Abstract
The multigene PE and PPE family represents about 10% of the genome of Mycobacterium tuberculosis. Here, we report that three members of the PE family, namely, Rv1169c, Rv0978c, and Rv1818c, elicit a strong, but differential, B-cell humoral response among different clinical categories of tuberculosis patients. The study population (n = 211) was comprised of different clinical groups of both adult and child patients: group 1 (n = 94) patients with pulmonary infection, group 2 (n = 30) patients with relapsed infection, group 3 (n = 31) patients with extrapulmonary infections, and clinically healthy donors (n = 56). Among the PE proteins studied, group 1 adult patient sera reacted to Rv1818c and Rv0978c, while Rv1169c elicited immunoreactivity in group 3 children. However, all three PE antigens studied as well as the 19-kDa antigen did not demonstrate humoral reactivity with sera from group 2 patients with relapsed infection. The current study shows that while responsiveness to all three PE antigens is a good marker for M. tuberculosis infection, a strong response to Rv0978c or to Rv1818c by group 1 adult patients with pulmonary infection or largely restricted reactivity to Rv1169c antigen in child patients with extrapulmonary infections offers the possibility of differential utility in the serodiagnosis of tuberculosis.
Mycobacterium tuberculosis, the causative agent of pulmonary tuberculosis (TB), infects one-third of the world's population (22). Despite the multiplicity of antimicrobial responses mounted by its host, M. tuberculosis shows a remarkable ability to survive either by evoking survival strategies or by interfering with critical macrophage functions that are required to successfully respond to infection (12, 21, 30). One such immune evasion or survival strategy may be to express different sets of proteins during various clinical stages of the disease in infected macrophages of granulomas, which provides survival advantages amid robust host immune responses. A group of genes carried by M. tuberculosis that are expressed upon infection of macrophages belongs to the PE family (1, 4, 9). This family is comprised of about 100 genes, scattered throughout the genome, with highly homologous sequences corresponding to a signature Pro-Glu (PE) amino acid sequence near the amino terminus (1, 4, 9). In many proteins, the PE domain is often linked to a unique domain of various lengths that is rich in alanine and glycine amino acids, termed the PGRS domain (PE_PGRS subfamily). It is generally believed that the PGRS domain of PE family genes could be a source of antigenic variability (5, 10, 13, 26, 29). The uniqueness of the PE genes is further illustrated by the fact that these genes are restricted to mycobacteria (4). However, despite their abundance in mycobacteria, very little is known regarding the expression or the functions of PE family genes. Recent studies have provided some insights into functional roles of selected PE family proteins. It has been shown that Mycobacterium marinum expresses a homologue of the M. tuberculosis PE (PE_PGRS) Rv1651c gene in infected granulomas (27). Mutation studies have shown that Rv1818c, a PE (PE_PGRS) gene product, may play a role during growth in liquid medium as well as in the infection of macrophages (5). In addition, aerosol infection of mice with virulent M. tuberculosis strains generates a humoral response to the Rv1818c protein (10). The involvement of PE family genes in the virulence of the pathogen has also been reported, and many members of PE family proteins, including Rv1818c, are reported be localized on the surface of M. tuberculosis bacilli (3, 11, 27). Additionally, it has been suggested that the PE_PGRS subfamily of PE genes is enriched in genes with a high probability of being essential for M. tuberculosis (19). Although those studies strongly support a role for the PE family proteins in the biology, and possibly pathogenesis, of M. tuberculosis, an understanding of the function of PE proteins will require extensive investigations.
In the current study, Rv1169c, Rv0978c, and Rv1818c were selected based on the following observations. These PE family proteins display a differential antigenic profile and are associated with pathological conditions, as evident from DNA microarray expression data (17, 26, 28, 31, 32). Furthermore, Rv0978c and Rv1169c were upregulated in TB bacilli upon infection of macrophages (28, 31). As mentioned above, a mutation in Rv1818c affects the growth of bacilli as well as the infection of macrophages (5). Recently published studies suggested that the ectopic expression of Rv1818c in a nonpathogenic Mycobacterium smegmatis strain results in specific properties more typical of virulent mycobacteria, including increased survival in macrophages and host tissues (13). In addition, Rv1818c and Rv1169c have been detected in M. tuberculosis bacilli isolated from the granulomas of lungs of human pulmonary TB patients (26). Rv0978c was demonstrated to be a member of a group of genes called in vivo-expressed genomic island genes, which were shown to be upregulated in M. tuberculosis bacilli during infection of mice (31). Rv0978c was also shown to be upregulated, at least eightfold, in human brain microvascular endothelial cell-associated M. tuberculosis, suggesting a role for endothelial cell invasion and intracellular survival (17). Furthermore, Rv0978c and Rv1169c are expressed upon infection by pathogenic species such as M. tuberculosis and Mycobacterium bovis only and not by environmental mycobacteria like M. smegmatis or Mycobacterium avium. Similarly, Rv1818c is constitutively expressed (5) by strains of M. tuberculosis and M. bovis but not by environmental mycobacteria like M. smegmatis or M. avium. In the current investigation, we report that the above-mentioned antigens of the PE family elicited differential humoral antibody reactivities in a panel of human sera obtained from different clinical categories of TB patients as measured by enzyme-linked immunosorbent assay (ELISA).
