Abstract
In response to the rising incidence of Haemophilus influenzae type b (Hib) disease in the United Kingdom, a national campaign to give a booster dose of single-antigen Hib conjugate vaccine to children aged 6 months to 4 years was undertaken in 2003. Children (n = 386) eligible for Hib vaccine in the campaign were recruited. Hib antibody concentrations were measured before boost and at 1 month, 6 months, 1 year, and 2 years after boost and were analyzed according to children's ages at booster dose and whether a Hib combination vaccine containing acellular pertussis (aP) or whole-cell pertussis (wP) components was given in infancy. The geometric mean antibody concentrations (GMCs) before the booster declined as the time since primary immunization increased (P < 0.001), and GMCs were threefold higher in recipients of wP-Hib than aP-Hib combination vaccines (P < 0.001). GMCs 1 month after the booster increased with age (P < 0.001) as follows: 6 to 11 months; 30 μg/ml (95% confidence interval [CI], 22 to 40); 12 to 17 months, 68 μg/ml (95% CI, 38 to 124); and 2 to 4 years, 182 μg/ml (151 to 220), with no difference according to the type of priming vaccine received. Antibody levels declined after the booster, but 2 years later, GMCs were more than 1.0 μg/ml for all age groups. By extrapolating data for the decline in antibody levels, we found the GMCs 4 years after boosting were predicted to be 0.6, 1.4, and 2.6 μg/ml for those boosted at 6 to 11 months, 12 to 17 months, and 2 to 4 years, respectively, with levels of at least 0.15 μg/ml in about 90% of individuals. A booster dose of Hib vaccine given after the first year of life should provide long-lasting protection.
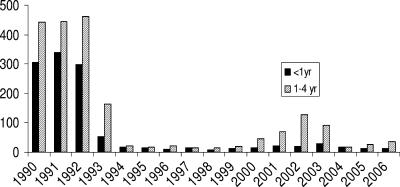
Prior to the inclusion of the conjugate vaccine in the routine schedule in October 1992, Haemophilus influenzae type b (Hib) was one of the three most common organisms causing bacterial meningitis in the United Kingdom (23). Unlike the situation with other developed countries, Hib vaccine was routinely given in the United Kingdom as an accelerated primary immunization schedule at 2, 3, and 4 months of age without a booster. Despite an initial dramatic reduction in disease incidence, a resurgence of cases occurred in 1999 (Fig. 1); of the 480 laboratory-confirmed Hib infections in England and Wales of children under 5 years of age between 1994 and 2002, around half occurred in 2001 and 2002 (www.hpa.org.uk/infections/topics_az/haemophilus_influenzae/data_lab_age_qtr.htm), with the majority occurring in fully vaccinated children (19). A number of factors are believed to have contributed to this increase.
FIG. 1.
Number of laboratory reports of Hib disease in England and Wales by age, 1990 to 2006 (data are from www.hpa.org.uk).
First, when Hib vaccine was introduced into the routine infant immunization schedule in 1992 there was a catch-up campaign in which all children between 1 and 4 years of age were offered a single dose of Hib vaccine. The program had a profound effect on disease incidence and reduced oropharyngeal carriage of Hib in the preschool population (15). Carriage has been shown to be important in the development of natural immunity (2), and following immunization, the reduction in carriage may have reduced potential opportunities for natural boosting.
Second, estimates of direct protection from the vaccine in the United Kingdom were lower than those predicted from an early intervention study (4), with efficacy waning from 61% in the first 2 years after 3 doses of vaccine in infancy to 27% thereafter (19). In addition, there was evidence of higher efficacy in children vaccinated at 1 to 4 years of age, with a single dose during the catch-up campaign, and less evidence of waning protection over time than in those vaccinated in infancy.
