Abstract
It has been repeatedly observed that mixing Haemophilus influenzae type b (Hib) conjugate vaccines with acellular pertussis-containing vaccines (diphtheria-tetanus-acellular pertussis [DTPa]) resulted in a reduced magnitude of the anti-polyriboseribitolphosphate antibody response compared to that obtained when Hib vaccines were administered separately and not mixed. Nevertheless, the quality and functionality of the immune responses have been shown to be the same. With the purpose of investigating the quality of the anti-Hib immune responses that are elicited under different vaccination regimens, we report here four primary and booster-based pediatric clinical trials in which Hib vaccine was either mixed with DTPa or diphtheria-tetanus-whole-cell pertussis (DTPw)-based vaccines or was coadministered. Our results show that avidity maturation of the antibodies was lower when primary vaccination involved DTPa mixed with Hib compared to when DTPa and Hib were coadministered. No such difference was observed between mixed and separately administered Hib when associated with DTPa-hepatitis B virus-inactivated poliovirus or DTPw-based vaccines. All different combinations and regimens elicited the same opsonophagocytic and bactericidal activity as well as the same ability to protect in a passive infant rat protection assay. The functional activity of mixed DTPa-based and Hib vaccines was similar to that of mixed DTPw-based/Hib combinations. In conclusion, in vitro and in vivo data as well as postmarketing vaccine effectiveness data attest to the ability of DTPa-based/Hib combination vaccines to effectively prevent Hib-induced disease in children.
The effectiveness of Haemophilus influenzae type b (Hib) conjugate vaccines in preventing Hib disease in young children has been conclusively demonstrated. In Europe, the annual incidence of Hib meningitis in children <5 years of age prior to the availability of Hib conjugate vaccines was between 11 and 40 per 100,000 (25). After widespread implementation of vaccination, the incidence has fallen to 0 to 8 per 100,000 in this age group (25). In The Gambia, the incidence of Hib meningitis fell from over 200/100,000 in children <1 year of age to 20/100,000 within 2 years after the introduction of Hib conjugate vaccine into the routine vaccination schedule (1).
In order to facilitate immunization practices, combinations of Hib conjugate with diphtheria-tetanus-acellular pertussis (DTPa) or diphtheria-tetanus-whole-cell pertussis (DTPw) vaccines are commonly used (6). However, reduced antibody responses to the Hib capsular polysaccharide polyriboseribitolphosphate (PRP) have been reported following vaccination with these combinations, being more pronounced when Hib is combined with DTPa-based vaccines (6, 14).
It is recognized that the continuous presence of low levels of circulating anti-PRP antibody is required for protection from Hib disease and that B-cell memory alone is insufficient for protection, although immune memory explains to some extent why Hib conjugate vaccines are protective at lower antibody levels than plain Hib polysaccharide (3, 6). The protective serum level of anti-PRP antibody has been postulated to be between 0.05 and 1 μg/ml (2). Since the quality of anti-PRP antibody increases from the postprimary to the prebooster/postbooster time period following vaccination with conjugate vaccines (8, 26), we have recently proposed that the protective level of mature antibody is in the range of 0.05 μg/ml (27). This is in line with findings from Finnish Hib conjugate efficacy trials where the observed protective efficacy of 90% more closely approximated the proportion of subjects with anti-PRP antibody concentrations of ≥0.06 μg/ml (85%), compared to ≥0.15 μg/ml (70%) after the three-dose primary vaccination (5). Increased functional activity of antibody following conjugate vaccination is another factor explaining why conjugate vaccines afford similar protection with lower antibody levels compared to polysaccharide vaccines (17).
Previously, we reported that combined hexavalent DTPa-hepatitis B virus (HBV)-inactivated poliovirus (IPV)/Hib PRP-tetanus toxoid (TT) vaccines induce a lower quantity of anti-PRP antibodies but a similar quality compared to those induced when PRP-TT and DTPa-HBV-IPV are injected separately (27). PRP-TT also induces anti-PRP antibodies of an increased quality compared to those of the licensed efficacious PRP-Neisseria meningitidis outer membrane protein (OMP) conjugate vaccine (20, 29). In this report, we extend our previous findings by describing the results from four randomized clinical trials consisting of primary and booster vaccinations in infants (4, 31, 35) in which several DTPa-based vaccines were administered with Hib vaccines separately or combined as a mix. We also describe results from a clinical trial in which PRP-TT was administered mixed with DTPw (13). Our aim was to analyze and compare the quality of the anti-PRP responses induced by these different vaccination protocols. For that purpose, the avidity, the bactericidal and opsonophagocytic activity, and the in vivo protection of the anti-PRP antibodies in Hib-challenged infant rats were evaluated.
MATERIALS AND METHODS
All assays were performed at the GSK Biologicals laboratory in Belgium, with the exception of bactericidal testing, which was performed in the Department of Microbiology and Immunology, University of Rochester Medical Center, under the responsibility of Michael E. Pichichero, and avidity testing, which was performed at the laboratory of D. Goldblatt, Institute of Child Health, University College London.
Study population, vaccines, and samples.
Sera were obtained from volunteers participating in four randomized controlled primary and booster vaccination studies conducted in the United States, Germany, and Myanmar (Table 1). Subjects received primary and booster vaccination with a DTPa-based vaccine administered separately from or mixed with an Hib conjugate vaccine. The subjects in the Myanmar study received primary and booster vaccination with DTPw/Hib containing 10 μg or 2.5 μg of PRP conjugated to TT. All vaccines were manufactured by GSK Biologicals, with the exception of OmniHIB (Sanofi Pasteur). All Hib vaccines were conjugated to TT. Studies were conducted according to Good Clinical Practice guidelines (http://www.emea.europa.eu/pdfs/human/ich/013595en.pdf) and the Declaration of Helsinki (Somerset West 1996). Informed consent was obtained from parents/guardians of subjects before enrolment. Blood samples were collected prior to and 1 month after the completion of the primary vaccination and 4 to 6 weeks after the booster dose.
TABLE 1.