MATERIALS AND METHODS
Patients and control subjects.
The study population (n = 211) was comprised of TB-infected patients reporting to the National Jalma Institute of Leprosy and Other Mycobacterial Diseases in Agra, India. The patient population was categorized into different clinical groups as follows. Group 1 (n = 94) patients were diagnosed with pulmonary TB for the first time and had no history of chemotherapeutic intervention. Group 1 had 69 adults and 25 children. Group 2 (n = 30) patients had relapsed infection, and all recruited patients were adults. Group 3 (n = 31) patients with extrapulmonary TB infections consisted of 9 adults and 22 children. The patients were categorized according to guidelines of the National TB Control Program, Central TB Division, Government of India. Pulmonary TB in patients in groups 1 and 2 was confirmed by the presence of acid-fast bacilli in at least two initial sputum smear examinations and growth of bacilli in BACTEC cultures. Patients with active TB infection were also examined for radiological abnormalities by chest X-ray. In the case of group 2 patients with relapsed infection, all patients were adults and were diagnosed with pulmonary TB. After diagnosis, these patients had a full course of antitubercular chemotherapeutic treatment but had a recurrence of the infection and disease symptoms after therapy. On the other hand, group 1 patients were patients with active pulmonary TB at the onset of disease who were attending the Outpatient Department at the National Jalma Institute of Leprosy and Other Mycobacterial Diseases, Agra, India, and had not received any antitubercular chemotherapeutic intervention previously. Members from group 3 had primarily abdominal TB infection and tubercular meningitis. The diagnosis of extrapulmonary TB was carried out by histological examination as well as with culture positivity of the bacillus in specimens obtained from extrapulmonary sites. The age ranges of adults and children were 18 to 60 years and 2 to 15 years, respectively. In the case of pulmonary TB patients as well as patients belonging to other categories, samples were obtained from active untreated patients (both adults and children) at the time of diagnosis. Samples were taken prior to commencement of chemotherapy. Relapsed patients were those patients who had a recurrence of symptoms after taking a full course of antitubercular treatment. Sera were separated from blood and kept at −20°C until use. The samples were obtained from all donors upon entry into the study, and samples from human immunodeficiency virus-positive subjects were excluded from the study. The healthy donors (n = 56) included in the study were recruited after radiological and clinical examination to exclude individuals with active TB. Additionally, healthy donors included in the study were age and gender matched to the different clinical groups. The included subjects had given written consent, and the current study was carried out after approval from the institutional bioethics committee.
Cloning, expression, and in silico analysis of Rv0978c, Rv1169c, and Rv1818c.
The selected PE genes were PCR amplified from genomic DNA using gene-specific primers: 5′-GCGGATCCATGTCGTTTGTCAACGTGGC-3′ (forward) and 5′-CGCTCGAGAGCTGATTACCGACACCGTGT-3′ (reverse) for Rv0978c, 5′-TGTCTTTTGTCACCACACGG-3′ (forward) and 5′-GGTGGAGGTGCCCGCGCGGTT-3′ (reverse) for Rv1169c, and 5′-CCGGAATTCATGTCATTTGTGGTCACG-3′ (forward) and 5′-CGCGGATCCCGGTAACCCGTTCATCCC-3′ (reverse) for Rv1818c. The amplified PE gene PCR product was ligated into the pGEMT-Easy vector (Promega Inc.), and the recombinant clones carrying the appropriate PE gene insert were confirmed by DNA sequencing. The PE gene inserts were subcloned into cloned pRSET series vectors for protein expression and purification. Escherichia coli BL21 cells carrying recombinant plasmids were induced with IPTG (isopropyl-β-d-thiogalactopyranoside), and His-tagged recombinant proteins were purified with Ni-nitrilotriacetic acid columns (QIAGEN). In addition, recombinant E. coli clones expressing Rv1818c and 19-kDa antigens were kind gifts from M. J. Brennan, FDA, and Neil Reiner, University of British Columbia, Canada, respectively.
In silico analyses of Rv0978c, Rv1169c, and Rv1818c were carried out according to a method described previously by Jameson and Wolf (18). The program generates values for surface accessibility parameters and combines these values with those obtained for regional backbone flexibility and predicted secondary structure. Furthermore, the program offers a reliable prediction of potential antigenic determinants.
Serological characterization of Rv0978c, Rv1169c, and Rv1818c.
ELISAs were carried out with 96-well microtiter plates (Nunc) using the above-mentioned recombinant PE proteins. The plates were incubated with recombinant PE proteins (1.25 μg/ml) overnight at 4°C, followed by three washes with phosphate-buffered saline (PBS)-Tween 20 (0.05%) buffer. After being blocked with 3% bovine serum albumin in PBS, wells were incubated with human sera (1:400 dilution in blocking buffer) for 1 h at 37°C, followed by washing with PBS-Tween 20 buffer. The above-mentioned serum dilution was selected after careful titration experiments with different dilutions of the patient sera were carried out. The plates were further incubated with anti-human immunoglobulin (Ig)-horseradish peroxidase, followed by development with o-phenylenediamine tetrahydrochloride. The absorbance values were measured at 492 nm by using an ELISA reader (Molecular Devices).