Third, due to shortages of diphtheria/tetanus/whole-cell pertussis/Hib (DTwP/Hib) combination vaccines in late 1999 in the United Kingdom, an acellular pertussis (aP)-containing combination vaccine (DTaP/Hib, Infanrix-Hib) was used instead; this vaccine comprised around 50% of the doses distributed in the United Kingdom over the period of 2000 to 2002 (19). Although some aP-containing combination vaccines have a reduced immunogenicity of the Hib component, particularly when given only 1 month apart (24), these vaccines still induce immunological memory, which was argued to be a more appropriate correlation of protection for a conjugate vaccine (5). However, a case-control study in the United Kingdom showed that infants who received 3 doses of DTaP/Hib were at an 8.4-fold-higher risk of vaccine failure than those fully immunized with DTwP/Hib vaccine (14).
Because of the increased risk of vaccine failure in the DTaP/Hib-vaccinated cohorts and evidence of waning protection even in those vaccinated with DTwP/Hib in infancy (18), the United Kingdom Department of Health offered a booster (fourth) dose of Hib vaccine to all children aged 6 months to 4 years in a catch-up campaign in 2003 (Chief Medical Officer Letter PL CMO (2003)1 [www.dh.gov.uk/en/publicationsandstatistics/lettersandcirculars/professionalletters/chiefmedicalofficerletters/dh_4004814] and Chief Medical Officer Letter PL CMO (2003)2 [www.dh.gov.uk/en/publicationsandstatistics/lettersandcirculars/professionalletters/chiefmedicalofficerletters/dh_4004833]) and, in September 2006, a routine Hib booster was introduced at 12 months of age (Chief Medical Officer Letter PL CMO (2006)1 [www.dh.gov.uk/en/publicationsandstatistics/lettersandcirculars/professionalletters/chiefmedicalofficerletters/dh_4137171]).
The observed population effect of introducing a less immunogenic vaccine suggests that lower levels of circulating antibody are associated with poorer protection. This study is the first to measure the persistence of Hib antibody since priming and the response to a booster dose in children of different ages and varied primary vaccination histories.
MATERIALS AND METHODS
Study population.
Children aged 6 months to 4 years, attending immunization clinics in Hertfordshire and Gloucestershire, England, and who were eligible for a fourth dose of Hib vaccine in the national campaign of 2003 were recruited. Criteria for study participation included the receipt of three doses of Hib vaccine in infancy and the provision of written informed consent by a parent or guardian. In line with United Kingdom recommendations (20), children who had a history of severe reaction to a previous dose of Hib vaccine were excluded from the study. Immunization was deferred if a child had an acute illness and/or a temperature of more than 38°C on the day of vaccination.
Treatment and follow-up schedule.
All participants received a booster dose of single-antigen Hib vaccine conjugated to tetanus toxoid (TT) as used in the national campaign (Hiberix; GlaxoSmithKline). All doses given in the study were from a single batch. The DTP/Hib vaccine that had been given for primary immunization was recorded, together with the batch number/manufacturer of any concomitant meningococcal C conjugate (MCC) vaccine. Three MCC vaccines were in use in the United Kingdom when the study cohort was eligible for primary immunization; two were MCC-CRM (CRM is a nontoxic mutant of the diphtheria toxin isolated from cultures of Corynebacterium diphtheriae) conjugates (Meningitec and Menjugate), and the third was an MCC-TT conjugate (NeisVacC). The MCC-TT vaccine contains the same carrier protein as the Hib vaccine does, and this may affect the response after the primary immunization (13).
Blood samples were collected prior to booster vaccination and at 1 month (±1 week), 6 months (±1 week), 1 year (±1 month), and 2 years (±1 month) later. Serious adverse events were collected throughout the study period.
Serology.
Hib-specific antibodies (immunoglobulin G [IgG]) were quantified using a standardized enzyme-linked immunosorbent assay (ELISA) at the Immunoassay Laboratory, Centre for Emergency Preparedness and Response, Health Protection Agency, Porton Down, Wiltshire, England (17). Sera were titrated against an international Hib reference serum (lot 1983; Center for Biologics and Evaluation Research) in which the quantity of specific antibody was known.
To fulfill a duty of care, a further dose of Hiberix was offered to any subject with a Hib IgG concentration 4 to 6 weeks postvaccination below the putative protective threshold of 0.15 μg/ml (12).
Analyses.