Clinical trials with DTPa/Hib and DTPw/Hib vaccinesa
| Study (study date [day-mo-yr]) | Schedule | Primary vaccination | Booster vaccination | Study objective |
|---|---|---|---|---|
| United States, DTPa-HBV-IPV-Hib-001 (245917/001) (24-07-1996 to 28-04-1998) | 2, 4, 6 mo; booster, 15-18 mo | DTPa-HBV-IPV/Hib (Infanrix HeXa) | DTPa/Hib (Infanrix/Hib) | Comparison of the immune responses to a combination of components and immune responses to the components injected separately |
| DTPa + HBV + OPV + Hib (Hib: OmniHIB) | DTPa + Hib (Hib: OmniHIB) | |||
| Germany A, Hib-006 (208108/006) (20-08-1993 to 28-08-1995) | 3, 4, 5 mo; booster, 15-27 mo | DTPa/Hib (Hib: Hiberix) | DTPa/Hib | Comparison of the immune responses against Hib whether combined or coadministered with DTPa |
| DTPa + Hib (Hib: Hiberix) | DTPa + Hib (Hib: Hiberix) | |||
| Germany B, Hib-026 (208108/026) (09-06-1994 to 02-04-1996) | 3, 4, 5 mo; booster, 18-24 mo | DTPa-HBV + Hib (Hib: Hiberix) | dtpa-HBV + PRP (plain polysaccharide) | Comparison of the immune responses against Hib when coadministered or combined with DTPa-HBV and to evaluate immunological priming with plain polysaccharide booster |
| DTPa-HBV/Hib (Hib: Hiberix) | dtpa-HBV/PRP (plain polysaccharide) | |||
| Myanmar, Hib-052 (208108/052) (16-01-1998 to 11-10-1999) | 6, 10, 14 wk; booster, 15-19 mo | DTPw-HBV/Hib2.5 | DTPw-HBV/Hib2.5 + MMR | Comparison of two different doses of Hib in combination with DTPw-HBV |
| DTPw-HBV/Hib (TritanrixHepB/Hiberix) | DTPw-HBV/Hib (TritanrixHepB/Hiberix) + MMR |
dtpa, experimental DTPa vaccine with reduced antigen content; Hib2.5, Hib vaccine containing 2.5 μg PRP conjugated to TT; MMR, combined measles mumps rubella vaccine; OPV, oral poliovirus vaccine. All vaccines were manufactured by GSK Biologicals, with the exception of OmniHIB (Sanofi Pasteur). All Hib vaccines were conjugated to TT. In the vaccine formulations, a hyphen indicates the components of a solution, a slash indicates the reconstitution of a lyophilisate, and a plus indicates coadministration.
Measurement of total immunoglobulin (Ig) and IgG subclasses. (i) RABA.
The anti-PRP radioimmunoantigen binding assay (RABA) measures total anti-PRP antibodies in human serum by a Farr precipitation assay. Radioiodinated tyraminated PRP antigen was mixed with dilutions of human sera and incubated. Unbound radioiodinated PRP was separated from radioiodinated PRP bound to specific antibodies by ammonium sulfate precipitation. Radioactivity in the supernatant (containing unbound radioiodinated PRP) was measured and was inversely proportional to the quantity of PRP-specific antibodies present in the serum. The level of specific PRP antibody present in test serum was determined by comparison with a standard serum (U.S. FDA anti-PRP reference lot 1983). The cutoff value was 0.15 μg/ml.
(ii) ELISA.
Polystyrene microtiter plates were incubated overnight with tyraminated PRP antigen (0.1 μg/ml in bicarbonate buffer). Human sera were diluted and added to the PRP-coated plates. Antibodies specific for PRP were allowed to bind to the coated antigen for 30 min. Next, IgG antibodies were detected with an anti-human IgG peroxidase-labeled antibody (A2290; Sigma-Aldrich, Bornem, Belgium). IgG1 (IgG2) antibodies were detected using an anti-human IgG1 (IgG2) mouse antibody (I5385 for anti-human IgG1 and I9513 for anti-human IgG2; Sigma-Aldrich), followed by an anti-mouse biotin-labeled antibody (RPN 1177; GE Healthcare-Biosciences, Uppsala, Sweden) and an Extravidin peroxidase complex (Sigma-Aldrich). All incubations lasted for 30 min. The reaction was visualized by the addition of tetramethylbenzidine substrate. The color intensity was directly proportional to the amount of antibody present in the serum. Absorbance readings were measured at 450 nm in a spectrophotometer. For all determinations, the level of the specific antibody present in the unknown fraction was determined by comparison to reference serum (human serum calibrated against U.S. FDA anti-PRP reference lot 1983). Four-parameter logistic-log function was used for forming the reference and samples curves. This enzyme-linked immunosorbent assay (ELISA) has been fully validated according to ICH Guidance documents, as it was compared to the RABA. Taking into account the normal variation of the ELISA and RABA methods, a very good correspondence was observed over the whole range for both assays. The cutoff value for positivity was defined as 0.15 μg/ml.
Antibody avidity assay (ELISA).
The avidity measurement assay is a modification of the standard anti-PRP ELISA, with the inclusion of the use of a chaotropic agent, isothiocyanate (8). Polystyrene microtiter plates were incubated overnight with a PRP-human serum albumin-phosphate-buffered saline (PBS) solution. Human sera were prediluted to a final concentration of 0.5 μg/ml and then further diluted to obtain a 1:10 dilution. The diluted sera and ammonium thiocyanate diluted to concentrations of 0.8, 0.4, 0.2, 0.1, and 0.05 M were added to the plates. Antibodies specific for PRP bound to the coated antigen. IgG antibodies were detected with an anti-human IgG alkaline phosphatase conjugate. The reaction was visualized by the addition of p-nitrophenyl phosphate. The reaction was stopped when wells without ammonium thiocyanate reached an optical density of 1.0. Antibody avidity was expressed as the avidity index corresponding to the molar concentration of ammonium thiocyanate required to produce a 50% reduction in absorbance.
Infant rat passive protection assay.
Hib strain Eagan was grown for approximately 2 h in sterile filtered tryptic soy broth (TSB) plus 3 μg/ml NAD, 3 μg/ml hemin, 1% polyvitex, and 1% inactivated horse serum (enriched TSB). Next, bacteria were diluted in the same medium to obtain a suspension of 5 × 105 CFU/ml. Study groups each comprised eight 7-day-old rats randomly mixed between litters before immunization. Immunization was done by intraperitoneal injection of 200 μl of a human serum pool (from subjects of a same group who had received separate or mixed administration of Hib and DTPa-based vaccines in the clinical trials described above) diluted in sterile PBS in order to reach an anti-PRP antibody concentration of 2 μg/ml (by RABA). Each rat, therefore, received 0.4 μg of anti-PRP antibodies. Serum antibody levels obtained by such a dose were undetectable (<0.15 μg/ml), indicating the sensitivity of this assay. Control rats received an intraperitoneal injection of PBS. On the next day, animals were challenged with 100 μl (5 × 104 CFU) of the bacterial suspension, and 24 h later, 250 μl of blood was obtained by cardiac puncture after anesthesia and was mixed with 2.25 ml of TSB. Serial 10-fold dilutions were made, 100-μl aliquots were plated onto chocolate agar, and the number of bacteria in the blood was estimated. Weighted means were calculated for each animal. The lower detection limit for CFU was 0.85. Group means were compared using the one-way analysis of variance (ANOVA) Tukey-honestly significant difference test.