Statistical analysis.
Student's t test was used for analysis of statistical significance (P value). The data for serological reactivities of different categories of patients were compared with data for healthy controls. Graphpad Quickcalcs (online t test calculator [http://www.graphpad.com/quickcalcs/ttest1.cfm]) was used for this purpose. Only P values that were less than 0.05 were considered to be significant. Comparisons of immunoreactivities within clinical groups were carried out using Bonferroni's multiple-comparison test (Graphpad Prism).
RESULTS
Expression and purification of Rv0978c, Rv1169c, and Rv1818c.
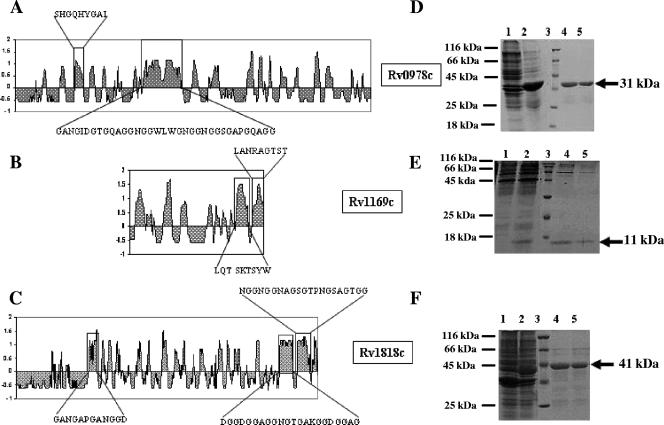
The recombinant PE proteins Rv0978c, Rv1169c, and Rv1818c were cloned, expressed, and purified as described in Materials and Methods (Fig. 1). In silico analysis of PE Rv0978c (331 amino acids [aa]), Rv1169c (100 aa), and Rv1818c (498 aa) was performed according to a method described previously by Jameson and Wolf (18), which predicts the topological features of a protein directly from its primary amino acid sequence. The output of this algorithm, the antigenic index, is used to create a linear surface contour profile of the protein. Since most of the antigenic sites are located within surface-exposed regions of a protein, the program offers a reliable means of predicting potential antigenic determinants. The antigenic index is calculated by summing several weighted measures of secondary structure. The hypothetical PE open reading frames Rv0978c, Rv1169c, and Rv1818c have a high antigenic profile score, as obtained by in silico analysis, revealing the regions with a high antigenic index for potential antigenic determinants (Fig. 1A to C). Among all the proteins belonging to the PE family analyzed, the selected PE proteins Rv1169c, Rv0978c, and Rv1818c exhibited very high antigenic indices for potential antigenic determinants as mentioned in the legend of Fig. 1A to C. Furthermore, only 8 PE proteins out of 37 PE proteins and 23 PE_PGRS proteins out of 62 proteins exhibited similarly high antigenic indices. However, according to recent reports in the literature (26, 32), Rv1169c, Rv0978c, and Rv1818c are among nine PE family proteins that were shown to be expressed in M. tuberculosis bacilli upon infection of macrophages. As shown in Fig. 1, experiments utilizing the peptides with boxed protein sequences for assessing reactivity with humoral and cell-mediated immune responses of TB patients are under way.
FIG. 1.
Cloning, expression, and in silico analyses of Rv0978c, Rv1169c, and Rv1818c. In silico analysis of Rv0978c (A), Rv1169c (B), and Rv1818c (C) reveals the regions with high antigenic indices for potential antigenic determinants. The boxed areas represent potential regions that might elicit variable immune reposes among the above-mentioned three PE family proteins. (D, E, and F) The protein gels represent the expression and purification of Rv0978c, Rv1169c, and Rv1818c, respectively. Lane 1, uninduced E. coli; lane 2, induced E. coli; lane 3, marker; lanes 4 and 5, purified protein. The arrow indicates the position of the recombinant protein.
Rv0978c, Rv1169c, and Rv1818c elicit differential B-cell responses during infection with TB.
