Abstract
Among 125 clinical isolates of Mycobacterium tuberculosis collected in Hong Kong and Shanghai, China, between 2002 and 2004, IS6110 typing revealed that 71 strains (57%) belonged to the Beijing family. The intracellular growth of the strains in human peripheral blood monocyte-derived macrophages was measured ex vivo on days 0, 3, 6, and 10. Among all tested strains, three hypervirulent strains showed significant increases in intracellular growth after 10 days of incubation. With an initial bacterial load of 104 CFU, most of the clinical isolates and H37Ra (an avirulent strain) exhibited no intracellular survival on day 10, while the three hypervirulent strains together with H37Rv (a virulent strain) showed on average a two- to fourfold rise in CFU count. These three hypervirulent strains belonging to a non-Beijing family were isolated from patients suffering from tuberculosis meningitis. Cytokines secreted by gamma interferon-activated macrophages were measured daily after challenge with selected strains of M. tuberculosis. The levels of tumor necrosis factor alpha were elevated after 24 h of infection among all strains, but the levels were significantly lower among the three hypervirulent strains, whereas interleukin 10 (IL-10) and IL-12 were not detected. Results were concordant with the differential expression of the corresponding cytokine genes in activated macrophages, as monitored by real-time PCR. Our findings highlighted that these three hypervirulent strains may possess an innate mechanism for escaping host immunity, which accounts for their characteristic virulence in patients presenting with a more severe form of disease.
Despite continuous efforts in monitoring and treating tuberculosis, the disease remains a major public health issue in many parts of the world, with over 50% of all new cases being reported in Asian countries (31). Recent studies of several repetitive insertion sequences (ISs) in Mycobacterium tuberculosis have indicated that these elements are associated with DNA polymorphism in the mycobacterial genome. Among these IS elements, the IS6110 restriction fragment length polymorphism (RFLP) demonstrates high potential for the fingerprinting of M. tuberculosis isolates (2, 5, 27, 28). Several epidemiological studies have provided valuable information on the global transmission of the disease and the identification of particular strains with high-level infectivity, virulence, and multidrug resistance properties (6, 7, 19, 30). In addition, the analysis of the IS6110 RFLP may provide insight in the study of the bacterium's evolutionary history and the emergence of particular genotypes in various geographic localities.
The characterization of virulence determinants of M. tuberculosis that are relevant to the pathogenesis of human tuberculosis has gained widespread attention and recognition (22). Although animal models using guinea pigs and mice are useful for the characterization of M. tuberculosis infection and the study of bacterial virulence, an assessment of the bacterium's ability to persist in human macrophages is essential for determining the virulence of the pathogen. The lung is the major portal for the entry of M. tuberculosis, the cause of pulmonary tuberculosis. Once inhaled into the lung, the pathogen may enter the alveoli of lungs and be phagocytosed by defensive alveolar macrophages. Unlike nonpathogens, M. tuberculosis may resist killing by macrophages and even survive in a latent form. Quantitative measurement of the intracellular growth of M. tuberculosis in human macrophages was not invariable among various strains of M. tuberculosis (23, 32). For study of the early stages of tuberculosis, human macrophages derived from peripheral blood monocytes can be used because they mimic the situation in humans. Activated macrophages in the presence of T cells and cytokines will become efficient in bacterial killing and hence contain the infection. However, various M. tuberculosis strains may have evolved mechanisms that interfere with macrophage killing and allow them to grow inside host macrophages. These virulent strains can evade the host's immune barriers within the lungs and may subsequently spread to other organs and cause a more severe form of disease, such as extrapulmonary or miliary tuberculosis. Therefore, the identification of specific virulent M. tuberculosis strains among clinical isolates may assist in further elucidating the pathogenesis of M. tuberculosis. In this study, we analyzed clinical isolates of M. tuberculosis collected in Hong Kong and Shanghai between 2002 and 2004 and characterized the immune escape mechanisms observed in some virulent strains.
MATERIALS AND METHODS
Mycobacterium tuberculosis strains.