In all analyses, the outcome of interest was the Hib antibody concentration at the various blood sampling time points and whether this value was at least 0.15 μg/ml (the putative protective level) or more than 1.00 μg/ml (which is considered predictive of longer-term protection [12]). The main explanatory variables of interest were as follows: (i) age at boosting (stratified into 6 to 11 months, 12 to 17 months, and 2 to 4 years), (ii) time from completion of the primary vaccination to obtaining the prebooster blood sample, (iii) number of doses of MCC-TT given as part of the primary immunization schedule, (iv) number of doses of DTwP and DTaP given as part of the primary immunization schedule (only children boosted at 2 to 4 years of age received primary vaccination at a time when DTwP was available), and (v) time from boosting to the retrieval of each subsequent blood sample at about 1 month, 6 months, 1 year, and 2 years.
The age groups were chosen to reflect possible ages at which a fourth dose might be routinely scheduled. Other explanatory variables examined were which particular nurse administered the vaccination, the sex of the patient, and the antibody concentration before the booster.
Within groups of interest, data were analyzed by the calculation of geometric means with 95% CIs and proportions of at least 0.15 μg/ml or of more than 1.00 μg/ml. Groups were compared using a t test/Kruskal-Wallis test (for GMCs) or chi-squared test/Fisher's exact test (for proportions). Results at different time points were compared using paired t tests. Multivariable analysis was performed on log10 titers using normal error regression or, where many results were less than 0.15 μg/milliliter, probit regression. The decline in antibody levels after primary vaccination and booster was also modeled as a function of time since vaccination by using regression.
Sample size.
The original recruitment target was 850 individuals (450 children aged 2 to 4 years and 400 children aged 6 to 18 months). This relatively large sample size was based on the outcome measure being the proportion with concentrations of more than 1.0 μg/ml with the aim to detect meaningful differences (e.g., 30% versus 13%, with 80% power at a 5% significance level) in such proportions across ages (as a trend) and according to the DTP vaccines received in infancy. In the 2-month time frame for the campaign, the recruitment was less at 388. This reduced sample size, along with the high proportions achieving concentrations of more than 1.0 μg/ml after the booster, meant that GMCs were used as the main outcome for comparisons. More than 80% power was used to detect twofold differences in GMC across ages and according to vaccine used for priming.
RESULTS
Study population.
A total of 388 children were recruited to the study; their distribution across the age groups is shown in Table 1. The same children did not necessarily contribute to sampling at each point due to failed venepuncture and nonattendance. Of the 2- to 4-year-old cohort, 124 participants had been primed exclusively with DTwP/Hib, 70 participants were primed with DTaP/Hib, and 73 participants had received mixed courses. Concomitant MCC-TT had been given to 71 children, of whom 40 had received MCC-TT for all 3 doses, the remainder either had received MCC-CRM or had no record of concomitant MCC vaccine administration.
TABLE 1.
Hib IgG GMCs before boosting and after a booster dose of single-antigen Hib vaccine
| Age at booster | na | GMC μg/ml (95% CI)
|
||||
|---|---|---|---|---|---|---|
| Before booster | At indicated time after booster
|
|||||
| One mo | Six mo | One yr | Two yr | |||
| 6-11 mo | 89 | 1.98 (1.45-2.70) | 29.87 (22.26-40.08) | 5.28 (4.06-6.87) | 2.48 (1.91-3.23) | 1.30 (1.00-1.70) |
| 12-17 mo | 32 | 0.89 (0.32-2.43) | 68.41 (37.63-124.37) | 11.70 (5.59-24.50) | 6.69 (3.14-14.26) | 4.99 (1.98-12.60) |
| 2-4 yr | 267 | 0.38 (0.31-0.47) | 182.36 (151.31-219.78) | 23.70 (20.00-28.08) | 9.22 (7.51-11.32) | 5.92 (4.84-7.24) |
n is the no. of participants who provided at least one blood sample.
Serology.