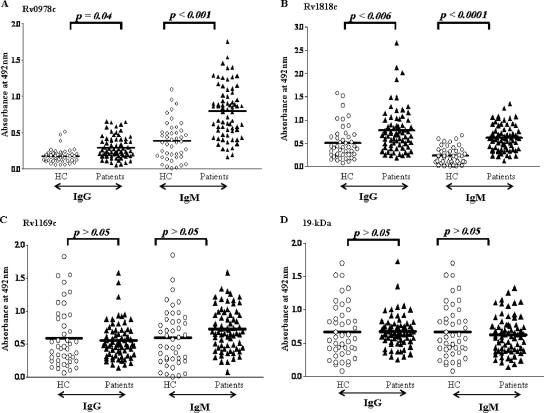
Humoral immune responses of group 1 patients (those reporting pulmonary TB disease symptoms for the first time) to the recombinant proteins Rv0978c, Rv1169c, and Rv1818c and the 19-kDa antigen were evaluated along with healthy controls as shown in Fig. 2A to D. In the case of group 1 adult pulmonary TB patients, only Rv0978c and Rv1818c showed statistically significant IgG and IgM immunoreactivities, while Rv1169c or the 19-kDa antigen did not react to the humoral response in either the IgG or IgM subtype. However, in the case of children in group 1, only Rv0978c, but not Rv1818c, Rv1169c, or the 19-kDa antigen, elicited IgG antibody reactivity (Fig. 3). In contrast, all three PE proteins studied as well as the 19-kDa antigen did not demonstrate statistically significant reactivity to IgG and IgM antibodies (Fig. 4 and data not shown, respectively) in sera from adult patients with relapsed infection in group 2. The sera derived from group 2 children were not available for our studies.
FIG. 2.
Differential humoral reactivity of PE proteins with sera from group 1 adult TB patients. The recombinant Rv0978c and Rv1818c PE family proteins elicit a strong and differential antibody response in M. tuberculosis-infected patients (diagnosed for pulmonary TB) in group 1 as opposed to healthy controls (HC). However, the antibody responses to Rv1169c (C) and the 19-kDa antigen (D) are not statistically significant (P > 0.5) compared to those to Rv0978c (P = 0.04 [IgG]; P < 0.001 [IgM]) (A) and Rv1818c (P < 0.006 [IgG]; P < 0.0001 [IgM]) (B).
FIG. 3.
Among studied PE proteins, only Rv0978c elicited reactivity in child pulmonary TB patients in group 1. The group 1 child patients showed statistically significant humoral IgG antibody reactivity to Rv0978c (P = 0.0348) and the 19-kDa antigen (P = 0.0174) but not to Rv1169c or Rv1818c (P > 0.05). The elicited immune response comprises only IgG but not the IgM class of humoral response. HC, healthy controls.
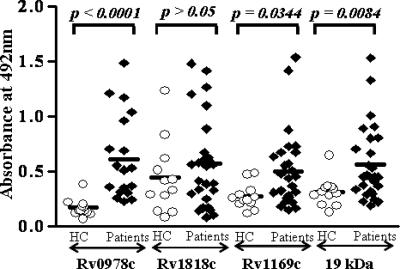
FIG. 4.
The studied PE proteins did not react with sera from group 2 adult patients with relapsed TB infection. The selected PE antigen Rv0978c, Rv1169c, or Rv1818c or the 19-kDa antigen (P > 0.05) did not demonstrate significant immunoreactivities in sera obtained from adult patients with relapsed TB infections. The lack of serum immunoreactivities was observed in both the IgG and IgM classes of the humoral responses, and data for the IgG class of antibodies are presented in this figure. HC, healthy controls.
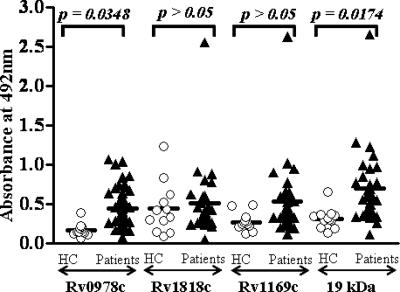
In the case of group 3 patients who reported extrapulmonary infections, only children and not adults elicited significant IgG immunoreactivity (Fig. 5 and data not shown). Among the PE antigens studied, only Rv0978c and Rv1169c showed strong reactivity to IgG, but not to IgM, in sera from group 3 children over healthy controls (Fig. 5 and Table 1). However, the lack of reactivity to Rv1818c suggests the possibility of stage-specific expression of Rv1818c in group 1 patients reporting pulmonary TB infection. Similarly, the specific reactivity of Rv1169c with sera from patients with extrapulmonary TB infection indicates the likelihood of the stage-specific expression of some of the above-mentioned PE antigens.
FIG. 5.
Rv1169c is recognized only by sera from patients with extrapulmonary infections. The children reporting extrapulmonary infections belonging to group 3 mounted significant IgG responses to Rv0978c (P < 0.001), Rv1169c (P = 0.0344), and the 19-kDa antigen (P = 0.0084) but not to the Rv1818c antigen (P > 0.5). Among the clinical categories studied, Rv1169c elicited immunoreactivity only with group 3 children. The data represented here are comprised of IgG antibody reactivities in sera of patients. HC, healthy controls.
TABLE 1.