Bactericidal assay.
The bactericidal assay evaluates the capacity of anti-Hib antibodies present in the serum of a patient to bind and activate complement, leading to the killing of the bacteria. Hib strain Eagan was cultivated, harvested, and diluted to a concentration of around 104 CFU/ml. Eleven serial twofold dilutions of the serum to be tested (starting at 1:4) were mixed with precolostral calf serum complement (obtained from Virginia Polytechnic Institute School of Veterinarian Medicine) and 25 μl of bacteria. After 45 min of incubation, the number of surviving bacteria was determined by plating 5 μl onto chocolate agar and counting the colonies. The bactericidal titer of the serum was defined as the inverse of the highest dilution that led to >50% bacterial killing and was compared to that of negative control serum. The cutoff value was 4. Groups were compared by ANOVA with a significance level of a P value of 0.05. The geometric mean ratio (GMR) for each group was defined as the bactericidal titer-to-RABA ratio obtained by using individual data of positive bactericidal samples only (i.e., responders above the detection limit). The means of each group were compared using one-way ANOVA Tukey-honestly significant difference test.
Opsonophagocytosis assay.
Hib strain Eagan was grown in enriched TSB (see above) for approximately 2 h. Bacteria were then diluted in the same medium to obtain a 104 CFU/ml suspension. Polymorphonucleocytes (PMN) were prepared from heparinized blood from human volunteers isolated by density gradient centrifugation with Ficoll Paque (catalog number 17-0840-02; Pharmacia Biotech). PMN were diluted in Hanks balanced salt solution (catalog number 1425-050; Gibco) to obtain a suspension of 107 PMN/ml. Twofold dilutions of heat-inactivated infant sera to be tested were prepared using Hanks balanced salt solution starting with a 1:5 dilution (40 μl). Forty microliters of a solution containing 4 × 105 PMN, 10 μl of baby rabbit complement, and 20 μl of 200 CFU of Hib were added to the serum dilutions. After 2 h of incubation at 37°C under gentle agitation, the opsonophagocytic reaction was stopped by putting the reaction plate on ice. Five microliters of the suspension was collected and plated onto chocolate agar plates. The opsonic titer of a serum sample corresponding to a Hib killing activity of 50% was calculated as follows: (mean CFU negative control − CFU sample)/mean CFU negative control × 100. The cutoff value was 4. GMRs (opsonophagocytic geometric mean titer [GMT] to RABA GMT) were calculated for each group using individual data for positive opsonophagocytic samples only.
RESULTS
Antibody isotypes and subclasses.
An increase in total Ig was observed between postprimary and postbooster time points that was relatively higher for IgG than for total Ig (Table 2). This indicates that IgG antibodies represent a higher proportion of total antibodies after booster vaccination than after primary vaccination. The relative increase in IgG from postprimary to postbooster time points suggests antibody maturation over time. Postprimary anti-PRP antibody concentrations were considerably lower following vaccination with DTPa-based Hib than with DTPw-based Hib vaccines. However, the four-dose schedule with combined DTPa/Hib delivered results that are comparable to those obtained with the four-dose Hib separate schedule (Table 2). The exception was the Germany A study, where a significantly lower anti-PRP antibody geometric mean concentration (GMC) was observed after the fourth dose in combined vaccines versus separate ones. Nonetheless, even the lowest GMCs in this study were high compared to those in the U.S. study (Table 2).
TABLE 2.
Serum anti-PRP antibody concentrations in four clinical trials at postprimary and booster time pointsa
| Study | Group | No. of subjects tested | GMC (μg/ml) (95% CI) for:
|
Fold increase
|
||
|---|---|---|---|---|---|---|
| IgG | RABA Ig | IgG | Total Ig | |||
| United States | ||||||
| Postprimary | DTPa + HBV + OPV + Hib* | 53 | 4.27 (2.76-6.6) | 6.21 (4.276-9.023) | ||
| DTPa-HBV-IPV/Hib | 51 | 2.1 (1.51-2.92) | 3.27 (2.481-4.321) | |||
| Postbooster | DTPa + Hib* | 33 | 33.63 (20.68-54.70) | 28.37 (17.612-45.716) | 7.87 | 4.56 |
| DTPa/Hib | 33 | 42.89 (29.71-61.92) | 26.52 (18.433-38.148) | 20.42 | 8.09 | |
| Germany A | ||||||
| Postprimary | DTPa + Hib | 53 | 5.74 (4.07-8.1) | 8.93 (6.74-11.8) | ||
| DTPa/Hib | 64 | 1.13 (0.81-1.57) | 2.46 (1.77-3.42) | |||
| Postbooster | DTPa + Hib | 35 | 73.5 (46.4-117) | 89.4 (56.6-141) | 12.8 | 10.01 |
| DTPa/Hib | 40 | 36.4 (22.8-58.4) | 38.1 (25.8-56.4) | 32.2 | 15.5 | |
| Germany B | ||||||
| Postprimary | DTPa-HBV + Hib | 21 | 7.79 (4.53-13.41) | |||
| DTPa-HBV/Hib | 25 | 1.67 (1.11-2.53) | ||||
| Prebooster | DTPa-HBV + Hib | 21 | 0.754 (0.42-1.36) | |||
| DTPa-HBV/Hib | 25 | 0.21 (0.15-0.29) | ||||
| Postbooster | dtpa-HBV + PRP | 21 | 35.28 (15.04-82.73) | 4.53 | ||
| dtpa-HBV/PRP | 25 | 9.63 (4.84-19.15) | 5.77 | |||
| Myanmar | ||||||
| Postprimary | DTPw-HBV/Hib2.5 | 25 | 20.32 (12.968-31.864) | |||
| DTPw-HBV/Hib | 25 | 18.67 (12.331-28.270) | ||||
| Postbooster | DTPw-HBV/Hib2.5 | 25 | 44.10 (28.062-69.309) | 2.17 | ||
| DTPw-HBV/Hib | 25 | 49.91 (27.716-79.390) | 2.67 | |||
Blood samples were collected 1 month after the three-dose primary series and 4 to 6 weeks after the booster dose. CI, confidence interval; Hib2.5, Hib vaccine containing 2.5 μg PRP conjugated to TT; OPV, oral poliovirus vaccine. *, OmniHIB.
The analysis of IgG subclasses showed that IgG1 was dominant in postprimary and postbooster sera, whatever the mode of administration, mixed or separate, of the Hib vaccine (Table 3).
TABLE 3.