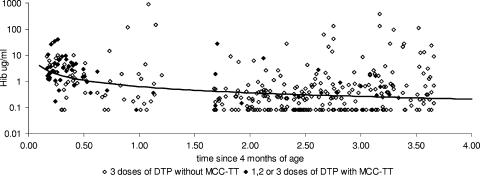
The GMCs and proportions of at least 0.15 and of more than 1.00 μg/ml in the prebooster sample showed a significant decrease (P < 0.001) with age at booster (Tables 1 and 2), this being a surrogate for time since the last dose of Hib vaccine in infancy. This decline with time since primary vaccination was initially rapid and then more gradual, and it was modeled as a log-log relationship that gave an estimate of a 42% decline in antibody concentration for every twofold change in time (Fig. 2). Taking into account the time since vaccination, the severalfold difference for those who had at least one dose of MCC-TT compared to those who had none was 2.1 (95% CI, 1.1 to 3.9) for those aged under 1 year at the prebooster bleed and 1.0 (95% CI, 0.4 to 2.6) for those aged over 1 year at the time of the prebooster bleed.
TABLE 2.
Percentages of children with antibody concentrations of ≥0.15 μg/ml and >1.0 μg/ml by age at boosting for each blood sample
| Age at booster | % of children with indicated antibody concn (μg/ml)a
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Before booster
|
At indicated time after booster
|
|||||||||
| One mo
|
Six mo
|
One yr
|
Two yr
|
|||||||
| ≥0.15 | >1.0 | ≥0.15 | >1.0 | ≥0.15 | >1.0 | ≥0.15 | >1.0 | ≥0.15 | >1.0 | |
| 6-11 mo | 94 (75/80) | 75 (60/80) | 99 (80/81) | 99 (80/81) | 100 (78/78) | 95 (74/78) | 100 (71/71) | 77 (55/71) | 99 (66/67) | 54 (36/67) |
| 12-17 mo | 77 (20/26) | 38 (10/26) | 100 (29/29) | 100 (29/29) | 96 (22/23) | 96 (22/23) | 95 (20/21) | 95 (20/21) | 100 (14/14) | 86 (12/14) |
| 2-4 yr | 64 (162/253) | 19 (48/253) | 100 (235/235) | 99 (233/235) | 100 (199/199) | 99 (197/199) | 99 (157/158) | 97 (153/158) | 100 (158/158) | 91 (143/158) |
Values in parentheses are no. of children with the indicated antibody concentration/total no. of children.
FIG. 2.
Decline in Hib IgG antibody concentration after primary vaccination with fitted trend line and according to primary MCC vaccination.
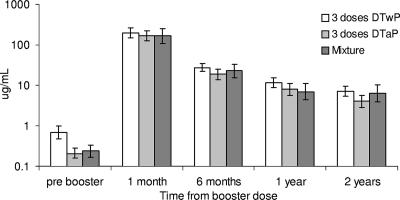
In the group aged 2 to 4 years, the GMCs before the booster differed significantly (P < 0.001) according to the type of vaccine received in infancy, with levels more than threefold higher in those primed exclusively with DTwP/Hib vaccine (GMC, 0.70 μg/ml; 95% CI, 0.49 to 1.00) compared with those primed exclusively with DTaP/Hib (GMC, 0.21 μg/ml; 95% CI, 0.16 to 0.29) (Fig. 3). Similarly, the proportions that were at least 0.15 μg/ml and more than 1.0 μg/ml were significantly higher (P < 0.001) in participants primed exclusively with DTwP/Hib vaccine (76% and 33%, respectively) than in those primed exclusively with DTaP/Hib (53% and 4%, respectively). After the booster, there were no significant differences between those primed with DTaP/Hib and DTwP/Hib vaccines (Fig. 3), and the data for these groups are therefore combined in the tables.
FIG. 3.
Hib IgG geometric mean concentrations and 95% CIs according to type of Hib combination received for primary immunization and timing of blood sample in children aged 2 to 4 years at time of boosting. Error bars indicate standard deviations.