A total of 125 clinical isolates of M. tuberculosis were obtained from three general hospitals in Hong Kong and one general hospital in Shanghai, People's Republic of China, from 2002 to 2004. These strains were isolated from patients suffering from tuberculosis and included 65 respiratory specimens and 60 extrapulmonary specimens (14 cerebrospinal fluid [CSF] specimens, 10 tissue specimens, 8 early-morning urine specimens, 6 wound swab specimens, 5 biopsy specimens, 4 lymph node aspirate specimens, 4 stool specimens, 3 pus specimens, 2 peritoneal fluid specimens, 1 abscess specimen, 1 ear swab specimen, 1 blood culture specimen, and 1 bone marrow specimen). Cultures positive for acid-fast bacilli were identified as M. tuberculosis by the AccuProbe hybridization assay (Gen-Probe, San Diego, CA), according to the manufacturer's instructions.
IS6110 RFLP fingerprinting.
The extraction of total M. tuberculosis genomic DNA and Southern blotting with a labeled IS6110 DNA probe were performed according to standardized methods (1). In brief, the total genomic DNA was extracted by enzymatic lyses, followed by phenol chloroform extraction. Each isolate was inoculated onto two Middlebrook 7H10 selective agar plates supplemented with oleic acid-albumin-dextrose-catalase (Beckon Dickinson) and incubated for 3 weeks at 37°C with 5% CO2. Cultures were harvested with 10 ml Tris-EDTA buffer (pH 8.0), and suspensions were centrifuged at 4,000 × g for 20 min at 4°C. The pellet was held at −20°C for a minimum of 4 h to weaken the cell wall. Then, it was resuspended in 5 ml of Tris-EDTA buffer (pH 8.0) with the addition of an equal volume of chloroform-methanol in a ratio of 2:1. After centrifugation at 4,000 rpm for 20 min at 4°C, the bacterial pellet at the interphase was collected and dried at 55°C for 15 min. It was then homogenized with 5 ml of Tris-EDTA buffer (pH 8.0), followed by the addition of 500 μl of 1 M Tris-HCl (pH 9.0) and 55 μl of lysozyme (10 mg/ml; Sigma). Suspensions were incubated at 37°C for 18 to 24 h. Then, 55 μl of proteinase K (10 mg/ml; Sigma) and 550 μl of 10% sodium dodecyl sulfate (Sigma) were added. The mixture was incubated at 55°C for 3 h. Suspensions were centrifuged at 12,000 × g for 30 min at 4°C first with a phenol-chloroform-isoamyl alcohol mixture in the ratio of 25:24:1 and then with a chloroform-isoamyl alcohol mixture in the ratio of 24:1. Then, the DNA was precipitated by adding 0.1 volume of 3 M sodium acetate (pH 5.2) and an equal volume of absolute isopropanol, followed by overnight incubation at 4°C. DNA was precipitated using 2 ml of cold 70% alcohol. After centrifugation, the dried pellet was dissolved in 200 μl of Tris-EDTA buffer (pH 8.0). The concentration of DNA extract was measured using a UV spectrophotometer and then adjusted to 200 μg/ml. Chromosomal DNA was digested by overnight incubation at 37°C with the endonuclease PvuII. The PvuII-digested DNA product was precipitated and electrophoresed for at least 22 h at 4°C in a cold room at 20 V. Southern hybridization was set up using a digoxigenin-labeled PCR probe at 60°C in a shaking water bath for 18 to 24 h. Hybridized signal on a Hybond-N+ membrane was detected by the chemiluminescence method using CSPD (Roche Diagnostics GmbH, Germany) as the substrate.
Measurement of M. tuberculosis growth inside human macrophages.