Serum anti-PRP IgG subclass concentrations at postprimary and booster time pointsa
| Study | Group | No. of subjects tested | GMC (μg/ml) (95% CI)
|
IgG1/IgG2 ratio | |
|---|---|---|---|---|---|
| IgG1 | IgG2 | ||||
| United States | |||||
| Postprimary | DTPa + HBV + OPV + Hib* | 21 | 1.83 (0.62-5.36) | 1.01 (0.59-1.72) | 1.81 |
| DTPa-HBV-IPV/Hib | 17 | 1.15 (0.56-2.37) | 0.27 (0.17-0.44) | 4.17 | |
| Postbooster | DTPa + Hib* | 33 | 17.77 (8.77-36.01) | 3.615 (2.33-5.6) | 4.91 |
| DTPa/Hib | 31 | 15.48 (7.9-30.29) | 5.09 (3.25-7.95) | 3.18 | |
| Germany B | |||||
| Postprimary | DTPa-HBV/Hib | 25 | 0.547 (0.31-0.97) | 0.213 (0.17-0.27) | 3.5 |
| DTPa-HBV + Hib | 21 | 5.204 (2.16-12.53) | 0.614 (0.36-1.04) | 8.5 | |
| Prebooster | DTPa-HBV/Hib | 25 | 0.253 (0.17-0.37) | 0.155 (0.14-0.17) | 3.0 |
| DTPa-HBV + Hib | 25 | 0.679 (0.3-1.51) | 0.182 (0.14-0.24) | 7.2 | |
| Postbooster | dtpa-HBV-PRP | 25 | 6.175 (2.34-16.3) | 1.967 (0.98-3.95) | 3.7 |
| dtpa-HBV + PRP | 21 | 21.254 (6.27-72.04) | 4.774 (2.01-11.35) | 4.5 | |
Not evaluated in Germany A and Myanmar studies. Blood samples were collected 1 month after the three-dose primary series and 4 to 6 weeks after the booster dose. CI, confidence interval; dtpa, experimental DTPa vaccine with reduced antigen content; OPV, oral poliovirus vaccine. *, OmniHIB.
Antibody avidity.
In studies for which data are available, an increase in anti-PRP IgG antibody avidity was evidenced for all groups in the interval between postprimary vaccination and prebooster. No further increase was observed 1 month after the booster dose (Table 4). Anti-PRP antibodies from volunteers vaccinated with DTPa/Hib showed statistically significantly lower pre- and postbooster avidity than those from subjects vaccinated with DTPa and Hib administered separately. In contrast, there was no difference in avidity whether the subjects received Hib mixed or separate from DTPa-HBV-IPV. Avidity results with DTPw-Hib combinations were found to be in the same range as those obtained with separate DTPw-HBV and Hib.
TABLE 4.
Geometric mean avidity indices of anti-PRP IgG antibodiesa
| Study | Group | No. of subjects tested | GMAI (95% CI) | Statistical difference between groups (P value)b |
|---|---|---|---|---|
| United States | ||||
| Postprimary | DTPa + HBV + OPV + Hib* | 40 | 0.126 (0.106-0.150) | 0.0794 |
| DTPa-HBV-IPV/Hib | 40 | 0.105 (0.094-0.118) | ||
| Prebooster | DTPa + Hib* | 21 | 0.192 (0.143-0.257) | 0.8164 |
| DTPa/Hib | 23 | 0.183 (0.135-0.250) | ||
| Postbooster | DTPa + Hib* | 34 | 0.189 (0.145-0.246) | 0.8067 |
| DTPa/Hib | 37 | 0.182 (0.136-0.193) | ||
| Germany A | ||||
| Postprimary | DTPa + Hib | 33 | 0.094 (0.092-0.096) | 1 |
| DTPa/Hib | 45 | 0.094 (0.094-0.095) | ||
| Prebooster | DTPa + Hib | 21 | 0.292 (0.221-0.387) | 0.0189 |
| DTPa/Hib | 18 | 0.183 (0.138-0.242) | ||
| Postbooster | DTPa + Hib | 31 | 0.252 (0.202-0.313) | <0.0001 |
| DTPa/Hib | 59 | 0.126 (0.115-0.138) | ||
| Myanmar | ||||
| Postprimary | DTPw-HBV/Hib2.5 | 25 | 0.085 (0.070-0.103) | 0.2398 |
| DTPw-HBV/Hib | 25 | 0.069 (0.051-0.094) | ||
| Postbooster | DTPw-HBV/Hib2.5 | 25 | 0.207 (0.167-0.258) | 0.0744 |
| DTPw-HBV/Hib | 25 | 0.283 (0.214-0.374) |
Not evaluated in the Germany B study. Blood samples were collected 1 month after the three-dose primary series and before and 4 to 6 weeks after the booster dose. GMAI, geometric mean avidity index; Hib2.5, Hib vaccine containing 2.5 μg PRP conjugated to TT; OPV, oral poliovirus vaccine. *, OmniHIB.
Two-sided P value using one-way ANOVA to show a difference between groups.
Infant rat passive protection.
The protective capacity of sera pooled from immune volunteers in an infant rat passive protection model from three clinical trials is presented in Table 5. No statistically significant difference was observed whether the serum pools originated from separate Hib and DTPa-based vaccines or combined DTPa-based Hib vaccines. This was shown indiscriminately with postprimary and postbooster serum pools, indicating that anti-PRP antibodies induced by the mixed or separate Hib vaccine regimens have the same functional capacities in the infant rat passive protection assay.
TABLE 5.
Protective capacity of pooled sera measured in the infant rat passive protection assaya
| Study | Group | Mean log10 CFU/100 μl blood (SD) | P value for difference compared to positive reference (Hib coadministered) |
|---|---|---|---|
| United States | |||
| Postprimary | Control | 4.27 (0.52) | <0.05 |
| DTPa + HBV + OPV + Hib* | <0.85 (0.00) | NS | |
| DTPa-HBV-IPV/Hib | 2.55 (1.65) | NS | |
| Postbooster | Control | 4.37 (0.90) | <0.01 |
| DTPa + Hib* | 1.09 (0.64) | NS | |
| DTPa/Hib | <0.85 (0.00) | NS | |
| Germany A | |||
| Postprimary | Control | 4.89 (0.29) | <0.01 |
| DTPa + Hib | 2.82 (0.73) | NS | |
| DTPa/Hib | 2.07 (1.72) | NS | |
| Postbooster | Control | 4.95 (0.95) | <0.01 |
| DTPa + Hib | <0.85 (0.00) | NS | |
| DTPa/Hib | <0.85 (0.00) | NS | |
| Germany B | |||
| Postprimary | Control | 5.43 (0.53) | <0.01 |
| DTPa-HBV + Hib | 1.48 (0.71) | NS | |
| DTPa-HBV/Hib | 2.43 (2.21) | NS | |
| Postbooster | Control | 5.43 (0.53) | <0.01 |
| dtpa-HBV + PRP | <0.85 (0.00) | NS | |
| dtpa-HBV/PRP | <0.85 (0.00) | NS |
Not evaluated in the Myanmar study. NS, no statistically significant difference; dtpa, experimental DTPa vaccine with reduced antigen content; OPV, oral poliovirus vaccine. *, OmniHIB.