One month after the booster, all age groups achieved a significant increase in GMC compared to the levels before the booster (P < 0.001) (Table 1). GMCs also increased as the age at boosting increased, with severalfold differences relative to the group aged 6 to 11 months of 2.3 (95% CI, 1.3 to 4.2) and 6.1 (95% CI, 4.3 to 8.7) for the groups aged 12 to 17 months and 2 to 4 years, respectively. Lower prevaccination concentrations in the older age groups did not explain this age effect since there was a positive correlation between pre- and postvaccination concentrations with a 1.25-fold increase in the postvaccination micrograms per milliliter for every 10-fold increase in prevaccination micrograms per milliliter.
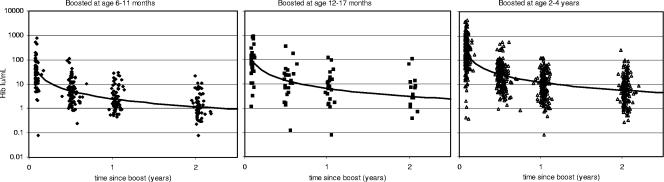
Significant declines in antibody concentrations after the booster were seen between sampling points (P < 0.001). As with the decline after the primary immunization, these declines followed a log-log relationship with time and were similar between age groups, with a 53% decline for every twofold change in time since boosting (Fig. 4). This age-independent pattern of decline meant that severalfold differences between age groups after the booster remained similar at each time point, averaging 2.5- and 4.7-fold differences for the groups aged 12 to 17 months and 2 to 4 years, respectively, relative to the group aged 6 to 11 months. We extrapolated the data for the decline in antibody levels to 4 years after boosting to give predicted geometric means of 0.6, 1.4, and 2.6 μg/ml for those boosted at 6 to 11 months, 12 to 17 months, and 2 to 4 years, respectively (Fig. 4). It is also predicted that at 4 years after the booster, no more than 10% of individuals would have levels below 0.15 μg/ml irrespective of age at boosting. The decline after the booster was more rapid than the 42% seen after the primary vaccination, but the higher initial concentrations meant that concentrations remained at a higher level.
FIG. 4.
Decline in Hib IgG antibody concentration by age at boosting and by the time since boosting with fitted trend lines.
One month after the booster, all children, apart from one in the group aged 6 to 11 months, had achieved antibody concentrations of at least 0.15 μg/ml, with the majority achieving concentrations of more than 1.0 μg/ml (Table 2). The proportion with antibody concentrations of at least 0.15 μg/ml was maintained for the 2-year sample, but proportions of more than 1.0 μg/ml fell over time, particularly in the group aged 6 to 11 months, of whom only 54% were above this level 2 years after boosting.
One child who was vaccinated at 7 months of age was given an extra dose in line with the protocol and did not attend for subsequent sample collection.
No effect of which nurse administered the vaccine or of the sex of the vaccinee was found in the multivariable regression analyses.
Serious adverse events.
No serious adverse events were recorded in the period of 4 to 6 weeks following vaccination. Hospitalizations during the trial, for rotaviral enteritis and Henoch-Schonlein purpura at 20 months and 26 months after Hib vaccination, respectively, were deemed unrelated to the vaccination.
DISCUSSION
This study aimed to assess differences in the antibody responses to a booster dose of Hib vaccine according to age at booster, time since priming, and nature of the priming vaccine. In addition, the analysis of persistence of antibodies in the prebooster samples by time since priming and by nature of vaccine received allowed the effect of these factors on immunogenicity to be compared with their known effect on clinical protection. We showed that in those primed with DTaP/Hib vaccine in infancy, the persistence of antibody at 2 to 4 years of age was poorer than in those primed with DTwP/Hib vaccine, consistent with the greater risk of vaccine failure observed in these cohorts (14). There was no significant difference, either in the immediate antibody response or in the persistence of antibody at 2 years, between these groups after the booster, indicating that the differences in protection after the priming course are unlikely to result in differences in long-term protection after the booster.