Our protocol was adopted from a previous study of Zhang et al. (32). Normal human blood was provided by Blood Transfusion Services. Peripheral blood monocytes were isolated from mononuclear cells by plastic adhesion followed by plating onto 24-well plates at 106 cells/well in RPMI medium containing 5% autologous serum. The monocytes were refed by fresh medium every 2 days and allowed to differentiate into macrophages for 10 to 12 days in vitro. M. tuberculosis cultured in 7H10 medium for 7 to 10 days was suspended in RPMI medium and adjusted to 103 to 104 CFU/ml. Clumps of bacteria were dispersed using an ultrasonic cell disrupter or vortexed, and suspensions were centrifuged at 400 × g for 20 min. Then, 0.5 ml supernatant was used to infect human macrophages in each well. After overnight incubation at 37°C with 5% CO2, the macrophages were washed with 1 ml RPMI medium twice to remove extracellular bacilli. This was considered day 0. On days 0, 3, 6, and 10, infected macrophages were lysed by aspirating them from the RPMI medium, and then 1 ml of distilled water was added and the mixture was incubated for 10 min. Then, 20 μl of 10% sodium dodecyl sulfate was added, and the mixture was incubated for 10 min, followed by the addition of 1 ml of 20% bovine serum albumin. The mixture was centrifuged at 4,000 × g for 20 min, and the supernatant was discarded. One milliliter of distilled water was added to disperse the sediment. Finally, 10 μl of suspension was inoculated onto 7H10 agar to allow enumeration of the mycobacteria after 3 weeks of incubation at 37°C with 5% CO2. Each strain was tested with three different batches of human macrophages. The growth of M. tuberculosis inside human macrophages was determined by counting the number of CFU per ml. The growth index was defined as the number of CFU per ml divided by the number of CFU per ml on day 0. A growth index of greater than 1 indicated that the strain exhibited multiplication inside human macrophages, whereas a growth index of less than 1 indicated that the bacteria were engulfed and gradually killed by macrophages.
Cytokine detection.
Twenty-three strains of M. tuberculosis from different specimens (14 CSF and 9 respiratory specimens) exhibiting various IS6110 RFLP patterns were selected. H37Rv (ATCC 25618) and H37Ra (ATCC 25177) were included as controls. Supernatants from macrophages preactivated by gamma interferon (500 IU/ml) and challenged by M. tuberculosis were collected on days 0, 1, 2, 3, 4, and 5. All supernatants were deep-frozen immediately at −70°C before being tested. Cytokine assays were performed for interleukin 10 (IL-10), IL-12, and tumor necrosis factor alpha (TNF-α) according to the manufacturer's instructions (DuoSet enzyme-linked immunosorbent assay development system; R&D Systems, Inc.). Briefly, the 96-well microplate was precoated with the corresponding capture antibody and incubated at room temperature for 12 to 18 h. After being washed with buffer, samples and standards were added in duplicate to each well and incubated at room temperature for 2 h. The plate was washed with buffer, and biotinylated detection antibody was added, followed by incubation at room temperature for 2 h. After incubation of the reaction mixture with horseradish peroxidase and the substrate (H2O2 with tetramethylbenzidine), the reaction was stopped by adding 2 N H2SO4. The optical density at 450 nm of each well was determined.
Real-time PCR for mRNA expression of cytokine genes.
Three strains from CSF specimens with a growth index inside macrophages of greater than 1 and a strain from a respiratory specimen with a growth index of less than 1 were selected. H37Rv and H37Ra were also included as controls. Total RNA was extracted from M. tuberculosis-infected macrophages at 3, 6, and 9 h after infection. The quantitative measurement of mRNA expressed by macrophages excreting β-actin, TNF-α, IL-10, and IL-12 was performed by a two-step real-time RT-PCR technique. The reaction mixture contained 1 μl of an oligo(dT)12-18 primer (Invitrogen), 1 μl of a 10 mM concentration of a deoxynucleoside triphosphate (Invitrogen), and 10 μl of RNA extract. The mixture was incubated at 65°C for 5 min, and the reaction tubes were transferred to ice immediately. The first-strand cDNA was synthesized by adding 4 μl of 5× first-strand buffer (Invitrogen), 2 μl of 0.1 M dithiothreitol (Invitrogen), 1 μl of distilled water, and 1 μl of Superscript II reverse transcriptase (Invitrogen) to each tube. The mixture was incubated at 42°C for 50 min and then at 70°C for a further 15 min.