Bactericidal activity.
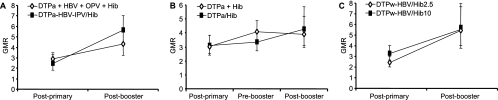
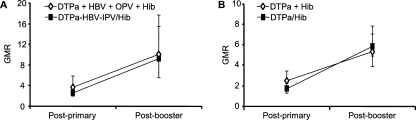
Following primary vaccination with DTP-based Hib combination vaccines, the bactericidal GMTs were statistically lower than those after separately administered Hib vaccinations (Table 6). This difference was no longer present prior to and after the booster dose. These data are in line with the anti-PRP RABA and ELISA results. Anti-PRP antibodies in infants who received mixed Hib/DTPa-based vaccines or coadministered Hib conjugate vaccine had a similar functional capacity in the bactericidal assay, as evaluated by the GMR of serum bactericidal activity and RABA (Fig. 1). Similar GMRs at postprimary and postbooster time points were observed in DTPa-based Hib vaccinees and in those vaccinated with DTPw-based Hib vaccines. In all study groups, regardless of the vaccine given, an increase in bactericidal capacity (GMR) occurred in sera between postprimary and postbooster time points, suggesting maturation of the antibody response over time.
TABLE 6.
GMTs of bactericidal and opsonophagocytic activitiesa
| Study | Group | Bactericidal activity
|
Opsonophagocytic activity
|
||
|---|---|---|---|---|---|
| No. of responders above detection limit/total no. of subjects | GMT (95% CI) | No. of responders above detection limit/total no. of subjects | GMT (95% CI) | ||
| United States | |||||
| Postprimary | DTPa + HBV + OPV + Hib* | 16/26 | 20.89 (13.16-33.16)b | 33/57 | 22.24 (12.911-38.310)b |
| DTPa-HBV-IPV/Hib | 11/23 | 7.53 (5.51-10.29) | 25/60 | 7.42 (5.078-10.842) | |
| Postbooster | DTPa + Hib* | 32/33 | 122.73 (72.27-208.44) | 33/33 | 286.11 (112.41-728.12) |
| DTPa/Hib | 33/33 | 148.27 (102.59-214.31) | 33/33 | 245.1 (124.13-483.94) | |
| Germany B | |||||
| Postprimary | DTPa-HBV + Hib | 29/40 | 16.00 (11.84-21.63)b | ||
| DTPa-HBV/Hib | 19/39 | 7.58 (6.03-9.54) | |||
| Prebooster | DTPa-HBV + Hib | 9/39 | 6.35 (4.97-8.11) | ||
| DTPa-HBV/Hib | 4/39 | 4.61 (4.09-5.20) | |||
| Postbooster | dtpa-HBV + PRP | 35/40 | 76.11 (45.87-126.28) | ||
| dtpa-HBV/PRP | 37/40 | 130.24 (84.78-200.06) | |||
| Myanmar | |||||
| Postprimary | DTPw-HBV/Hib2.5 | 23/25 | 38.9 (23.4-64.4) | ||
| DTPw-HBV/Hib | 25/25 | 51.3 (34.6-76.0) | |||
| Postbooster | DTPw-HBV/Hib2.5 | 25/25 | 216.8 (126.7-370.9) | ||
| DTPw-HBV/Hib | 24/25 | 235.6 (138.5-400.6) | |||
| Germany A | |||||
| Postprimary | DTPa + Hib | 41/56 | 21.4 (13.84-33.68)b | ||
| DTPa/Hib | 14/72 | 3.74 (3.15-4.45) | |||
| Postbooster | DTPa + Hib | 34/34 | 462.2 (266.7-801.1)b | ||
| DTPa/Hib | 64/64 | 215.9 (158.3-294.6) | |||
Blood samples were collected 1 month after the three-dose primary series and before and 4 to 6 weeks after the booster dose. CI, confidence interval; dtpa, experimental DTPa vaccine with reduced antigen content; Hib2.5, Hib vaccine containing 2.5 μg PRP conjugated to TT; OPV, oral poliomyelitis vaccine; *, OmniHIB.
P < 0.05 for comparisons between groups receiving the mixed and separately administered vaccines at the specified time points (ANOVA for postbooster time points and the postprimary time point for the Myanmar study and Kruskal-Wallis test for all other postprimary and prebooster time points).
FIG. 1.
Ratio of bactericidal GMT to RABA GMT (GMR) with 95% confidence intervals in three clinical trials. (A) U.S. study. (B) Germany B study. (C) Myanmar study. No statistically significant differences between the groups were observed, except for the postprimary time point for the Myanmar study (P = 0.0367).
Opsonophagocytic activity.
The opsonic activity of a subset of individual sera from subjects who participated in the U.S. and Germany A studies is presented in Table 6 and Fig. 2. Postprimary opsonophagocytic GMTs were significantly lower after primary vaccination with DTPa-based Hib vaccines than after separately administered Hib vaccines in both studies. Postbooster opsonophagocytic GMTs were also significantly lower after DTPa/Hib vaccination in the Germany A study. These results are in line with the anti-PRP RABA and ELISA data, including the booster responses in the Germany A study. No statistically significant differences in GMR were observed between subjects receiving mixed Hib and DTPa-based vaccines and subjects receiving Hib and DTPa separately. GMRs were higher after the booster than after the primary vaccination, indicating that a maturation of the immune response had occurred.
FIG. 2.
Ratio of opsonophagocytic GMT to RABA GMT (GMR) with 95% confidence intervals in two clinical trials. (A) U.S. study. (B) Germany A study. No statistically significant differences between the groups were observed.
DISCUSSION
Efficacy studies to evaluate new Hib conjugate vaccines are no longer tenable now that highly effective Hib vaccines are widely available. The understanding and the use of surrogate markers of protection have thus become key factors in supporting the licensure of new combination Hib vaccines. Measurement of antibody concentrations against PRP remains the cornerstone for the putative demonstration of protection. However, it is generally accepted that antibody quality and functional activity are equally important as or possibly more important than serum IgG concentrations alone. Antibody functional capacity depends on concentration, avidity, and isotype, including the IgG subclass, which is also possibly modulated by the age of the subject and the form of antigen stimulation (conjugate/nonconjugate/naturally acquired) (21). In this study, we have characterized the anti-PRP antibody response induced by a PRP-TT conjugate vaccine administered concomitantly or mixed with various DTPa-based and DTPw-based vaccines.