Previous United Kingdom studies of Hib antibody persistence after infant immunization at 2, 3, and 4 months have also found higher levels after primary immunization with DTwP/Hib than DTaP/Hib. Johnson et al. measured antibody concentrations in 2 to 4 year olds prior to their receiving a booster in the 2003 campaign; the GMC in DTwP/Hib recipients was 0.61 μg/ml (95% CI, 0.41 to 0.92), compared with 0.30 μg/ml (95% CI, 0.19 to 0.49) in DTaP/Hib recipients (11). These levels are remarkably close to those reported here. In two previous studies by our group in the same Hertfordshire and Gloucestershire populations in 1996 to 1997, 401 infants vaccinated with DTwP/Hib had a Hib GMC of 0.41 (95% CI, 0.35 to 0.47) at 12 to 18 months of age (6), with a significantly lower GMC of 0.25 (95% CI, 0.21 to 0.30) among 120 DTaP/Hib vaccinees bled at a median age of 16 months (7). While the severalfold differences between DTwP/HIb and DTaP/Hib groups are similar in the various studies (around two- to threefold), the absolute levels are significantly higher in the more recent cohorts. This difference was not explained by augmentation of the initial Hib response by concomitant MCC-TT, as this effect did not persist past the first year of life. It may therefore reflect a greater opportunity for natural boosting through carriage in the more recent cohorts who were followed up during a period of Hib resurgence. This result is supported by the findings of Johnson et al. (11), who swabbed children before they received the booster and found a pharyngeal carriage rate of 2.1%, with a further 4.5% of children with high antibody levels suggestive of recent boosting through colonization. Studies of Hib antibody concentrations and disease incidence in adults over the period of 1991 to 2003 also suggest an important role for boosting through carriage in determining population immunity (16).
The approximate twofold difference in antibody levels of less than 0.15 μg/ml persisting since priming between DTwP/Hib and DTaP/Hib recipients seems inconsistent with an 8.4-fold difference in the risk of vaccine failure as reported by McVernon et al. (14). However, a more recent case-control study of host and environmental risk factors for Hib disease in which controls were matched by district of residence found a lower odds ratio of 2.88 (95% CI, 0.99 to 8.37) for the risk of vaccine failure in DTaP/Hib compared with the ratio for DTwP/Hib recipients, which is more in line with the difference in immunogenicity (J. McVernon, personal communication).
The height of the antibody response and persistence after boosting were shown to be related to the age at administration, with better responses in those boosted after the first year of life. However, irrespective of the age at boosting, almost all children had antibody concentrations of at least 0.15 μg/ml 2 years after boosting, with around 90% predicted to be above this level at 4 years. This proportion is similar to that reported in a recent study of 341 children in Germany in which Hib antibody persistence was assessed 3 years after a fourth booster dose of a DTaP/Hib vaccine combined with hepatitis B and inactivated poliovirus vaccine given in the second year of life. This formulation contained the same Hib component vaccine as that used in the present study, and encouragingly, more than 90% of the children had antibody levels persisting above the putative protective level of at least 0.15 μg/ml (9).
The log-log relationship observed in all age groups between antibody decline and time since vaccination with an initial rapid decline, followed by a more gradual fall, suggests that the underlying biological mechanism of antibody persistence is similar. The decline in antibody levels with time since priming also followed a log-log relationship, albeit at a lower level than the booster curves. The initial rapid decline in antibody titers could be due to the dominance of the early response by short-lived plasma cells (half-life of 3 to 14 days in mice) that produce antibody shortly after antigen exposure (10). Some plasma cells have a longer half-life (typically 3 to 4 months in mouse models [21]) and these, together with memory B cells, may be responsible for maintaining serum antibody levels over a longer period of time. It is still not clear what the stimulus might be for the ongoing secretion of antigen-specific antibody, but this might be due to bystander stimulation of memory B cells by unrelated antigens (3) or by the encounter with antigen (perhaps via nasopharyngeal carriage) over time (8). Persistence of antibody may thus partly rely on the presence of immunological memory, although both vaccine-induced and natural priming for memory against Hib in the absence of circulating antibody may still leave an individual vulnerable to infection (1). Higher antibody titers were reached in older individuals following boosting, which may be due to natural maturation of the immune system, and were reflected in a higher number of B cells being recruited and more robust memory being laid down, although the mechanisms responsible for this remain elusive. Higher titers may, however, translate into greater persistence of clinical protection, as has been seen for meningococcal C conjugate in the United Kingdom (22).