Real-time PCR was performed in an ABI7700 PCR cycler (Applied Biosystems) by setting up a 10-μl PCR mixture containing 5 μl of the TaqMan reaction mix, 1 μl of distilled water, 1 μl of a 10 μM concentration of the forward primer, 1 μl of a 10 μM concentration of the reverse primer, 1 μl of a 1:40 dilution of the TaqMan probe, and 1 μl of the RNA extract. The serial dilution of the plasmid standard ranged from 10 to 107 copies per μl.
RESULTS
IS6110 RFLP patterns of M. tuberculosis isolated in Hong Kong and Shanghai.
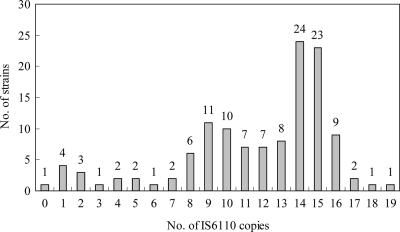
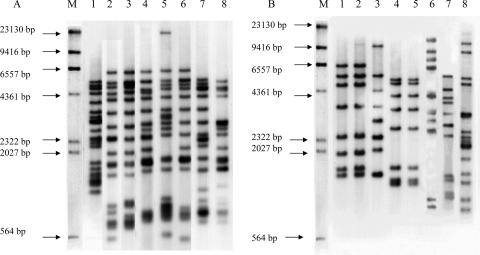
The RFLP patterns of isolates revealed that the number of IS6110 copies harbored in each strain ranged from 0 to 19 (Fig. 1). Seventy-one strains (57%) belonged to the Beijing-like family (Fig. 2A), and the other 54 strains were non-Beijing-like family types (Fig. 2B). There was no documented outbreak of tuberculosis during the study period, and no specific clusters of isolates were indicated in the phylogenetic analysis (data not shown).
FIG. 1.
Distribution of IS6110 copy numbers among 125 strains of M. tuberculosis.
FIG. 2.
IS6110 RFLP patterns of M. tuberculosis isolates. Lanes M contain the digested products of the digoxigenin II molecular marker. (A) Lanes 1 to 8 contain representative isolates with IS6110 RFLP patterns belonging to the Beijing family. (B) Lanes 1 to 8 contain representative isolates with IS6110 RFLP patterns belonging to a non-Beijing family. Lanes 1, 2, and 6 contain CSF isolates (H107, H108, and H112, respectively).
Macrophage challenge.
Table 1 shows quantitative measurements of the multiplication of M. tuberculosis inside human macrophages. The growth index was defined as the number of CFU per ml at day x normalized to the number of CFU per ml on day 0. Among the 125 tested strains, three isolates (H107, H108, and H112) and H37Rv (ATCC 25618) exhibited a growth index of >1 (Fig. 3). The other 122 strains and H37Ra (ATCC 25177) showed a growth index of <1. The three strains with a growth index of >1 were named hypervirulent strains. These three strains were all isolated from patients suffering from tuberculosis meningitis and belonged to a non-Beijing family (Fig. 2B). The three patients were subjected to timely antitubular treatment for 9 to 12 months with good prognoses. Strain H112 was collected in Hong Kong, while H107 and H108 were collected in Shanghai. Strains H107 and H108, showing identical IS6110 RFLP patterns, were collected from two patients in 2002 and 2004. They resided in different regions outside Shanghai and had no epidemiological link with regard to their family or work history.
TABLE 1.
Quantitative measurement of the intracellular multiplication of M. tuberculosis organisms inside human macrophages
| Strain category | Strain | Specimen type | Growth index at:
|
|||
|---|---|---|---|---|---|---|
| Day 0 | Day 3 | Day 6 | Day 10 | |||
| Hypervirulent clinical isolates | H107 | CSF | 1.0 (±0) | 1.85 (±5) | 2.45 (±4) | 3.65 (±3) |
| H108 | CSF | 1.0 (±0) | 1.45 (±7) | 1.8 (±11) | 2.8 (±4) | |
| H112 | CSF | 1.0 (±0) | 1.35 (±7) | 1.6 (±13) | 2.2 ± (8) | |
| Standard virulent strain | H37Rv (ATCC 27294) | 1.0 (±0) | 1.2 (±17) | 1.5 (±13) | 2.4 (±4) | |
| Standard avirulent strain | H37Ra (ATCC 25177) | 1.0 (±0) | 0.45 (±14) | 0.2 (±17) | 0.1 (±9) | |
| Other clinical isolates | CSF (n = 11) | 1.0 (±0) | 0.4 (±29) | 0.2 (±17) | 0.1 (±9) | |
| Other (n = 111) | 1.0 (±0) | 0.5 (±27) | 0.2 (±58) | 0.1 (±36) | ||
aThe growth indexes were normalized by dividing the number of CFU at day x by that at day 0. Standard errors (percentages) for repeated experiments with three batches of macrophages are shown in parentheses.