Higher postprimary anti-PRP antibody GMCs were observed after vaccination with a Hib vaccine administered separately than with mixed vaccination with a DTPa-based vaccine. This corroborates the observation that DTPa/Hib combinations typically induce lower postprimary anti-PRP responses (6). It was also observed, in the Myanmar study, that anti-PRP antibody GMCs were considerably higher following DTPw-based Hib vaccinations, which we may attribute to the use of whole-cell pertussis, which was already known to elicit higher antibody titers than acellular pertussis (11). However, after the booster dose, these differences between vaccines were no longer present, in line with a recent study in which there was no association between postbooster anti-PRP antibody concentrations and the vaccine (DTPw/Hib and/or DTPa/Hib) used for primary vaccination (15). On the other hand, the DTPa/Hib Germany A and B studies demonstrated lower postbooster antibody levels for the DTPa/Hib combination than with separate administration (Table 2).
Antibody avidity is a measurement of the binding strength of the antibodies to the polysaccharide antigen. Booster responses are characterized by the production of high-avidity antibodies, and antibody avidity has been proposed as a surrogate marker for the induction of successful priming (8). There is evidence that avidity may develop in the absence of a detectable primary response to DTPa-based Hib vaccines (9). A similar range of avidity indices was observed in subjects vaccinated with Hib, whether administered mixed with or separate from DTPa-based vaccines, and in subjects who received DTPw-based Hib vaccines, with most maturation evident by the time of the booster dose. However, there appeared to be differences between the maturation of avidity following primary vaccination with DTPa/Hib and that with DTPa-HBV-IPV/Hib or DTPw-based Hib vaccination. The avidity index was statistically significantly lower prior to and after the booster dose of DTPa/Hib than that of coadministered DTPa and Hib, whereas no such difference was observed between the mixed and separately administered DTPa-HBV-IPV and Hib vaccines. The lower booster response in the Germany A DTPa/Hib study is in line with the lower avidity maturation and confirms that antibody avidity is a surrogate marker for immune memory for conjugate vaccines (8). This observation is in accordance with a recent report by Johnson et al. (15), who noted a reduced Hib conjugate postbooster antibody avidity when the primary vaccination was done with DTPa/Hib compared to primary vaccination with DTPw/Hib. We have previously reported the absence of any difference in avidity maturation following primary vaccination with the larger combined DTPa-HBV-IPV/Hib vaccine (27). Taken together, our data, and those described previously by Johnson et al., support the notion that the DTPa/Hib vaccine may have a reduced ability to induce avidity maturation compared with those of DTPa-HBV-IPV/Hib and DTPw-based Hib vaccines. We have evidence that IPV acts as an immunostimulant, which might explain the differences between DTPa/Hib and DTPa-HBV-IPV/Hib (data not shown).
Despite this reduced antibody avidity after primary vaccination with DTPa/Hib versus DTPa-HBV-IPV/Hib or DTPw-based Hib vaccinations, we observed no difference between vaccines with regard to antibody quality and function, as measured by bactericidal activity, opsonophagocytic activity (GMR), and the ability to protect in a passive rat protection assay. Significantly lower postprimary opsonophagocytic and bactericidal GMTs in DTPa-based Hib vaccine recipients and lower postbooster GMTs in DTPa/Hib recipients in the Germany A study reflect the lower antibody levels achieved at these time points. There were no observed differences in GMR, indicating that the antibodies had similar functional activities regardless of their concentrations. Maturation of the bactericidal and opsonic responses evolved over time in a similar fashion in all groups. The reduced avidity maturation and booster responses following DTPa/Hib vaccination versus those of the other vaccines tested contrasts with the maturation of the bactericidal and opsonic responses for all DTPa-based Hib combinations, including DTPa/Hib. The reduced avidity and reduced booster responses with DTPa/Hib raise the question of whether the protective efficacy of DTPa/Hib may also be reduced. This seems unlikely, given the absence of any differences in bactericidal activity, opsonophagocytosis, and protection in the infant rat model. Furthermore, similar observations (lower avidity with comparable bactericidal and opsonic activity as well as reduced booster responses than other Hib vaccines) were also made for infants vaccinated with the licensed PRP-OMP vaccine. The protective efficacy of PRP-OMP was 95% in a placebo-controlled trial conducted with high-risk children in the United States that included a booster immunization (6, 20, 29), suggesting no impact of the reduced avidity maturation on efficacy.
There is a great deal of uncertainty about the importance of direct bactericidal activity or opsonophagocytosis in the human anti-Hib defense mechanism. In a study involving young adults, virtually all samples displayed opsonophagocytic activity, while only half of them had demonstrable bactericidal activity (23). In mice, C5-deficient but not C3-depleted mice showed normal clearance of Hib from the bloodstream, indicating the critical role of opsonophagocytosis in Hib clearance (24). Due to the observation that individuals with late complement pathway deficiencies are not at increased risk of clinical Hib disease, it has been suggested that opsonic activity alone is sufficient for protection (7).
The clinical effectiveness of DTPa-based Hib combinations in preventing Hib disease has been conclusively demonstrated in Germany, where the vaccine effectiveness was 96.7% after primary immunization and 98.5% after the booster dose in the second year of life (16). Prior to the introduction of DTPa-based Hib combinations in Germany, it was recognized that the absence of a booster dose was associated with an increase in Hib disease (34) and with a reduction in the prevention of Hib colonization (18). The importance of the booster dose in consolidating immunity to Hib has been more recently demonstrated in the United Kingdom, where vaccine effectiveness of DTPw/Hib administered by a 2-, 3-, and 4-month schedule without a booster was rather low (67 to 88%) within the first 2 years and absent (no effectiveness) after 2 years (28). The rather low effectiveness was initially obscured due to the installation of a catch-up Hib conjugate campaign in children less than 4 years of age in 1992 (12, 28). No booster dose was administered at that time in the United Kingdom, and an increase in Hib disease was observed starting in 1999 and exacerbated after the introduction of the DTPa/Hib vaccine in 2000 (28). The absence of a Hib booster has been identified as the single most important factor leading to the increased incidence of Hib disease in the United Kingdom (32). Within the United Kingdom context of suboptimal control of Hib disease, vaccination with a combined DTPa/Hib vaccine was associated with an increased risk for Hib disease (22). Although the absence of a Hib booster is a critical factor, anti-PRP antibody GMCs and the proportion of vaccinated United Kingdom subjects with anti-PRP antibodies of ≥0.15 μg/ml were, nevertheless, atypically low for DTPa/Hib combinations (10).
