm, S. M. Coyle, S. A. Corbett, S. E. Calvano, and S. F. Lowry. 2002. Human Toll-like receptor 4 mutations but not CD14 polymorphisms are associated with an increased risk of gram-negative infections. J. Infect. Dis. 186:1522-1525. [DOI] [PubMed] [Google Scholar]
- 2.Arbour, N. C., E. Lorenz, B. C. Schutte, J. Zabner, J. N. Kline, M. Jones, K. Frees, J. L. Watt, and D. A. Schwartz. 2000. TLR4 mutations are associated with endotoxin hyporesponsiveness in humans. Nat. Genet. 25:187-191. [DOI] [PubMed] [Google Scholar]
- 3.Ausiello, C. M., R. Lande, F. Urbani, A. La Sala, P. Stefanelli, S. Salmaso, P. Mastrantonio, and A. Cassone. 1999. Cell-mediated immune responses in four-year-old children after primary immunization with acellular pertussis vaccines. Infect. Immun. 67:4064-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banus, H. A., H. J. van Kranen, F. R. Mooi, B. Hoebee, N. J. Nagelkerke, P. Demant, and T. G. Kimman. 2005. Genetic control of Bordetella pertussis infection: identification of susceptibility loci using recombinant congenic strains of mice. Infect. Immun. 73:741-747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banus, H. A., R. J. Vandebriel, H. de Ruiter, J. A. Dormans, N. J. Nagelkerke, F. R. Mooi, B. Hoebee, H. J. van Kranen, and T. G. Kimman. 2006. Host genetics of Bordetella pertussis infection in mice: significance of Toll-like receptor 4 in genetic susceptibility and pathobiology. Infect. Immun. 74:2596-2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barrett, J. C., B. Fry, J. Maller, and M. J. Daly. 2005. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21:263-265. [DOI] [PubMed] [Google Scholar]
- 7.Bastiaanssen, J. M., R. A. de Bie, C. H. Bastiaenen, A. Heuts, M. E. Kroese, G. G. Essed, and P. A. van den Brandt. 2005. Etiology and prognosis of pregnancy-related pelvic girdle pain: design of a longitudinal study. BMC Public Health 5:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bruss, J. B., and G. R. Siber. 2002. Quantitative priming with inactivated pertussis toxoid vaccine in the aerosol challenge model. Infect. Immun. 70:4600-4608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cassone, A., C. M. Ausiello, F. Urbani, R. Lande, M. Giuliano, A. La Sala, A. Piscitelli, S. Salmaso, and The Progetto Pertosse-CMI Working Group. 1997. Cell-mediated and antibody responses to Bordetella pertussis antigens in children vaccinated with acellular or whole-cell pertussis vaccines. Arch. Pediatr. Adolesc. Med. 151:283-289. [DOI] [PubMed] [Google Scholar]
- 10.Cassone, A., P. Mastrantonio, and C. M. Ausiello. 2000. Are only antibody levels involved in the protection against pertussis in acellular pertussis vaccine recipients? J. Infect. Dis. 182:1575-1577. [DOI] [PubMed] [Google Scholar]
- 11.Cherry, J. D., J. Gornbein, U. Heininger, and K. Stehr. 1998. A search for serologic correlates of immunity to Bordetella pertussis cough illnesses. Vaccine 16:1901-1906. [DOI] [PubMed] [Google Scholar]
- 12.de Greeff, S. C., J. F. Schellekens, F. R. Mooi, and H. E. de Melker. 2003. Pertussis in The Netherlands, 2001-2002. RIVM Report. RIVM, Bilthoven, The Netherlands.
- 13.de Greeff, S. C., J. F. Schellekens, F. R. Mooi, and H. E. de Melker. 2005. Effect of vaccination against pertussis on the incidence of pertussis in The Netherlands, 1996-2003. Ned. Tijdschr. Geneeskd. 149:937-943. (In Dutch.) [PubMed] [Google Scholar]
- 14.de Melker, H. E., F. G. Versteegh, M. A. Conyn-van Spaendonck, L. H. Elvers, G. A. Berbers, Z. A. van Der, and J. F. Schellekens. 2000. Specificity and sensitivity of high levels of immunoglobulin G antibodies against pertussis toxin in a single serum sample for diagnosis of infection with Bordetella pertussis. J. Clin. Microbiol. 38:800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Health Council of The Netherlands. 2004. Vaccination against pertussis. Publication no. 2004/04E, p. 1-98. Health Council of The Netherlands, The Hague, The Netherlands. http://www.gr.nl/pdf.php?ID=964&p=1.