FIG. 3.
Growth indexes reflecting the multiplication of M. tuberculosis organisms inside human macrophages. H107, H108, and H112 are hypervirulent strains, and H37Rv exhibited a growth index of >1. H37Ra and other strains (65 pulmonary and 57 extrapulmonary isolates) exhibited growth indexes of <1. Each strain was tested with three batches of human macrophages. The data represent means ± standard deviations.
Cytokine quantification.
Quantitative measurement of the cytokines TNF-α, IL-10, and IL-12 revealed that the maximal amount of TNF-α secreted by macrophages was detected on day 1 of infection. H107, H108, H112, and H37Rv had TNF-α concentrations secreted by activated macrophages of less than 400 pg/ml. Other strains exhibited higher concentrations of TNF-α. Experiments were repeated with similar findings, and mean values are shown in Fig. 4. The three hypervirulent strains and H37Rv induced significantly lower levels of TNF-α secretion in activated macrophages than other isolates and H37Ra. The amounts of IL-10 and IL-12 excreted by macrophages after challenge with M. tuberculosis were below the detection limit (less than 100 pg/ml). Most of the strains at various time points had nondetectable levels of IL-10 and IL-12 (data not shown).
FIG. 4.
Mean levels of TNF-α excreted by activated macrophages challenged with M. tuberculosis after 24 h (n = 2).
Real-time PCR for differential gene expression.
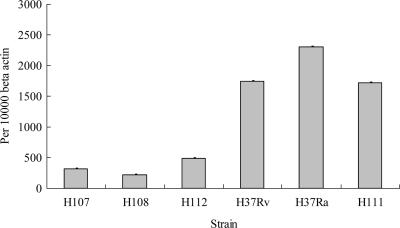
The levels of mRNAs encoding TNF-α, IL-10, and IL-12 were expressed as numbers of copies normalized per 10,000 copies of β-actin, which is the housekeeping gene. It was found that maximal amounts of TNF-α gene expression by macrophages challenged with H107, H108, H112, and H37Rv were relatively low compared with those of macrophages challenged with H37Ra and H111. Experiments were repeated with similar findings, and mean values are shown in Fig. 5. No significant difference in IL-10 and IL-12 gene expression was exhibited by all tested strains (data not shown).
FIG. 5.
TNF-α gene expression (mean values) in macrophages challenged with M. tuberculosis (n = 2).
DISCUSSION
In our collections of M. tuberculosis isolates in Hong Kong and Shanghai, 57% of all isolates belong to the Beijing family. Our finding is similar to that of other studies indicating that the Beijing family originating in China dominates in the surrounding regions (6, 14, 29). In the macrophage challenge experiment, three hypervirulent strains were very distinctly confined to isolates from patients suffering from tuberculosis meningitis, a more severe form of infection, whereas none of the pulmonary isolates were hypervirulent. Strains H107 and H108, showing identical IS6110 RFLP patterns, were collected from two patients with no epidemiological link in Shanghai. Some strains of the Beijing family were reported to show high transmissibility and cause more-severe forms of infections in animals (16), but all three hypervirulent strains in this study belonged to the non-Beijing family.