Of note, a strikingly similar observation was recently made in the United Kingdom with respect to meningococcal C conjugate immunization. Indeed, a primary immunization according to the 2-, 3-, and 4-month schedule without booster resulted in deficient protection against serogroup C meningococcal disease in children >1 year of age (33). Primary immunization according to the less immunologically challenging 2-, 4-, and 6-month schedule without booster in Spain was associated with a better persistence of protection (19).
Although the mixing of DTPa and Hib conjugate vaccine resulted in a reduced magnitude of the anti-PRP antibody response, combined vaccines demonstrated anti-PRP antibody levels, seroprotection rates, and antibody avidity that were equal to or higher than those observed after primary vaccination with the licensed and efficacious PRP-OMP vaccine (29). In the present study, we have also demonstrated that antibody quality, maturation, and protective capacity are equivalent in subjects who received primary and booster vaccination with Hib vaccine, independently of the mode of administration with DTPa-based vaccine. Furthermore, these responses were also similar to those induced by the DTPw-based Hib vaccine. These data, along with postmarketing confirmation of ongoing vaccine effectiveness in Germany (30), attest for the excellent clinical effectiveness of DTPa-based Hib combination vaccines in preventing Hib disease in infants and children.
Acknowledgments
We thank Joanne Wolter for assistance with preparation of the manuscript.
Infanrix, HeXa, and Hiberix are trademarks of the GlaxoSmithKline group of companies; OmniHIB is a trademark of Sanofi-Pasteur.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.Adegbola, R. A., O. Secka, G. Lahai, N. Lloyd-Evans, A. Njie, S. Usen, C. Oluwalana, S. Obaro, M. Weber, T. Corrah, K. Mulholland, K. McAdam, B. Greenwood, and P. J. Milligan. 2005. Elimination of Haemophilus influenzae type b (Hib) disease from The Gambia after the introduction of routine immunisation with a Hib conjugate vaccine: a prospective study. Lancet 366:144-150. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, P. 1984. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J. Infect. Dis. 149:1034-1035. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, P., D. L. Ingram, M. E. Pichichero, and G. Peter. 2000. A high degree of natural immunologic priming to the capsular polysaccharide may not prevent Haemophilus influenzae type b meningitis. Pediatr. Infect. Dis. J. 19:589-591. [DOI] [PubMed] [Google Scholar]
- 4.Blatter, M. M., K. S. Reisinger, D. R. Terwelp, F. J. DelBuono, and B. J. Howe. 1998. Immunogenicity of a combined diphtheria-tetanus-acellular pertussis (DT-tricomponent Pa)-hepatitis B (HB)-inactivated poliovirus (IPV) admixed with Haemophilus influenzae type b (Hib) vaccine in infants. Pediatr. Res. 43:141A. [Google Scholar]
- 5.Eskola, J., H. Käyhty, A. K. Takala, H. Peltola, P. R. Ronnberg, E. Kela, E. Pekkanen, P. H. McVerry, and P. H. Makela. 1990. A randomized, prospective field trial of a conjugate vaccine in the protection of infants and young children against invasive Haemophilus influenzae type b disease. N. Engl. J. Med. 323:1381-1387. [DOI] [PubMed] [Google Scholar]
- 6.Eskola, J., J. Ward, R. Dagan, D. Goldblatt, F. Zepp, and C. A. Siegrist. 1999. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 354:2063-2068. [DOI] [PubMed] [Google Scholar]
- 7.Frasch, C. E. 1995. Haemophilus influenzae type b conjugate and combined vaccines. Clin. Immunother. 4:376-386. [Google Scholar]
- 8.Goldblatt, D., A. R. J. P. M. Pinto Vaz, and E. Miller. 1998. Antibody avidity as a surrogate marker of successful priming by Haemophilus influenzae type b conjugate vaccines following infant immunization. J. Infect. Dis. 177:1112-1115. [DOI] [PubMed] [Google Scholar]
- 9.Goldblatt, D., P. Richmond, E. Millard, C. Thornton, and E. Mille. 1999. The induction of immunologic memory after vaccination with Haemophilus influenzae type b conjugate and acellular pertussis-containing diphtheria, tetanus, and pertussis vaccine combination. J. Infect. Dis. 180:538-541. [DOI] [PubMed] [Google Scholar]
- 10.Goldblatt, D., J. Southern, L. Ashton, P. Richmond, P. Burbidge, J. Tasevska, A. Crowley-Luke, N. Andrews, R. Morris, R. Borrow, K. Cartwright, and E. Miller. 2006. Immunogenicity and boosting after a reduced number of doses of a pneumococcal conjugate vaccine in infants and toddlers. Pediatr. Infect. Dis. J. 25:312-319. [DOI] [PubMed] [Google Scholar]
- 11.Gylca, R., V. Gylca, O. Benes, A. Melnic, V. Chicu, C. Weisbecker, P. Willems, and A. Kaufhold. 2000. A new DTPa-HBV-IPV vaccine co-administered with Hib, compared to a commercially available DTPw-IPV/Hib vaccine co-administered with HBV, given at 6, 10 and 14 weeks following HBV at birth. Vaccine 19:825-833. [DOI] [PubMed] [Google Scholar]
- 12.Heath, P. T., R. Booy, H. J. Azzopardi, M. P. Slack, J. Bowen-Morris, H. Griffiths, M. E. Ramsay, J. J. Deeks, and E. R. Moxon. 2000. Antibody concentration and clinical protection after Hib conjugate vaccination in the United Kingdom. JAMA 284:2334-2340. [DOI] [PubMed] [Google Scholar]
- 13.Hla, K. H., S. A. Thein, A. Aye, H. H. Han, H. L. Bock, M. P. David, and L. Schuerman. 2006. Reactogenicity and immunogenicity profiles of a novel pentavalent DTPw-HepB/Hib vaccine with reduced PRP antigen content: a randomised dose-range trial. Pediatr. Infect. Dis. J. 25:706-712. [DOI] [PubMed] [Google Scholar]
- 14.Hoppenbrouwers, K., R. Lagos, B. Swennen, C. Ethevenaux, J. Knops, M. M. Levine, and J. Desmyter. 1998. Safety and immunogenicity of an Haemophilus influenzae type b-tetanus toxoid conjugate (PRP-T) and diphtheria-tetanus-pertussis (DTP) combination vaccine administered in a dual-chamber syringe to infants in Belgium and Chile. Vaccine 16:921-927. [DOI] [PubMed] [Google Scholar]