Immunoreactivities to the PE antigens studieda
| Age category and group | Rv0978c
|
Rv1818c
|
Rv1169c
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IgG
|
IgM
|
IgG
|
IgM
|
IgG
|
IgM
|
|||||||
| OD ± 2 SEM | % of patients | OD ± 2 SEM | % of patients | OD ± 2 SEM | % of patients | OD ± 2 SEM | % of patients | OD ± 2 SEM | % of patients | OD ± 2 SEM | % of patients | |
| Adults, group 1 | 0.29 ± 0.036 | 69.5 | 0.79 ± 0.08 | 78.2 | 0.78 ± 0.1 | 59.2 | 0.62 ± 0.06 | 89.8 | 0.55 ± 0.06 | 18.8 | 0.72 ± 0.06 | 46.3 |
| Children | ||||||||||||
| Group 1 | 0.45 ± 0.06 | 60.7 | 0.75 ± 0.16 | 8 | 0.51 ± 0.14 | 16.6 | 0.67 ± 0.16 | 12 | 0.53 ± 0.16 | 20 | 0.85 ± 0.16 | 48 |
| Group 3 | 0.61 ± 0.16 | 84.2 | 0.98 ± 0.8 | 22.2 | 0.56 ± 0.14 | 22.2 | 0.8 ± 0.16 | 27.2 | 0.50 ± 0.12 | 55.55 | 0.85 ± 0.22 | 40.9 |
OD, optical density. % of patients, percentage of patients with ELISA absorbance values greater than the means plus 2 standard errors of the means for healthy controls.
The specificity and sensitivity of IgG and IgM antibody reactivity to Rv0978c, Rv1169c, and Rv1818c were calculated in order to address the serodiagnostic efficacy of these antigens. The specificities for Rv1818c and Rv0978c immunoreactivity in the case of adult patients belonging to group 1 or group 3 were 93% and 94%, respectively, for IgG and 98% and 96%, respectively, for IgM. However, Rv1169c did not elicit immunoreactivity in the case of group 1 or group 3 adult patients. The specificity for Rv1169c and Rv0978c immunoreactivity in the case of child patients belonging to group 3 was 84% for IgG, and the immunoreactivity of IgM antibodies to Rv1169c or Rv0978c was not statistically significant. In addition, Rv1818c did not elicit IgG or IgM reactivity in the case of group 3 children. The sensitivity was generally over 85% for the above-mentioned cases where specificity was over 80%.
To compare the serological sensitivities (both IgG and IgM) to Rv0978c, Rv1169c, and Rv1818c, the data presented in Fig. 2 to 5 were recalculated as percentages of individuals showing ELISA absorbance values above the means plus 2 standard errors of the means for healthy controls (Table 1). Among all patients, Rv0978c and Rv1818c elicited strong IgM antibody responses in a very high percentage of adult group 1 individuals (78% for Rv0978c and 90% for Rv1818c), while Rv1169c elicited IgG responses (56%) more frequently than it elicited IgM responses (41%) in group 3 children with extrapulmonary infection. In addition, when the immunoreactivities to two selected PE_PGRS antigens were compared within the clinical groups of patients, Rv1818c elicited specific reactivity in sera from group 1 adult pulmonary TB patients (Fig. 6A and B), while Rv0978c elicited immunoreactivity in sera from adult and child patients with pulmonary TB infection belonging to group 1 as well as in sera from children with extrapulmonary infection in group 3. However, Rv1169c elicited specific reactivity in sera from group 3 patients only (data not shown). The data presented in Fig. 6 represent the immunoreactivities of the IgG class of the humoral response.
FIG. 6.
Immunoreactivities comprised of differential antibody responses to two selected PE_PGRS antigens, Rv1818c and Rv0978c. Shown is a comparison of the immunoreactivities of adult patients to Rv1818c (A) and to Rv0978c (B) and of child patients to Rv1818c (C) and to Rv0978c (D). The IgG antibody immunoreactivities are represented in the figure. HC, healthy controls.
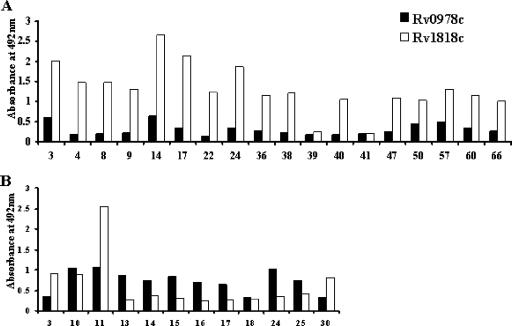
These results indicate that while Rv0978c is recognized by sera from pulmonary TB patients belonging to group 1 or by sera from child patients belonging to group 3, Rv1818c and Rv1169c elicit specific reactivity with adult group 1 patients and group 3 patients, respectively. More importantly, the immunoreactivity elicited in patient sera demonstrated differential reactivities of the humoral antibodies to Rv1818c versus Rv0978c (Fig. 6 and 7). The data presented in Fig. 6 and 7 represent the immunoreactivities of the IgG class of the humoral response. The ratios of responses to Rv1818c and Rv0978c are quite different in adult and child pulmonary TB patients belonging to group 1. The ratio of responses to Rv1818c and Rv0978c in the case of adult pulmonary TB patients was anywhere between two- and ninefold, while the ratio was much less than onefold in the case of child pulmonary TB patients. The possible reasons for such a discrepancy in the ratio of reactivity are described above, as Rv1818c clearly demonstrated significant immunoreactivity only with adult, not with the majority of child, group 1 patients. Overall, this differential reactivity to Rv1818c and to Rv0978c clearly suggests the degree of difference in recognition by patient sera even though they share similar glycine-rich C-terminal regions.