- 16.Higgins, S. C., A. G. Jarnicki, E. C. Lavelle, and K. H. Mills. 2006. TLR4 mediates vaccine-induced protective cellular immunity to Bordetella pertussis: role of IL-17-producing T cells. J. Immunol. 177:7980-7989. [DOI] [PubMed] [Google Scholar]
- 17.Higgins, S. C., E. C. Lavelle, C. McCann, B. Keogh, E. McNeela, P. Byrne, B. O'Gorman, A. Jarnicki, P. McGuirk, and K. H. Mills. 2003. Toll-like receptor 4-mediated innate IL-10 activates antigen-specific regulatory T cells and confers resistance to Bordetella pertussis by inhibiting inflammatory pathology. J. Immunol. 171:3119-3127. [DOI] [PubMed] [Google Scholar]
- 18.Kerfoot, S. M., E. M. Long, M. J. Hickey, G. Andonegui, B. M. Lapointe, R. C. Zanardo, C. Bonder, W. G. James, S. M. Robbins, and P. Kubes. 2004. TLR4 contributes to disease-inducing mechanisms resulting in central nervous system autoimmune disease. J. Immunol. 173:7070-7077. [DOI] [PubMed] [Google Scholar]
- 19.Kimman, T. 2001. Genetics of infectious disease susceptibility. Kluwer Academic Publishers, Dordrecht, The Netherlands.
- 20.Kimman, T., R. J. Vandebriel, and B. Hoebee. Genetic variation in the response to vaccination. Community Genet., in press. [DOI] [PubMed]
- 21.Kummeling, I., C. Thijs, J. Penders, B. E. Snijders, F. Stelma, J. Reimerink, M. Koopmans, P. C. Dagnelie, M. Huber, M. C. Jansen, R. de Bie, and P. A. van den Brandt. 2005. Etiology of atopy in infancy: the KOALA Birth Cohort Study. Pediatr. Allergy Immunol. 16:679-684. [DOI] [PubMed] [Google Scholar]
- 22.Mahon, B. P., M. T. Brady, and K. H. Mills. 2000. Protection against Bordetella pertussis in mice in the absence of detectable circulating antibody: implications for long-term immunity in children. J. Infect. Dis. 181:2087-2091. [DOI] [PubMed] [Google Scholar]
- 23.Mann, P. B., D. Wolfe, E. Latz, D. Golenbock, A. Preston, and E. T. Harvill. 2005. Comparative Toll-like receptor 4-mediated innate host defense to Bordetella infection. Infect. Immun. 73:8144-8152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mills, K. H., M. Ryan, E. Ryan, and B. P. Mahon. 1998. A murine model in which protection correlates with pertussis vaccine efficacy in children reveals complementary roles for humoral and cell-mediated immunity in protection against Bordetella pertussis. Infect. Immun. 66:594-602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poltorak, A., X. He, I. Smirnova, M. Y. Liu, C. Van Huffel, X. Du, D. Birdwell, E. Alejos, M. Silva, C. Galanos, M. Freudenberg, P. Ricciardi-Castagnoli, B. Layton, and B. Beutler. 1998. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science 282:2085-2088. [DOI] [PubMed] [Google Scholar]
- 26.Racke, M. K., W. Hu, and A. E. Lovett-Racke. 2005. PTX cruiser: driving autoimmunity via TLR4. Trends Immunol. 26:289-291. [DOI] [PubMed] [Google Scholar]
- 27.RIVM. 2005. Reported cases of whooping cough in The Netherlands. CIE, RIVM, Bilthoven, The Netherlands. http://www.rivm.nl/isis/ggd/openbaar/diag/aa/gr_aa_PERT.html. Accessed 23 February 2005.
- 28.RIVM. 2004. Vaccination coverage in The Netherlands. Zorgatlas, RIVM, Bilthoven, The Netherlands. http://www.rivm.nl/vtv/data/atlas/vaccinaties/dktp_vacc_03.html. Accessed 23 February 2005.
- 29.Schroder, N. W., and R. R. Schumann. 2005. Single nucleotide polymorphisms of Toll-like receptors and susceptibility to infectious disease. Lancet Infect. Dis. 5:156-164. [DOI] [PubMed] [Google Scholar]
- 30.Storsaeter, J., H. O. Hallander, L. Gustafsson, and P. Olin. 1998. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 16:1907-1916. [DOI] [PubMed] [Google Scholar]
- 31.Taranger, J., B. Trollfors, T. Lagergard, V. Sundh, D. A. Bryla, R. Schneerson, and J. B. Robbins. 2000. Correlation between pertussis toxin IgG antibodies in postvaccination sera and subsequent protection against pertussis. J. Infect. Dis. 181:1010-1013. [DOI] [PubMed] [Google Scholar]
- 32.Turvey, S. E., and T. R. Hawn. 2006. Towards subtlety: understanding the role of Toll-like receptor signaling in susceptibility to human infections. Clin. Immunol. 120:1-9. [DOI] [PubMed] [Google Scholar]