- 15.Johnson, N. G., J. U. Ruggeberg, G. F. Balfour, Y. C. Lee, H. Liddy, D. Irving, J. Sheldon, M. P. Slack, A. J. Pollard, and P. T. Heath. 2006. Haemophilus influenzae type b reemergence after combination immunization. Emerg. Infect. Dis. 12:937-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalies, H., T. Verstraeten, V. Grote, N. Meyer, A. Siedler, H. J. Schmitt, T. Breuer, L. H. Moulton, R. von Kries, and the Erhebungseinheit fur Seltene Pädiatrische Erkrankungen in Deutschland Study Group. 2004. Four and one-half-year follow-up of the effectiveness of diphtheria-tetanus toxoids-acellular pertussis/Haemophilus influenzae type b and diphtheria-tetanus toxoids-acellular pertussis-inactivated poliovirus/H. influenzae type b combination vaccines in Germany. Pediatr. Infect. Dis. J. 23:944-950. [DOI] [PubMed] [Google Scholar]
- 17.Käyhty, H., H. Peltola, V. Karanko, and P. H. Makela. 1983. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J. Infect. Dis. 147:1100. [DOI] [PubMed] [Google Scholar]
- 18.Käyhty. H. 1994. Difficulties in establishing a serological correlate of protection after immunization with Haemophilus influenzae conjugate vaccines. Biologicals 22:397-402. [DOI] [PubMed] [Google Scholar]
- 19.Larrauri, A., R. Cano, M. Garcia, and S. Mateo. 2005. Impact and effectiveness of meningococcal C conjugate vaccine following its introduction in Spain. Vaccine 23:4097-4100. [DOI] [PubMed] [Google Scholar]
- 20.Lucas, A. H., and D. M. Granoff. 1995. Functional differences in idiotypically defined IgG1 anti-polysaccharide antibodies elicited by vaccination with Haemophilus influenzae type B polysaccharide-protein conjugates. J. Immunol. 154:4195-4202. [PubMed] [Google Scholar]
- 21.Makela, O., P. Mattila, N. Rautonen, I. Seppala, J. Eskola, and H. Käyhty. 1987. Isotype concentrations of human antibodies to Haemophilus influenzae type b polysaccharide (Hib) in young adults immunized with the polysaccharide as such or conjugated to a protein (diphtheria toxoid). J. Immunol. 139:1999-2004. [PubMed] [Google Scholar]
- 22.McVernon, J., N. Andrews, M. P. Slack, and M. E. Ramsay. 2003. Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with acellular pertussis. Lancet 361:1521-1523. [DOI] [PubMed] [Google Scholar]
- 23.Musher, D., A. Goree, T. Murphy, A. Chapman, J. Zahradnik, M. Apicella, and R. Baughn. 1986. Immunity to Haemophilus influenzae type b in young adults: correlation of bactericidal and opsonizing activity of serum with antibody to polyribosylribitol phosphate and lipooligosaccharide before and after vaccination. J. Infect. Dis. 154:935-943. [DOI] [PubMed] [Google Scholar]
- 24.Noel, G. J., S. Katz, and P. J. Edelson. 1988. Complement-mediated early clearance of Haemophilus influenzae type b from blood is independent of serum lytic activity. J. Infect. Dis. 157:85-90. [DOI] [PubMed] [Google Scholar]
- 25.Peltola, H. 2000. Worldwide Haemophilus influenzae type b disease at the beginning of the 21st century: global analysis of the disease burden 25 years after the use of the polysaccharide vaccine and a decade after the advent of conjugates. Clin. Microbiol. Rev. 13:302-317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pichichero, M. E., T. Voloshen, D. Zajac, and S. Passador. 1999. Avidity maturation of antibody to Haemophilus influenzae type b (Hib) after immunization with diphtheria-tetanus-acellular pertussis-Hib-hepatitis B combined vaccine in infants. J. Infect. Dis. 180:1390-1393. [DOI] [PubMed] [Google Scholar]
- 27.Poolman, J., A. Kaufhold, D. De Grave, and D. Goldblatt. 2001. Clinical relevance of lower Hib response in DTPa-based combination vaccines. Vaccine 19:2280-2285. [DOI] [PubMed] [Google Scholar]
- 28.Ramsay, M. E., J. McVernon, N. J. Andrews, P. T. Heath, and M. P. Slack. 2003. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J. Infect. Dis. 188:481-485. [DOI] [PubMed] [Google Scholar]
- 29.Schlesinger, Y., D. M. Granoff, et al. 1992. Avidity and bactericidal activity of antibody elicited by different Haemophilus influenzae type b conjugate vaccines. JAMA 267:1489-1494. [PubMed] [Google Scholar]
- 30.Schmitt, H.-J., R. von Kries, B. Hassenpflug, M. Hermann, A. Siedler, W. Niessing, R. Clemens, and J. Weil. 2001. Haemophilus influenzae type b disease: impact and effectiveness of diphtheria-tetanus toxoids-acellular pertussis (-inactivated poliovirus)/H. influenzae type b combination vaccines. Pediatr. Infect. Dis. J. 20:767-774. [DOI] [PubMed] [Google Scholar]
- 31.Schmitt, H.-J., F. Zepp, S. Müschenborn, G. Sümenicht, A. Schuind, K. Beutel, M. Knuf, H. L. Bock, H. Bogaerts, and R. Clemens. 1998. Immunogenicity and reactogenicity of a Haemophilus influenza type b tetanus conjugate vaccine when administered separately or mixed with concomitant diphtheria-tetanus-toxoid and acellular pertussis vaccine for primary and for booster immunizations. Eur. J. Pediatr. 157:208-214. [DOI] [PubMed] [Google Scholar]
- 32.Steinhoff, M., and D. M. Goldblatt. 2003. Conjugate Hib vaccines. Lancet 361:360-361. [DOI] [PubMed] [Google Scholar]
- 33.Trotter, C. L., N. J. Andrews, E. B. Kaczmarski, E. Miller, and M. E. Ramsay. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364:365-367. [DOI] [PubMed] [Google Scholar]
- 34.von Kries, R., O. Bohm, and A. Windfuhr. 1997. Haemophilus influenzae b-vaccination: the urgency for timely vaccination. Eur. J. Pediatr. 156:282-287. [DOI] [PubMed] [Google Scholar]
- 35.Zepp, F., H.-J. Schmitt, A. Kaufhold, A. Schuind, M. Knuf, P. Habermehl, C. Meyer, H. Bogaerts, M. Slaoui, and R. Clemens. 1997. Evidence for induction of polysaccharide specific B-cell-memory in the 1st year of life: plain Haemophilus influenzae type b-PRP (Hib) boosters children primed with a tetanus-conjugate Hib-DTPa-HBV combined vaccine. Eur. J. Pediatr. 156:18-24. [DOI] [PubMed] [Google Scholar]