The characterization of virulence determinants of M. tuberculosis that are relevant to human diseases is a critical process in understanding the pathogenesis of tuberculosis. Recent study has shown the potential involvement of a virulent M. tuberculosis gene in the organism's survival rate inside human macrophages (17). The macrophage model has the advantage of allowing us to study the interaction of M. tuberculosis with the host in the early phase of human infection. In addition, the cytokine network plays a central role in the inflammatory response and the outcome of mycobacterial infections (26). TNF-α plays a crucial role in protective immunity and pathophysiology against tuberculosis (12). It synergizes with gamma interferon to increase the production of nitric oxide metabolites and facilitate mycobacterial killing and is essential for granuloma formation for the containment of mycobacterial infection. Recent studies indicated that cytokines level were higher in bronchoalveolar lavage fluid specimens from patients suffering from pulmonary tuberculosis than in those with a lower-grade disease (25). Another study suggested that cytokine analysis of blood can be used to discriminate between patients with active tuberculosis and nondiseased healthy controls (11). The role for TNF-α in controlling bacilli in the latent stage was evidenced by the increased reactivation of tuberculosis (including miliary and extrapulmonary disease) in patients with Crohn's disease and rheumatoid arthritis after treatment with monoclonal anti-TNF-α antibodies (13). Similar findings were also reported in a study using mice incapable of synthesizing TNF-α, which displayed an increased susceptibility to tuberculosis (3).
In this study, the amount of TNF-α secreted by activated macrophages challenged with the three hypervirulent strains was lower than in macrophages challenged with the less virulent strains. Differential protein expression was further confirmed by the differential gene expression of activated macrophages. An earlier study showed that maximal TNF-α was detected 2 days after infection (23), and the production of cytokines in tuberculosis patients has been controversial in the literature (15, 24). Modifications on the host's cytokine profile by bacteria may facilitate the escape of an antimycobacterial defense mechanism. In this study, the three hypervirulent strains showed down-regulated TNF-α protein and gene expression in macrophages. The low level of TNF-α may be responsible for the higher growth index detected inside macrophages.
Controversial results which indicated that the infection of macrophages with a virulent M. tuberculosis strain induced the secretion of a higher level of TNF-α than the levels exhibited with attenuated strains were obtained by others (9). Another study revealed that in the presence of iron, human monocytes treated with TNF-α suppressed the growth of M. tuberculosis. In the absence of iron, however, TNF-α had a growth-stimulating effect (4). A clinical trial reported that the use of a small-molecule inhibitor of TNF-α reduced the replication of human immunodeficiency virus type 1 and M. tuberculosis in patients coinfected with these two pathogens (10). It should be noted that most of the published data used laboratory strains to infect macrophages rather than clinical strains, unlike in our study.
Interestingly, minimal activities of IL-10 and IL-12 were detected. The lack of IL-12 production may be an immune escape mechanism for the suppression of Th-1 responses, and the lack of an IL-12 response is not due to the presence of IL-10. We hypothesized that these strains have developed mutant proteins that may suppress an innate mechanism in order to circumvent the host's protective immunity, accounting for their characteristic virulence in causing a more severe form of disease. Previous studies indicated that the mycobacterial cell wall components lipoarabinomannans (LAMs) were virulence factors which triggered the production of cytokines, such as IL-8 (20) and IL-12 (8), by macrophages and polymorphonuclear granulocytes. However, controversial findings indicated that LAMs antagonized and inhibited the production of cytokines by macrophages (18, 21). The exact role of LAMs in controlling M. tuberculosis infection remains unclear. Further study may elucidate the role of LAMs in the three hypervirulent strains with regard to infection and the host's protective immunity.
Our study highlights the advances toward a better understanding of the bacterial phenotypic virulence of M. tuberculosis and its epidemiological link and interaction with a host's protective immune system in the fight against tuberculosis.
Footnotes
Published ahead of print on 22 August 2007.