FIG. 7.
Individual patients demonstrate varied antibody responses to Rv0978c versus Rv1818c. Differential humoral responses to Rv0978c and Rv1818c from sera of selected TB adult (A) and child (B) group 1 patients (numbered) are shown. The figure represents the IgG antibody responses to Rv0978c and Rv1818c that varied to a high degree in selected patients even though both PE proteins (PE_PGRS) share similar glycine-rich C-terminal domains.
DISCUSSION
M. tuberculosis is the etiologic agent of TB and a major cause of morbidity and mortality worldwide, with one-third of the world's population estimated to be infected with this microorganism (22). After acquiring M. tuberculosis by inhaling aerosolized bacteria, the majority of healthy individuals develop a cellular immune response and arrest the growth and spread of the microorganism, without it progressing to clinical TB. This protective cellular immune response to M. tuberculosis is initiated in the lung and consists primarily of alveolar macrophages and activated T cells. However, M. tuberculosis survives for prolonged periods of time in the phagosomes of infected macrophages in a hypoxic environment within the host (21) in an asymptomatic latent state and can reactivate years later if the host's immune system wanes. In this context, M. tuberculosis was reported to upregulate the expression of many genes that might be important for its survival amid a hostile host response. One such set of genes is the PE family of genes (9-12, 14). As mentioned above, some members of the PE family are implicated in the replication and survival of bacilli in granulomas as well as being the source of antigenic variations upon infection. However, the exact nature of the role played by PE genes in providing survival benefits to macrophages harboring M. tuberculosis from surrounding T cells in granulomas or during pathogenesis associated with infection remains unclear.
The principal objective of the current study is to compare the selected PE proteins Rv1169c, Rv0978c, and Rv1818c to a well-characterized antigen, the 19-kDa antigen, in terms of the humoral immunoreactivities that they elicit in the sera of TB patients as well as healthy individuals. The 19-kDa antigen is a culture filtrate protein known to elicit strong humoral as well as cell-mediated host immune responses (6, 15, 16, 20). Furthermore, the 19-kDa antigen was suggested to exhibit several immunomodulatory functions such as the inhibition of major histocompatibility complex class II expression and antigen processing and the inhibition of gamma interferon-induced immune genes, etc. (23-25).
In the current investigation, Rv0978c, Rv1169c, and Rv1818c elicited statistically significant yet differential immunoreactivities among three selected categories of TB patients. Importantly, not-so-significant or poorer reactivities were observed against the three PE proteins in the sera of all healthy individuals tested. Furthermore, the immunoreactivity data presented in Fig. 2 to 6 suggest that Rv1818c or Rv1169c may be expressed during a specific stage of the disease. In contrast, Rv0978c seems to be immunoreactive with sera derived from either group 1 (pulmonary infection) or group 3 (extrapulmonary) patients. In order to understand the molecular mechanisms that underlie the humoral response elicited by PE antigens, we would like to assess patient T-cell reactivity with specific HLA class I and class II binding peptides derived from the above-described PE family antigens, as the antibody response in many cases is often regulated by initial T-cell responses during infection. However, we agree with the fact that assessments of T-cell reactivity may not necessarily be a logical step in the further establishment of the selected PE antigens as serodiagnostic tools.
The presence of antibodies to Rv0978c, Rv1169c, and Rv1818c in sera of TB patients clearly suggests that these PE proteins are expressed in vivo during active infection with M. tuberculosis and that the above-described PE molecules are immunogenic. It has been observed that some of the PE_PGRS proteins are antigenic, where antibodies raised against five PE_PGRS proteins by DNA vaccination reacted with the respective proteins expressed in epithelial cells or in reticulocyte extracts (3, 5). Some of the antibodies showed cross-reactivity with more than one PE_PGRS protein, suggesting the presence of common epitopes. However, the dot blot technique used in the above-mentioned study is not a very reliable method to ascertain either the size or the nature of the cross-reactivity of antibodies raised against any given PE_PGRS protein. In our studies, we have found that rabbit polyclonal antibodies generated against Rv0978c do not cross-react with recombinant Rv1818c (data not shown). In addition, Rv0978c contains novel tandem repeats, called AB repeats, in the C terminus, which are absent in most of the PE (or PE_PGRS subfamily) genes, including Rv1818c (2).
Other studies have demonstrated that sera from TB patients showed immunoreactivity to many of the PPE family proteins, in addition to PE family proteins (7, 8). Many studies in the literature clearly emphasized the observation of a lack of a sufficient immune response in TB patients against many well-characterized serodiagnostic antigens of TB bacilli. The above-mentioned condition is more severe in newer cases of pulmonary TB infection where the host immune system is not sufficiently primed to elicit a strong humoral response against most antigens of M. tuberculosis. Overall, our observations suggest the possible serodiagnostic potential of Rv1818c, Rv1169c, and Rv0978c during different stages of infection.