It is hoped that the routine Hib booster now being given in the second year of life will prevent a future resurgence of disease as seen in the United Kingdom in 1999 to 2002. The clinical protection achieved by the new booster program will be evaluated, which, in conjunction with antibody persistence and carriage studies, will contribute to improving our understanding of the role of vaccination and natural boosting in determining population immunity.
Acknowledgments
We thank the vaccine research nurse teams in Gloucester and Hertfordshire, the laboratory immunoassay team at the HPA Centre for Emergency Preparedness and Response, and the administrative team at the HPA Centre for Infections for their assistance in the conduct of the study.
We thank the Department of Health Research and Development Directorate for financial support of this study under grant 1217470.
Footnotes
Published ahead of print on 15 August 2007.
REFERENCES
- 1.Anderson, P., D. L. Ingram, M. E. Pichichero, and G. Peter. 2000. A high degree of natural immunologic priming to the capsular polysaccharide may not prevent Haemophilus influenzae type b meningitis. Pediatr. Infect. Dis. J. 19:589-591. [DOI] [PubMed] [Google Scholar]
- 2.Barbour, M. L., R. T. Mayon-White, C. Coles, D. W. Crook, and E. R. Moxon. 1995. The impact of conjugate vaccine on carriage of Haemophilus influenzae type b. J. Infect. Dis. 171:93-98. [DOI] [PubMed] [Google Scholar]
- 3.Bernasconi, N. L., E. Traggiai, and A. Lanzavecchia. 2002. Maintenance of serological memory by polyclonal activation of human memory B cells. Science 298:2199-2202. [DOI] [PubMed] [Google Scholar]
- 4.Booy, R., S. Hodgson, L. Carpenter, R. Mayon-White, M. Slack, J. Macfarlane, E. A. Haworth, M. Kiddle, S. Shribman, and J. S. Roberts. 1994. Efficacy of Haemophilus influenzae type b conjugate vaccine PRP-T. Lancet 344:362-366. [DOI] [PubMed] [Google Scholar]
- 5.Eskola, J., J. Ward, R. Dagan, D. Goldblatt, F. Zepp, and C. A. Siegrist. 1999. Combined vaccination of Haemophilus influenzae type b conjugate and diphtheria-tetanus-pertussis containing acellular pertussis. Lancet 354:2063-2068. [DOI] [PubMed] [Google Scholar]
- 6.Goldblatt, D., E. Miller, N. McCloskey, and K. Cartwright. 1998. Immunological response to conjugate vaccines in infants: follow up study. BMJ 16:1570-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goldblatt, D., P. Richmond, E. Millard, C. Thornton, and E. Miller. 1999. The induction of immunologic memory after vaccination with Haemophilus influenzae type b conjugate and acellular pertussis-containing diphtheria, tetanus, and pertussis vaccine combination. J. Infect. Dis. 180:538-541. [DOI] [PubMed] [Google Scholar]
- 8.Goldblatt, D., M. Hussain, N. Andrews, L. Ashton, C. Virta, A. Melegaro, R. Pebody, R. George, A. Soininen, J. Edmunds, N. Gay, H. Kayhty, and E. Miller. 2005. Antibody responses to nasopharyngeal carriage of Streptococcus pneumoniae in adults: a longitudinal household study. J. Infect. Dis. 192:387-393. [DOI] [PubMed] [Google Scholar]
- 9.Heininger, U., R. Sanger, J. M. Jacquet, L. Schuerman, et al. 2007. Booster immunization with a hexavalent diphtheria, tetanus, acellular pertussis, hepatitis B, inactivated poliovirus vaccine, and Haemophilus influenzae type b conjugate combination vaccine in the second year of life: safety, immunogenicity, and persistence of antibody responses. Vaccine 25:1055-1063. [DOI] [PubMed] [Google Scholar]
- 10.Ho, F., J. E. Lortan, I. C. MacLennan, and M. Khan. 1986. Distinct short-lived and long-lived antibody-producing cell populations. Eur. J. Immunol. 16:1297-1301. [DOI] [PubMed] [Google Scholar]