REFERENCES
- 1.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1994. Current protocols in molecular biology, vol. 1, p. 2.9.1-2.10.16. Wiley Interscience, Hoboken, NJ. [Google Scholar]
- 2.Barnes, P. F., and M. D. Cave. 2003. Molecular epidemiology of tuberculosis. N. Engl. J. Med. 349:1149-1156. [DOI] [PubMed] [Google Scholar]
- 3.Bean, A. G., D. R. Roach, H. Briscoe, M. P. France, H. Korner, J. D. Sedgwick, and W. J. Britton. 1999. Structural deficiencies in granuloma formation in THF gene-targeted mice underlie the heightened susceptibility to aerosol Mycobacterium tuberculosis infection, which is not compensated for by lymphotoxin. J. Immunol. 162:3504-3511. [PubMed] [Google Scholar]
- 4.Byrd, T. F. 1997. Tumor necrosis factor α (TNFα) promotes growth of virulent Mycobacterium tuberculosis in human monocytes. J. Clin. Investig. 99:2518-2529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cave, M. D., K. D. Eisenach, G. Templeton, M. Salfinger, G. Mazurek, J. H. Bates, and J. T. Crawford. 1994. Stability of DNA fingerprint pattern produced with IS6110 in strains of Mycobacterium tuberculosis. J. Clin. Microbiol. 32:262-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan, Y. M., C. M. Tam, H. Wong, C. C. Leung, J. Wang, W. W. Yew, C. W. Lam, and K. M. Kam. 2003. Molecular and conventional epidemiology of tuberculosis in Hong Kong: a population-based prospective study. J. Clin. Microbiol. 41:2706-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, T., B. Sommers, and M. Murrey. 2003. The effect of drug resistance on the fitness of Mycobacterium tuberculosis. Lancet Infect. Dis. 3:13-21. [DOI] [PubMed] [Google Scholar]
- 8.Dao, D. N., L. Kremer, Y. Guerardel, A. Molano, W. R. Jacobs, Jr., S. A. Porcelli, and V. Briken. 2004. Mycobacterium tuberculosis lipomannan induces apoptosis and interleukin-12 production in macrophages. Infect. Immun. 72:2067-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engele, M., E. Stoßel, K. Castiglione, N. Schwerdtner, M. Wagner, P. Bolcskei, M. Rollinghoff, and S. Stenger. 2002. Induction of TNF in human alveolar macrophages as a potential evasion mechanism of virulent Mycobacterium tuberculosis. J. Immunol. 168:1328-1337. [DOI] [PubMed] [Google Scholar]
- 10.Haraguchi, S., N. K. Day, W. Kamchaisatian, M. Beigier-Pompadre, S. Stenger, N. Tangsinmankong, J. W. Sleasman, S. V. Pizzo, and G. J. Cianciolo. 2006. LMP-420, a small-molecule inhibitor of TNF-alpha, reduces replication of HIV-1 and Mycobacterium tuberculosis in human cells. AIDS Res. Ther. 3:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hussain, R., A. Kaleem, and F. Shahid. 2002. Cytokine profiles using whole-blood assay can discriminate between tuberculosis patients and healthy endemic controls in a BCG-vaccinated population. J. Immunol. Methods 264:95-108. [DOI] [PubMed] [Google Scholar]
- 12.Jo, E. K., J. K. Park, and H. M. Dockrell. 2003. Dynamics of cytokine generation in patients with active pulmonary tuberculosis. Curr. Opin. Infect. Dis. 16:205-210. [DOI] [PubMed] [Google Scholar]
- 13.Keane, J., S. Gershon, R. P. Wise, E. Mirable-Levens, J. Kasznica, W. D. Schwieterman, J. N. Siegel, and M. W. Braun. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098-1104. [DOI] [PubMed] [Google Scholar]
- 14.Kremer, K., J. R. Glynn, T. Lilleback, S. Niemann, N. E. Kurepina, B. N. Kreiswirth, P. J. Bifani, and D. van Soolingen. 2004. Definition of the Beijing/W lineage of Mycobacterium tuberculosis on the basis of genetic markers. J. Clin. Microbiol. 42:4040-4049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin, Y., M. Zhang, F. M. Hofman, J. Gong, and P. F. Barnes. 1996. Absence of a prominent Th2 cytokine response in human tuberculosis. Infect. Immun. 64:1351-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lopez, B., D. Aguilar, H. Orozco, M. Burger, C. Espitia, V. Ritacco, L. Barrera, K. Kremer, R. Hernandez-Pando, K. Huygen, and D. van Soolingen. 2003. A marked difference in pathogenesis and immune response induced by different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 133:30-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miller, B. H., and T. M. Shinnick. 2000. Evaluation of M. tuberculosis genes involved in resistance to killing by human macrophages. Infect. Immun. 68:387-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nigou, J., C. Zelle-Rieser, M. Gilleron, M. Thurnher, and G. Puzo. 2001. Mannosylated lipoarabinomannans inhibit IL-12 production by human dendritic cells: evidence for a negative signal delivered through the mannose receptor. J. Immunol. 166:7477-7485. [DOI] [PubMed] [Google Scholar]