Acknowledgments
We are grateful to Kiran Katoch and D. S. Chauhan for the generous gift of TB patient sera. We are thankful to M. J. Brennan, FDA, and to Neil Reiner, University of British Columbia, Canada, for the kind gifts of recombinant expression clones for Rv1818c and 19-kDa antigens. We thank Dipankar Nandi for critical comments and Nagasuma Chandra, Rashmi Chaturvedi, and V. L. Suhas for their help during the preparation of the manuscript.
The current study was funded by the Sir Dorabji Tata Center for Research in Tropical Diseases to K.N.B. and V.M.K. as well as funds from the Council for Scientific and Industrial Research, Department of Biotechnology, and Department of Science and Technology, Government of India, to K.N.B. Y.N. and K.C.M. are supported by a fellowship from the Council for Scientific and Industrial Research and the University Grants Commission, Government of India, respectively.
Footnotes
Published ahead of print on 8 August 2007.
REFERENCES
- 1.Abou-Zeid, C., T. Garbe, R. Lathigra, H. G. Wiker, M. Harboe, G. A. Rook, and D. B. Young. 1991. Genetic and immunological analysis of Mycobacterium tuberculosis fibronectin-binding proteins. Infect. Immun. 59:2712-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adindla, S., and L. Guruprasad. 2003. Sequence analysis corresponding to the PPE and PE proteins in Mycobacterium tuberculosis and other genomes. J. Biosci. 28:169-179. [DOI] [PubMed] [Google Scholar]
- 3.Banu, S., N. Honore, B. Saint-Joanis, D. Philpott, M. C. Prevost, and S. T. Cole. 2002. Are the PE_PGRS proteins of Mycobacterium tuberculosis variable surface antigens? Mol. Microbiol. 44:9-19. [DOI] [PubMed] [Google Scholar]
- 4.Brennan, M. J., and G. Delogu. 2002. The PE multigene family: a ‘molecular mantra’ for mycobacteria. Trends Microbiol. 10:246-249. [DOI] [PubMed] [Google Scholar]
- 5.Brennan, M. J., G. Delogu, Y. Chen, S. Bardarov, J. Kriakov, M. Alavi, and W. R. Jacobs, Jr. 2001. Evidence that mycobacterial PE_PGRS proteins are cell surface constituents that influence interactions with other cells. Infect. Immun. 69:7326-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brightbill, H. D., D. H. Linraty, S. R. Krutzik, et al. 1999. Host defense mechanisms triggered by microbial lipoproteins through Toll like receptors. Science 285:732-736. [DOI] [PubMed] [Google Scholar]
- 7.Chakhaiyar, P., Y. Nagalakshmi, B. Aruna, K. J. Murthy, V. M. Katoch, and S. E. Hasnain. 2004. Regions of high antigenicity within the hypothetical PPE major polymorphic tandem repeat open-reading frame, Rv2608, show a differential humoral response and a low T cell response in various categories of patients with tuberculosis. J. Infect. Dis. 190:1237-1244. [DOI] [PubMed] [Google Scholar]
- 8.Choudhary, R. K., S. Mukhopadhyay, P. Chakhaiyar, N. Sharma, K. J. Murthy, V. M. Katoch, and S. E. Hasnain. 2003. PPE antigen Rv2430c of Mycobacterium tuberculosis induces a strong B-cell response. Infect. Immun. 71:6338-6343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cole, S. T., et al. 1998. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 393:537-544. [DOI] [PubMed] [Google Scholar]
- 10.Delogu, G., and M. J. Brennan. 2001. Comparative immune response to PE and PE_PGRS antigens of Mycobacterium tuberculosis. Infect. Immun. 69:5606-5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delogu, G., C. Pusceddu, A. Bue, G. Fadda, M. J. Brennan, and S. Zanetti. 2004. Rv1818c-encoded PE_PGRS protein of Mycobacterium tuberculosis is surface exposed and influences bacterial cell structure. Mol. Microbiol. 52:725-733. [DOI] [PubMed] [Google Scholar]
- 12.Deretic, V., and R. A. Fratti. 1999. M. tuberculosis phagosome. Mol. Microbiol. 31:1603-1609. [DOI] [PubMed] [Google Scholar]
- 13.Dheenadhayalan, V., G. Delogu, and M. J. Brennan. 2006. Expression of the PE_PGRS 33 protein in Mycobacterium smegmatis triggers necrosis in macrophages and enhanced mycobacterial survival. Microbes Infect. 8:262-272. [DOI] [PubMed] [Google Scholar]
- 14.Espitia, C., J. P. Laclette, M. Mondragon-Palomino, A. Amador, J. Campuzano, A. Martens, M. Singh, R. Cicero, Y. Zhang, and C. Moreno. 1999. The PE_PGRS glycine-rich proteins of Mycobacterium tuberculosis: a new family of fibronectin-binding proteins? Microbiology 145:3487-3495. [DOI] [PubMed] [Google Scholar]
- 15.Faith, A., R. Moreno, R. Lathigra, et al. 1991. Analysis of human T cell epitopes in the 19,000 MW antigen of Mycobacterium tuberculosis: influence of HLA-DR. Immunology 74:1-7. [PMC free article] [PubMed] [Google Scholar]