- 11.Johnson, N. G., J. U. Ruggeberg, G. F. Balfour, Y. C. Lee, H. Liddy, D. Irving, J. Sheldon, M. P. Slack, A. J. Pollard, and P. T. Heath. 2006. Haemophilus influenzae type b reemergence after combination immunization. Emerg. Infect. Dis. 12:937-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Käyhty, H., H. Peltola, V., Karanko, and P. H. Makela. 1983. The protective level of serum antibodies to the capsular polysaccharide of Haemophilus influenzae type b. J. Infect. Dis. 147:1100. [DOI] [PubMed] [Google Scholar]
- 13.Kitchin, N. R., J. Southern, R. Morris, F. Hemme, S. Thomas, M. W. Watson, K. Cartwright, and E. Miller. 2007. Evaluation of a diphtheria-tetanus-acellular pertussis-inactivated poliovirus-Haemophilus influenzae type b vaccine given concurrently with meningococcal group C conjugate vaccine at 2, 3 and 4 months of age. Arch. Dis. Child. 92:11-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McVernon, J., N. Andrews, M. P. Slack, and M. E. Ramsay. 2003. Risk of vaccine failure after Haemophilus influenzae type b (Hib) combination vaccines with acellular pertussis. Lancet 361:1521-1523. [DOI] [PubMed] [Google Scholar]
- 15.McVernon, J., A. J. Howard, M. P., Slack, and M. E. Ramsay. 2004. Long-term impact of vaccination on Haemophilus influenzae type b (Hib) carriage in the United Kingdom. Epidemiol. Infect. 132:765-767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McVernon, J., C. L. Trotter, M. P. Slack, and M. E. Ramsay. 2004. Trends in Haemophilus influenzae type b infections in adults in England and Wales: surveillance study. BMJ 329:655-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Phipps, D. C., J. West, R. Eby, M. Koster, D. V. Madore, and S. A. Quataert. 1990. An ELISA employing a Haemophilus influenzae type b oligosaccharide-human serum albumin conjugate correlates with the radioantigen binding assay. J. Immunol. Methods 135:121-128. [DOI] [PubMed] [Google Scholar]
- 18.Ramsay, M. E., E. Miller, N. Andrews, M. Slack, and P. Heath. 2004. Epidemiological data are essential. BMJ http://bmj.bmjjournals.com/cgi/eletters/bmj.38301.657014.79v1#893.
- 19.Ramsay, M. E., J. McVernon, N. J. Andrews, P. T. Heath, and M. P. Slack. 2003. Estimating Haemophilus influenzae type b vaccine effectiveness in England and Wales by use of the screening method. J. Infect. Dis. 88:481-485. [DOI] [PubMed] [Google Scholar]
- 20.Salisbury, D. M., and N. T. Begg (ed.). 1986. Immunisation against infectious disease—the green book. HMSO Publications, London, United Kingdom.
- 21.Slifka, M. K., R. Antia, J. K. Whitmire, and R. Ahmed. 1998. Humoral immunity due to long-lived plasma cells. Immunity 8:363-372. [DOI] [PubMed] [Google Scholar]
- 22.Trotter, C. L., N. J. Andrews, E. B. Kaczmarski, E. Miller, and M. E. Ramsay. 2004. Effectiveness of meningococcal serogroup C conjugate vaccine 4 years after introduction. Lancet 364:365-367. [DOI] [PubMed] [Google Scholar]
- 23.Urwin, G., M. F. Yuan, and R. A. Feldman. 1994. Prospective study of bacterial meningitis in North East Thames region, 1991-3, during introduction of Haemophilus influenzae vaccine. BMJ 309:1412-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vidor, E., A. Hoffenbach, and M. A. Fletcher. 2001. Haemophilus influenzae type b vaccine: reconstitution of lyophilised PRP-T vaccine with a pertussis-containing paediatric combination vaccine, or a change in the primary series immunisation schedule, may modify the serum anti-PRP antibody responses. Curr. Med. Res. Opin. 17:197-209. [DOI] [PubMed] [Google Scholar]