- 19.Pelly, T., D. A. J. Moore, R. Gilman, and C. Evans. 2004. Recent tuberculosis advances in Latin America. Curr. Opin. Infect. Dis. 17:397-403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riedel, D. D., and S. H. E. Kaufmann. 1997. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect. Immun. 65:4620-4623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rojas, M., L. F. Garcia, J. Nigou, G. Puzo, and M. Olivier. 2000. Mannosylated lipoarabinomannan antagonizes Mycobacterium tuberculosis-induced macrophages apoptosis by altering Ca+2-dependent cell signaling. J. Infect. Dis. 182:240-251. [DOI] [PubMed] [Google Scholar]
- 22.Smith, I. 2003. Mycobacterium tuberculosis pathogenesis and molecular determinants of virulence. Clin. Microbiol. Rev. 16:463-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Theus, S. A., M. D. Cave, and K. D. Eisenach. 2005. Intracellular macrophage growth rates and cytokine profiles of Mycobacterium tuberculosis strains with different transmission dynamics. J. Infect. Dis. 191:453-460. [DOI] [PubMed] [Google Scholar]
- 24.Torres, M., H. Herrera, H. Villareal, E. A. Rich, and E. Sada. 1998. Cytokine profiles for peripheral blood lymphocytes from patients with active pulmonary tuberculosis and healthy household contacts in response to the 30-kilodalton antigen of Mycobacterium tuberculosis. Infect. Immun. 66:176-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsao, T. C., C. C. Huang, and W. K. Chiou. 2002. Levels of interferon-gamma and interleukin-2 receptor-alpha for bronchoalveolar fluid and serum were correlated with clinical grade and treatment of pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 6:720-727. [PubMed] [Google Scholar]
- 26.van Crevel, R., T. H. M. Ottenhoff, and L. W. M. van der Meer. 2002. Innate immunity to Mycobacterium tuberculosis. Clin. Microbiol. Rev. 15:294-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van Embden, J. D. A., M. D. Cave, J. T. Crawford, J. W. Dale, K. D. Eisenach, B. Gicquel, P. Hermans, C. Martin, R. McAdam, T. M. Shinnick, and P. M. Small. 1993. Strain identification of Mycobacterium tuberculosis by DNA fingerprinting: recommendations for a standardized methodology. J. Clin. Microbiol. 31:406-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Soolingen, D., P. E. de Haas, P. E. Hermans, P. W. Groenen, and J. D. A. van Embden. 1993. Comparison of various repetitive DNA elements as genetic markers for strain differentiation and epidemiology of M. tuberculosis. J. Clin. Microbiol. 31:1987-1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Soolingen, D., L. Qian, E. W. Petra, P. E. de Haas, J. T. Douglas, H. Traore, F. Portaels, H. Z. Qing, D. Enkhsaikan, P. Nymadawa, and J. D. A. van Embden. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J. Clin. Microbiol. 33:3234-3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Warren, R. M., G. D. van der Spuy, M. Richardson, N. Beyers, C. Booysen, M. A. Behr, and P. D. van Helden. 2002. Evolution of the IS6110-based restriction fragment length polymorphism pattern during the transmission of Mycobacterium tuberculosis. J. Clin. Microbiol. 40:1277-1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Health Organization. 2006. Tuberculosis fact sheet. World Health Organization, Geneva, Switzerland. http://www.who.int/tb/publications/2006/tb_factsheet_2006_1_en.pdf.
- 32.Zhang, M., J. Gong, Y. Lin, and P. F. Barnes. 1998. Growth of virulent and avirulent Mycobacterium tuberculosis strains in human macrophages. Infect. Immun. 66:794-799. [DOI] [PMC free article] [PubMed] [Google Scholar]