- 16.Harris, D., H. Vordermeier, E. Roman, et al. 1991. Murine T cell stimulatory peptides from the 19-kDa antigen of Mycobacterium tuberculosis: epitope restricted homology with the 28kDa protein of Mycobacterium leprae. J. Immunol. 147:2706-2712. [PubMed] [Google Scholar]
- 17.Jain, S. K., M. Paul-Satyaseela, G. Lamichhane, K. S. Kim, and W. R. Bishai. 2006. Mycobacterium tuberculosis invasion and traversal across an in vitro human blood-brain barrier as a pathogenic mechanism for central nervous system tuberculosis. J. Infect. Dis. 193:1287-1295. [DOI] [PubMed] [Google Scholar]
- 18.Jameson, B. A., and H. Wolf. 1988. The antigenic index: a novel algorithm for predicting antigenic determinants. Comput. Appl. Biosci. 4:181-186. [DOI] [PubMed] [Google Scholar]
- 19.Lamichhane, G., M. Zignol, N. J. Blades, D. E. Geiman, A. Dougherty, J. Grosset, K. W. Broman, and W. R. Bishai. 2003. A postgenomic method for predicting essential genes at subsaturation levels of mutagenesis: application to Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 100:7213-7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyashchenko, K., R. Colangeli, M. Houde, H. Al Jahdali, D. Menzies, and M. L. Gennaro. 1998. Heterogeneous antibody responses in tuberculosis. Infect. Immun. 66:3936-3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manabe, Y. C., and W. R. Bishai. 2000. Latent Mycobacterium tuberculosis—persistence, patience, and winning by waiting. Nat. Med. 6:1327-1329. [DOI] [PubMed] [Google Scholar]
- 22.Murray, C. J. L., K. Styblo, and A. Rouillon. 1990. Tuberculosis in developing countries: burden, intervention and cost. Bull. Int. Union Tuberc. Lung Dis. 65:95-96. [PubMed] [Google Scholar]
- 23.Neyrolles, O., K. Gould, M. P. Gares, et al. 2001. Lipoprotein access to MHC class I presentation during infection of murine macrophages with live mycobacteria. J. Immunol. 166:447-457. [DOI] [PubMed] [Google Scholar]
- 24.Noss, E. H., R. K. Pai, T. J. Sellati, et al. 2001. Toll-like receptor 2-dependent inhibition of macrophage class II MHC expression and antigen processing by 19-kDa lipoprotein of Mycobacterium tuberculosis. J. Immunol. 167:910-918. [DOI] [PubMed] [Google Scholar]
- 25.Pai, R. K., M. Convey, T. A. Hamilton, W. H. Boom, and C. V. Harding. 2003. Inhibition of IFN-gamma-induced class II transactivator expression by a 19 kDa lipoprotein from Mycobacterium tuberculosis: a potential mechanism for immune evasion. J. Immunol. 171:175-184. [DOI] [PubMed] [Google Scholar]
- 26.Rachman, H., M. Strong, T. Ulrichs, L. Grode, J. Schuchhardt, H. Mollenkopf, G. A. Kosmiadi, D. Eisenberg, and S. H. Kaufmann. 2006. Unique transcriptome signature of Mycobacterium tuberculosis in pulmonary tuberculosis. Infect. Immun. 74:1233-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramakrishnan, L., N. A. Federspiel, and S. Falkow. 2000. Granuloma specific expression of Mycobacterium virulence proteins from the glycine-rich PE_PGRS family. Science 288:1436-1439. [DOI] [PubMed] [Google Scholar]
- 28.Schnappinger, D., E. Sabine, M. I. Voskuil, Y. Liu, J. A. Mangan, I. M. Monahan, G. Dolganov, B. Efron, P. D. Butcher, C. Nathan, and G. K. Schoolnik. 2003. Transcriptional adaptation of Mycobacterium tuberculosis within macrophages: insights into the phagosomal environment. J. Exp. Med. 198:693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singh, K. K., X. Zhang, A. S. Patibandla, P. Chien, Jr., and S. Laal. 2001. Antigens of Mycobacterium tuberculosis expressed during preclinical tuberculosis: serological immunodominance of proteins with repetitive amino acid sequences. Infect. Immun. 69:4185-4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sly, L. M., S. M. Hingley-Wilson, N. E. Reiner, and W. R. McMaster. 2003. Survival of Mycobacterium tuberculosis in host macrophages involves resistance to apoptosis dependent upon induction of antiapoptotic Bcl-2 family member Mcl-1. J. Immunol. 170:430-437. [DOI] [PubMed] [Google Scholar]
- 31.Talaat, A. M., R. Lyons, S. T. Howard, and S. A. Johnston. 2004. The temporal expression profile of Mycobacterium tuberculosis infection in mice. Proc. Natl. Acad. Sci. USA 101:4602-4607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Voskuil, M. I., D. Schnappinger, R. Rutherford, Y. Liu, and G. K. Schoolnik. 2004. Regulation of the Mycobacterium tuberculosis PE/PPE genes. Tuberculosis 84:256-262. [DOI] [PubMed] [Google Scholar]